Abstract
Energy status plays a key role in the health of athletes and exercising individuals. Energy deficiency/low energy availability (EA), referring to a state in which insufficient energy intake and/or excessive exercise energy expenditure has resulted in compensatory metabolic adaptations to conserve fuel, can affect numerous physiological systems in women and men. The Female Athlete Triad, Male Athlete Triad, and Relative Energy Deficiency in Sport (RED-S) models conceptualize the effects of energy deficiency in athletes, and each model has strengths and limitations. For instance, the Female Athlete Triad model depicts relationships between low EA, reproductive, and bone health, underpinning decades of experimental evidence, but may be perceived as limited in scope, while the more recent RED-S model proposes a wider range of potential health effects of low EA, though many model components require more robust scientific justification. This critical review summarizes current evidence regarding the effects of energy deficiency on athlete health by addressing the quality of the underlying science, the strengths and limitations of each model, and highlighting areas where future research is needed to advance the field. With the health and wellness of athletes and exercising individuals as the overarching priority, we conclude with specific steps that will help focus future research on the Female and Male Athlete Triad and RED-S, and encourage all researchers, clinicians, and practitioners to collaborate to support the common goal of promoting the highest quality science and evidence-based medicine in pursuit of the advancement of athletes’ health, well-being, and performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
All stakeholders—i.e., athletes, sports medicine physicians, athletic trainers, researchers, sports dietitians, sports psychologists, coaches, etc.—understand the current state of the science to support improvements in the health and clinical care of athletes with, and at risk for, energy deficiency/low energy availability. |
The establishment of a causal role for low energy availability in the development of Triad and RED-S outcomes needs to be a focus of high-quality research in these areas, inclusive of randomized controlled trials and studies of long-term effects when possible. |
While both the Triad and RED-S models have limitations, the advancement of the RED-S model will only be possible with rigorous experiments on non-Triad components, i.e., gastrointestinal, immunological, hematological, cardiovascular, etc., establishing causal relationships for low energy availability with these outcomes. |
1 Introduction
Energy status plays a key role in the health of athletes and exercising individuals. Energy deficiency/low energy availability (EA), referring to a state in which insufficient energy intake and/or excessive exercise energy expenditure has resulted in compensatory metabolic adaptations to conserve fuel, can affect numerous physiological systems in women and men. The deleterious physiological effects of chronic energy deficiency/low EA on an individual’s health, performance, and well-being are important concerns for athletes and exercising individuals and, therefore, warrant thorough investigation and understanding. As such, it is necessary that all stakeholders—i.e., athletes, sports medicine physicians, athletic trainers, researchers, sports dietitians, sports psychologists, coaches, etc.—understand the current state of the science to support improvements in the health and clinical care of athletes with, and at risk for, energy deficiency/low EA. This paper provides a critical review of the strengths, limitations, and gaps in the Female and Male Athlete Triad (Triad) and Relative Energy Deficiency in Sport (RED-S) models and encourages researchers and clinicians to collaborate to support the common goal of promoting the highest quality science and evidence-based medicine in pursuit of the advancement of athlete health and well-being.
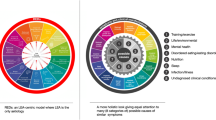
2 A Brief History of the Female Athlete Triad
The Female Athlete Triad (Triad), a syndrome characterized by the interrelated conditions of low EA, menstrual dysfunction, and compromised bone health, has been defined based on decades of scientific progress and evidence-based research. As early as the 1960s–1970s, the first scientific reports of menstrual disturbances in female athletes were published [1,2,3,4], laying the groundwork for future research delving into female-specific issues in exercise physiology. Dr. Barbara Drinkwater, a key pioneer, established the foundational understanding of the relationship between menstrual disturbances and compromised bone health in female athletes in the 1980s [5, 6]. Investigations of bone health and menstrual dysfunction continued (e.g. [7, 8]), leading to a Task Force on Women’s Issues of the American College of Sports Medicine (ACSM) in 1992, where researchers and clinicians devoted to the health of exercising women gathered to critically assess the evidence for a “Triad” of clinical disorders presenting in female athletes—disordered eating, amenorrhea, and osteoporosis—and hence the term the “Female Athlete Triad” was coined [9]. With the official recognition of a syndrome of three interrelated conditions [10], rigorous scientific inquiry progressed and our understanding of the mechanisms underpinning the Triad consequently evolved. The first ACSM Female Athlete Triad position stand, published in 1997 [11], reflected the most scientifically sound Triad research available to date and described the hypothesized mechanism that low EA disrupts the hypothalamic-pituitary-ovarian axis and suppresses menstruation, and that resultant hypoestrogenemia negatively affects bone mineral density [5, 7, 12, 13].
In 2007, the position stand was updated [14] to again reflect the most current science available; based on the evidence that had emerged, the Triad model was expanded to encompass a spectrum upon which each of the Triad components ranges from healthy (optimal EA, eumenorrhea, and optimal bone health) to pathological endpoints (low EA, functional hypothalamic amenorrhea, and osteoporosis). The updated Triad model was influenced by the work of Dr. Anne Loucks, who conducted high-quality, well-controlled laboratory experiments in which induced low EA resulted in a host of endocrine and metabolic adaptations, including altered luteinizing hormone (LH) pulsatility and altered concentrations of thyroid hormones, insulin-like growth factor-1 (IGF-1), insulin, cortisol, and bone-turnover markers [15,16,17,18,19]. The findings by Loucks and colleagues causally linked and described endocrinological underpinnings of low EA to the clinical reproductive and bone health outcomes in the Triad model [14]. Well-designed cross-sectional and longitudinal research studies in laboratory and free-living environments further bolstered the scientific evidence supporting the updated Triad model [17, 19,20,21,22,23,24,25,26,27,28,29,30]. In line with continued advancement in the scientific and clinical understanding of the Triad, an evidence-based consensus statement establishing treatment and return to play guidelines was published in 2014 by experts in the field representing the Female Athlete Triad Coalition [31, 32], a non-profit 501(c)(3) organization comprising an international group of clinicians and scientists.
3 Emergence of the Relative Energy Deficiency in Sport (RED-S) Model
In 2014, authors representing the International Olympic Committee (IOC) published a consensus statement that introduced new terminology: “Relative Energy Deficiency in Sport (RED-S)” [33] (updated in 2018 [34]). RED-S, defined as “energy deficiency relative to the balance between dietary intake and energy expenditure,” describes effects of low EA on reproductive and bone health, as outlined in the Triad model, as well as impairments in additional systems, including gastrointestinal (GI), immunological, hematological, growth and development, and psychological outcomes [33, 34]. This multi-system approach to the purported effects of energy deficiency is inclusive of male athletes. The RED-S model, which proposes a broader syndrome, is indeed an interesting concept that has sparked the interest of the sports medicine community.
The 2014 RED-S Consensus Statement also stated that “new terminology is required to more accurately describe the clinical syndrome originally known as the Female Athlete Triad” [33]. This language has led to confusion among stakeholders and resulted in the interpretation that the RED-S model supersedes and replaces the Triad model and that the Triad model no longer exists as a meaningful condition or diagnosis [35]. The path forward must include the understanding that the Female Athlete Triad and the emerging model of the Male Athlete Triad exist alongside and independent of RED-S. Additionally, the Female Athlete Triad and the RED-S Consensus Statements each include a different risk stratification algorithm, leaving practitioners to have to choose which approach to use.
It is important to note that the Triad components are the most robustly supported facets of the RED-S model, and represent clinically relevant outcomes backed by scientific evidence outlined in numerous expert consensus statements for diagnosis and treatment of functional hypothalamic amenorrhea [36], eating disorders [37], and osteoporosis [38]. Furthermore, the decades of research describing the interrelationships among energy, reproduction, and bone health in exercising women, and the endocrine and metabolic adaptations to energy deficiency highlighted in the experiments by Loucks and colleagues [15,16,17,18,19], are foundational to the RED-S model.
As the RED-S model is relatively new, several components that differentiate RED-S from the Triad (i.e., gastrointestinal, immunological, and hematological concerns) warrant well-designed research to investigate the true causal effects of low EA on these systems and the clinical relevance of proposed outcomes. This paper is designed to identify the gaps and areas of future research for both the Triad and RED-S and provide a direction forward.
4 Energy Deficiency in Men
Research emerged in the mid 1980s–early 1990s to indicate that a subset of male athletes presented with altered reproductive hormones (i.e., testosterone, LH, prolactin) [39,40,41,42] and poor bone health [43], suggesting an effect of energy deficiency in men. While the proposition that male athletes may also suffer from reproductive and bone health impairments in response to eating disorders was introduced in the 1997 Female Athlete Triad consensus statement [11], men were not a focal point of Triad research at that time. A most noteworthy aspect of RED-S was the explicit call to investigate the effects of energy deficiency in men, highlighting that the detrimental effects of energy deficiency are not limited to exercising girls and women. In response to the call for research into energy deficiency in men, researchers convened in a round-table meeting in 2017 to discuss current evidence and the basis for a Triad-like condition in men. The resultant product was a two-part consensus statement on the Male Athlete Triad, published in 2021, which introduced the interrelationship of energy deficiency/low EA with or without disordered eating/eating disorders, functional hypogonadotropic hypogonadism, and osteoporosis or low bone mineral density with or without bone stress injuries [44, 45]. An additional result of the roundtable and emphasis on required research in men was the renaming of the Female Athlete Triad Coalition as the Female and Male Athlete Triad Coalition in 2018.
5 Gaps to be Addressed in Triad Science
Scientific progress is dependent on developing and implementing well-designed research studies, reporting and interpreting novel results, and critically examining the quality of available evidence. In the early stages of Triad research, criticisms were raised regarding causality among conditions and overstating its prevalence and importance [46,47,48]. Such criticisms motivated researchers to develop well-designed laboratory-based studies [16, 18, 19, 22,23,24, 49,50,51,52] and prospective and randomized clinical studies [27,28,29,30, 53,54,55,56] to address specific concerns and to advance Triad science. Despite significant progress since that time, the understanding of Triad physiology and clinical consequences of low EA on reproductive and bone health is still incomplete. Further research is necessary to determine: (1) long-term effects on fertility, (2) long-term effects on bone health, including the state of bone health of previously amenorrhoeic athletes when reaching the menopausal transition and beyond, (3) preventive countermeasures, and (4) timing of and capacity for reversal of clinical endpoints. Ongoing work is aimed at establishing validated field-based measures to assess metabolic compensation as an indication of chronic energy deficiency. A clear gap in Triad literature is the impact of hormonal contraceptive therapy on Triad outcomes; recent randomized controlled trials interestingly demonstrate that non-oral routes of hormonal therapy may offer benefit to bone health in amenorrheic athletes, with improvements in bone density and structure [57,58,59]. In addition, gonadal steroid environment may impact psychological constructs such as cognitive flexibility [60], and may also impact verbal memory [60] and eating behavior [61]. It is imperative that future research also includes efforts to understand the impact of race, ethnicity, and disability on Triad-related outcomes. An emerging area of importance extends to understanding the effects of changing hormonal dynamics in transgender individuals on Triad outcomes. Lastly, recent advances have been made with the publication of two consensus papers on the Male Athlete Triad [44, 45], and future research will continue to build the Male Athlete Triad on the scaffolding of the Female Athlete Triad model.
6 Gaps to be Addressed in RED-S Science
Given that the RED-S model is still relatively new, it is important to similarly address its scientific concerns [62, 63] utilizing evidence-based, high-quality scientific approaches to test the model and advance knowledge in several key areas. Importantly, areas of the RED-S model that require additional scientific inquiry include: (1) confirmation of direct relationships between relative energy deficiency and non-Triad aspects of RED-S, i.e., identifying causal effects of energy deficiency (independent of exercise) on hematological, gastrointestinal and immunological health, growth and development, and psychological health (detailed in sect. 6.5); (2) differentiating benign physiological adaptations (i.e., physiological plasticity) to energy fluctuations from clinically concerning endpoints of RED-S; (3) distinguishing between the consequences of clinical eating disorders versus energy deficiency as a result of nonpathological eating behaviors; and (4) quantifiable and consistent definitions of non-Triad clinical outcomes that include recognized units of measure and established processes for measurement. Such research would seek to clarify the specific physiological endpoints, their units of measure, and their clinical relevance. This sentiment is reflected throughout the article.
Both the Triad and RED-S models require more research to understand how race, ethnicity, physical disabilities, and socioeconomic status affect energy deficiency-related health outcomes. Direct sex comparisons are also needed to further characterize effects of energy deficiency on unique male and female physiology. Furthermore, validation of accurate field-based assessments of energy deficiency would benefit all Triad and RED-S research. Key features pertaining to both models are compared between the Triad and RED-S, and areas of future research are summarized in Table 1.
Below, we outline the areas of the RED-S model that require the most additional research and clarifications to further our understanding of the consequences of energy deficiency.
6.1 Hematological
The intended outcomes of concern in the hematological domain of the RED-S model are unclear. The 2018 updated RED-S consensus statement suggests iron deficiency as the hematological concern of interest, and that iron deficiency may induce low EA and low EA may contribute to iron deficiency. Supporting references are limited to one review paper in which iron deficiency was examined with respect to the components of the Triad [64]. Other investigations include a cross-sectional questionnaire study that correlated survey surrogates of low EA with self-reported history of anemia; low hemoglobin, iron, or ferritin; and/or abnormal bruising [65]; and a case series that demonstrated no change in ferritin over a competitive season [66]. More investigations are required to test causal relationships between energy deficiency and low iron. Additionally, clearer definitions of hematological outcomes of clinical concern in response to RED-S are needed to guide clinical practice.
6.2 Gastrointestinal
The RED-S model proposes that gastrointestinal (GI) health may be impacted by low EA. To date, cross-sectional studies have demonstrated associations between self-reported GI symptoms and surrogates of low EA, though direct relationships between low energy availability and GI symptoms remain to be elucidated [65, 67, 68]. The presence of GI symptoms has been assessed in RED-S research [67] by the Low Energy Availability in Female Athletes Questionnaire (LEAF-Q) [69], which includes four questions related to gas/bloating, cramps/stomachache, frequency of bowel movements, and qualities of stool; however, the experience of any one of these non-specific GI symptoms could reflect an underlying illness that is independent of energy deficiency. For example, frequent, loose stools could reflect irritable bowel syndrome (a relatively common finding in 11% of people worldwide [70]), celiac disease, lactose or gluten intolerance, GI upset related to diet composition, or an infectious process [71]. Ruling out these conditions should be an essential part of initial subject screening for GI symptoms prior to assuming they are secondary to energy deficiency. Further, there is an established literature in athletes documenting GI issues with contributing factors inclusive of mechanical forces and neuroendocrine changes [72], altered blood flow [73, 74], “leaky gut” [75, 76], and inflammatory bowel disease [77]. As with the hematological outcomes, evidence from prospective experimental studies is needed to improve understanding of the relationship between low EA and GI health.
6.3 Immunological
Similar to the hematological and GI domains of the RED-S model, there is limited evidence to support a direct link between energy deficiency and immune function in athletes [63, 78], and in fact there are randomized trials that demonstrate that caloric restriction in healthy adults improves immunity [79]. Indeed, rarely have researchers linked immune responses in athletes to a clinically diagnosed illness, as most associations have also been based on self-report [80, 81] or have failed to objectively define illness [82,83,84]. Self-reported illness associated with survey-based surrogate measures of low EA suggest a need for further study into direct mechanisms linking EA and immune function [65, 67, 68]. Future studies must also take into account the general incidence of diseases, such as the common cold, influenza, and viral respiratory illnesses [85], and consider factors such as the seasonality of these illnesses [86, 87], housing conditions during Olympic competitions, or the risk associated with contracting illness during air travel, which may be common in athletes competing at high levels [88, 89]. Controlling for these numerous confounding factors is necessary to definitively demonstrate a causal relationship between energy deficiency and immune function.
6.4 Growth and Development
The 2018 RED-S updated consensus statement describes the “growth and development” domain of the model as encompassing the growth hormone (GH)/IGF-1 axis, citing anorexia nervosa literature and studies in amenorrheic athletes [90,91,92,93,94,95]. The authors point out a need for more research to understand implications of known physiological adaptations of the GH/IGF-1 axis on training and growth. The implication of the acquired state of GH resistance in conditions of EA beyond its impact on bone remains unclear.
6.5 Psychological
The relationship between psychology and energy deficiency is complex. It is well-documented that psychological constructs, such as a high drive for thinness and dietary cognitive restraint, result in eating behaviors that contribute to developing energy deficiency. However, the effect of energy deficiency on psychology is less well understood. Distinguishing whether energy deficiency can directly cause psychological impairments or exacerbate existing psychological impairments is warranted, and results of such studies will inform multidisciplinary treatment strategies in those with low EA and psychological disturbances.
7 Delineating Correlation from Causation
As mentioned, much of the current research assessing non-Triad outcomes of low EA proposed by RED-S is limited to correlational and self-report survey data. In some studies, energy status is not adequately measured, and yet individuals are presumed to be energy deficient based on the presence of a proposed physiological outcome. For instance, in a recent study by Rogers et al. [67] assessing the prevalence of RED-S symptoms, survey data suggested that 80% of athletes had RED-S, defined as any single component of the proposed outcomes of RED-S, with particularly high rates of impaired GI and immune function (> 30%). Despite a high rate of suggested RED-S outcomes, there was a very low prevalence of physiological indications of energy deficiency in the sample (i.e., only three of 107 individuals had a Cunningham resting metabolic rate ratio < 0.90) [67]. As such, it is unlikely that the GI and immune outcomes were actually a result of energy deficiency in this investigation. Thus, this study and others [65, 68, 96] do not provide causal evidence for a link between energy deficiency/low EA and GI or immune function, but rather imply that participants are in an energy-deficient state despite limited or no physiological evidence of energy deficiency. Importantly, evidence of an effect is not evidence of the cause, and evidence of two variables existing in the same group of individuals is not evidence that the two variables are at all related.
In order to move beyond correlational findings, more rigorous research study designs are needed to improve the overall quality of RED-S-related evidence [63, 97]. For instance, prospective experiments in which energy intake and expenditure are manipulated and specific outcomes are measured (i.e., GI or immune function) would be informative to determine direct effects of energy deficiency. Previous investigations that used short durations of EA perturbations (e.g., 4–5 days) [16,17,18,19, 98,99,100,101] provided fundamental causal evidence relating energy deficiency to surrogate measurements of metabolic, reproductive, and bone outcomes in men and women. Similar study designs can be utilized to investigate the additional RED-S outcomes and would provide important information regarding the causality and timing of effects of energy deficiency. Short-term studies would pave the way for longer-duration investigations or randomized controlled trials (RCTs) that mirror training cycles or other relevant time frames to further elucidate temporality. Importantly, in these studies, confounding etiologies contributing to the proposed effects of energy deficiency need to be assessed or explicitly controlled for. For instance, pre-existing medical conditions (e.g., screening for hyperandrogenism and other endocrinopathies), indirect effects of energy deficiency due to changes in estrogen, the physical stress of exercise itself, overtraining, training seasonality, micronutrient deficiencies, and macronutrient distribution may all contribute to physiological impairments as indirect effects of energy deficiency or even independent of energy deficiency, and should be considered. All scientists must be careful to assure that the message interpreted by the lay public is based on the best quality evidence. For certain facets of negative physiologic impact proposed by the RED-S model, such high-quality evidence has not, as of yet, been achieved. This creates a gap in knowledge and science that needs to be addressed, and also the risk that non-evidence-based messaging may perpetuate unsubstantiated fears in coaches, sports medicine practitioners, and other consumers. It is most appropriate to be concerned about the health consequences of energy deficiency, but assuming that common physiological impairments in athletes are all underpinned by energy deficiency can be misleading and further complicate proper medical evaluation, diagnosis, and treatment for athletes who may be truly experiencing an underlying condition independent of energy deficiency. Caution should be taken to avoid over-interpreting certain outcomes that could be used as surrogate markers, i.e., the use of LH pulsatility to indicate reproductive dysfunction, or the use of bone markers to indicate bone health, which should not be used to make direct statements on overall reproductive function or bone health. These surrogate markers cannot replace the use of long-term studies that assess menstrual cycles, bone density, or bone geometry when trying to claim effects of an independent variable such as energy deficiency on these clinically important outcomes.
8 Conclusion—Next Steps for the Field
With more research necessary to further understand the complex and widespread effects of energy deficiency, it is essential to recognize that a unifying interest exists: the health and wellness of athletes and exercising individuals. Just as RED-S does not replace the Triad, the Triad model does not preclude the existence of other potential physiological effects of energy deficiency as proposed by RED-S. Both the Triad and RED-S models can exist simultaneously, and the contributions, limitations, and advancements of each model deserve acknowledgement and recognition.
It is useful to recall that criticism during the early days of Triad research [46,47,48] sparked the necessary advancement and continued progression of our current understanding of the interrelationships among energy deficiency/low EA, reproductive dysfunction, and impaired bone health in exercising women. Thoughtful debate and critical discussion can serve as positive forces to continue to advance scientific rigor regarding Triad and RED-S health consequences, and thereby effectively address the health issues that are of most clinically relevant concern to athletes and exercising individuals. To this end, a suggested "pathway for progress" is illustrated in Fig. 1. It is suggested that systematic reviews be published using evidence-based grading strategies to assess the current status of Triad and RED-S literature. Validated approaches to achieve consensus are needed, and one that may be considered as a framework is the RAND modified-nominal group technique (NGT) [102]. This approach incorporates the independent anonymous structure of the Delphi method [103] with the ability to assemble (potentially remotely given our COVID-era technological flexibility) to encourage clarification and discussion. Consensus could lead to modifications of the Triad and RED-S models and spur new research addressing priority areas. Modification of the models that recognizes a hierarchy of clinical outcomes of most urgent concern when assessing athletes with energy deficiency is likely necessary. Progress ultimately requires that Triad and RED-S researchers and clinicians acknowledge and work toward the common goal to promote the best and highest quality science and evidence-based medicine in a cohesive manner.
References
Feicht CB, Johnson TS, Martin BJ, et al. Secondary amenorrhoea in athletes. Lancet. 1978;2(8100):1145–6.
Dale E, Gerlach DH, Wilhite AL. Menstrual dysfunction in distance runners. Obstet Gynecol. 1979;54(1):47–53.
Erdelyi G. Gynecologic survey of female athletes. J Sports Med Phys Fitness. 1962;2:174–9.
Erdelyi G. Effects of exercise on the menstrual cycle. Phys Sportsmed. 1976;4:79–81.
Drinkwater BL, Nilson K, Chesnut CH 3rd, et al. Bone mineral content of amenorrheic and eumenorrheic athletes. N Engl J Med. 1984;311(5):277–81.
Drinkwater BL, Nilson K, Ott S, Chesnut CH 3rd. Bone mineral density after resumption of menses in amenorrheic athletes. JAMA. 1986;256(3):380–2.
Drinkwater BL, Bruemner B, Chesnut CH 3rd. Menstrual history as a determinant of current bone density in young athletes. JAMA. 1990;263(4):545–8.
Loucks AB. Effects of exercise training on the menstrual cycle: existence and mechanisms. Med Sci Sports Exerc. 1990;22(3):275–80.
Yeager KK, Agostini R, Nattiv A, et al. The female athlete triad: disordered eating, amenorrhea, osteoporosis. Med Sci Sports Exerc. 1993;25(7):775–7.
Nattiv A, Agostini R, Drinkwater B, et al. The female athlete triad. The inter-relatedness of disordered eating, amenorrhea, and osteoporosis. Clin Sports Med. 1994;13(2):405–18.
Otis CL, Drinkwater B, Johnson M, et al. American College of Sports Medicine position stand. The Female Athlete Triad. Med Sci Sports Exerc. 1997;29(5):i–ix.
Lloyd T, Myers C, Buchanan JR, et al. Collegiate women athletes with irregular menses during adolescence have decreased bone density. Obstet Gynecol. 1988;72(4):639–42.
Marcus R, Cann C, Madvig P, et al. Menstrual function and bone mass in elite women distance runners. Endocrine and metabolic features. Ann Intern Med. 1985;102(2):158–63.
Nattiv A, Loucks AB, Manore MM, et al. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867–82.
Loucks AB, Heath EM. Dietary restriction reduces luteinizing hormone (LH) pulse frequency during waking hours and increases LH pulse amplitude during sleep in young menstruating women. J Clin Endocrinol Metab. 1994;78(4):910–5.
Loucks AB, Callister R. Induction and prevention of low-T3 syndrome in exercising women. Am J Physiol. 1993;264(5 Pt 2):R924–30.
Ihle R, Loucks AB. Dose-response relationships between energy availability and bone turnover in young exercising women. J Bone Miner Res. 2004;19(8):1231–40.
Loucks AB, Thuma JR. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J Clin Endocrinol Metab. 2003;88(1):297–311.
Loucks AB, Verdun M, Heath EM. Low energy availability, not stress of exercise, alters LH pulsatility in exercising women. J Appl Physiol. 1998;84(1):37–46.
De Souza MJ, Miller BE, Loucks AB, et al. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83(12):4220–32.
Bennell KL, Malcolm SA, Thomas SA, et al. Risk factors for stress fractures in track and field athletes. A twelve-month prospective study. Am J Sports Med. 1996;24(6):810–8.
De Souza MJ, Toombs RJ, Scheid JL, et al. High prevalence of subtle and severe menstrual disturbances in exercising women: confirmation using daily hormone measures. Hum Reprod. 2010;25(2):491–503.
Southmayd EA, Mallinson RJ, Williams NI, et al. Unique effects of energy versus estrogen deficiency on multiple components of bone strength in exercising women. Osteoporos Int. 2017;28(4):1365–76.
Southmayd EA, Williams NI, Mallinson RJ, et al. Energy deficiency suppresses bone turnover in exercising women with menstrual disturbances. J Clin Endocrinol Metab. 2019;104(8):3131–45.
Koltun KJ, De Souza MJ, Scheid JL, et al. Energy availability is associated with luteinizing hormone pulse frequency and induction of luteal phase defects. J Clin Endocrinol Metab. 2020;105:185–93.
Mallinson RJ, Williams NI, Hill BR, et al. Body composition and reproductive function exert unique influences on indices of bone health in exercising women. Bone. 2013;56(1):91–100.
Williams NI, Leidy HJ, Hill BR, et al. Magnitude of daily energy deficit predicts frequency but not severity of menstrual disturbances associated with exercise and caloric restriction. Am J Physiol Endocrinol Metab. 2015;308(1):E29-39.
Williams NI, Mallinson RJ, De Souza MJ. Rationale and study design of an intervention of increased energy intake in women with exercise-associated menstrual disturbances to improve menstrual function and bone health: the REFUEL study. Contemp Clin Trials Commun. 2019;14: 100325.
Williams NI, Helmreich DL, Parfitt DB, et al. Evidence for a causal role of low energy availability in the induction of menstrual cycle disturbances during strenuous exercise training. J Clin Endocrinol Metab. 2001;86(11):5184–93.
Williams NI, Caston-Balderrama AL, Helmreich DL, et al. Longitudinal changes in reproductive hormones and menstrual cyclicity in cynomolgus monkeys during strenuous exercise training: abrupt transition to exercise-induced amenorrhea. Endocrinology. 2001;142(6):2381–9.
De Souza MJ, Nattiv A, Joy E, et al. 2014 Female Athlete Triad Coalition Consensus Statement on Treatment and Return to Play of the Female Athlete Triad: 1st International Conference held in San Francisco, California, May 2012 and 2nd International Conference held in Indianapolis, Indiana, May 2013. Br J Sports Med. 2014;48(4):289.
De Souza MJ, Nattiv A, Joy E, et al. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad: 1st International Conference held in San Francisco, CA, May 2012, and 2nd International Conference held in Indianapolis, IN, May 2013. Clin J Sport Med. 2014;24(2):96–119.
Mountjoy M, Sundgot-Borgen J, Burke L, et al. The IOC consensus statement: beyond the Female Athlete Triad-Relative Energy Deficiency in Sport (RED-S). Br J Sports Med. 2014;48(7):491–7.
Mountjoy M, Sundgot-Borgen JK, Burke LM, et al. IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br J Sports Med. 2018;52(11):687–97.
Mountjoy M, Sundgot-Borgen J, Burke L, et al. Authors’ 2015 additions to the IOC consensus statement: Relative Energy Deficiency in Sport (RED-S). Br J Sports Med. 2015;49(7):417–20.
Gordon CM, Ackerman KE, Berga SL, et al. Functional hypothalamic amenorrhea: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(5):1413–39.
Ozier AD, Henry BW, American DA. Position of the American Dietetic Association: nutrition intervention in the treatment of eating disorders. J Am Diet Assoc. 2011;111(8):1236–41.
Osteoporosis prevention, diagnosis, and therapy. NIH Consensus Statement. 2000;17(1):1–45.
Wheeler GD, Wall SR, Belcastro AN, et al. Reduced serum testosterone and prolactin levels in male distance runners. JAMA. 1984;252(4):514–6.
MacConnie SE, Barkan A, Lampman RM, et al. Decreased hypothalamic gonadotropin-releasing hormone secretion in male marathon runners. N Engl J Med. 1986;315(7):411–7.
Hackney AC, Sinning WE, Bruot BC. Reproductive hormonal profiles of endurance-trained and untrained males. Med Sci Sports Exerc. 1988;20(1):60–5.
De Souza MJ, Arce JC, Pescatello LS, et al. Gonadal hormones and semen quality in male runners. A volume threshold effect of endurance training. Int J Sports Med. 1994;15(7):383–91.
Bilanin JE, Blanchard MS, Russek-Cohen E. Lower vertebral bone density in male long distance runners. Med Sci Sports Exerc. 1989;21(1):66–70.
Nattiv A, De Souza MJ, Koltun KJ, et al. The male athlete triad-A consensus statement from the female and male athlete triad coalition part 1: definition and scientific basis. Clin J Sport Med. 2021;31:335–48.
Fredericson M, Kussman A, Misra M, et al. The male athlete triad-A consensus statement from the female and male athlete triad coalition part II: diagnosis, treatment, and return-to-play. Clin J Sport Med. 2021. https://doi.org/10.1097/JSM.0000000000000948.
DiPietro L, Stachenfeld NS. The female athlete triad myth. Med Sci Sports Exerc. 2006;38(4):795 (author reply 6).
Di Pietro L, Stachenfeld N. Refutation of the myth of the female athlete triad. Br J Sport Med. 2007;41(1):57–8.
Loucks AB, Stachenfeld NS, DiPietro L. The female athlete triad: do female athletes need to take special care to avoid low energy availability? Med Sci Sports Exerc. 2006;38(10):1694–700.
Loucks AB, Verdun M. Slow restoration of LH pulsatility by refeeding in energetically disrupted women. Am J Physiol. 1998;275(4 Pt 2):R1218–26.
Strock NCA, De Souza MJ, Williams NI. Eating behaviours related to psychological stress are associated with functional hypothalamic amenorrhoea in exercising women. J Sports Sci. 2020;38(21):2396–406.
Strock NCA, Koltun KJ, Mallinson RJ, et al. Characterizing the resting metabolic rate ratio in ovulatory exercising women over 12 months. Scand J Med Sci Sports. 2020;30(8):1337–47.
Strock NCA, Koltun KJ, Southmayd EA, et al. Indices of resting metabolic rate accurately reflect energy deficiency in exercising women. Int J Sport Nutr Exerc Metab. 2020;29:1–11.
Williams NI, Bullen BA, McArthur JW, et al. Effects of short-term strenuous endurance exercise upon corpus luteum function. Med Sci Sports Exerc. 1999;31(7):949–58.
Bullen BA, Skrinar GS, Beitins IZ, von Mering G, Turnbull BA, McArthur JW. Induction of menstrual disorders by strenuous exercise in untrained women. N Engl J Med. 1985;312(21):1349–53.
De Souza MJ, Mallinson RJ, Strock NCA, et al. Randomized controlled trial of the effects of increased energy intake on menstrual recovery in exercising women with menstrual disturbances: the “REFUEL” Study. Hum Reprod. 2021;36:2285–97.
De Souza MJ, Ricker EA, Mallinson RJ, et al. Randomized controlled trial assessing the effects of increased energy intake on BMD changes in exercising women with menstrual disturbances: the “REFUEL” Study. Am J Clin Nutr. 2021 (in review).
Ackerman KE, Singhal V, Baskaran C, et al. Oestrogen replacement improves bone mineral density in oligo-amenorrhoeic athletes: a randomised clinical trial. Br J Sports Med. 2019;53(4):229–36.
Ackerman KE, Singhal V, Slattery M, et al. Effects of estrogen replacement on bone geometry and microarchitecture in adolescent and young adult oligoamenorrheic athletes: a randomized trial. J Bone Miner Res. 2020;35(2):248–60.
Allaway HCM, Misra M, Southmayd EA, et al. Are the effects of oral and vaginal contraceptives on bone formation in young women mediated via the growth hormone-IGF-I axis? Front Endocrinol (Lausanne). 2020;11:334.
Baskaran C, Cunningham B, Plessow F, et al. Estrogen replacement improves verbal memory and executive control in oligomenorrheic/amenorrheic athletes in a randomized controlled trial. J Clin Psychiatry. 2017;78(5):e490–7.
Plessow F, Singhal V, Toth AT, et al. Estrogen administration improves the trajectory of eating disorder pathology in oligo-amenorrheic athletes: a randomized controlled trial. Psychoneuroendocrinology. 2019;102:273–80.
De Souza MJ, Williams NI, Nattiv A, et al. Misunderstanding the female athlete triad: refuting the IOC consensus statement on Relative Energy Deficiency in Sport (RED-S). Br J Sports Med. 2014;48(20):1461–5.
Williams NI, Koltun KJ, Strock NCA, et al. Female athlete triad and relative energy deficiency in sport: a focus on scientific rigor. Exerc Sport Sci Rev. 2019;47(4):197–205.
Petkus DL, Murray-Kolb LE, De Souza MJ. The unexplored crossroads of the female athlete triad and iron deficiency: a narrative review. Sports Med. 2017;47(9):1721–37.
Ackerman KE, Holtzman B, Cooper KM, et al. Low energy availability surrogates correlate with health and performance consequences of Relative Energy Deficiency in Sport. Br J Sports Med. 2019;53:628–33.
Hooper DR, Mallard J, Wight JT, et al. Performance and health decrements associated with relative energy deficiency in sport for division i women athletes during a collegiate cross-country season: a case series. Front Endocrinol. 2021;12:12.
Rogers MA, Appaneal RN, Hughes D, et al. Prevalence of impaired physiological function consistent with Relative Energy Deficiency in Sport (RED-S): an Australian elite and pre-elite cohort. Br J Sports Med. 2021;55(1):38–45.
Mathisen TF, Heia J, Raustol M, et al. Physical health and symptoms of relative energy deficiency in female fitness athletes. Scand J Med Sci Sports. 2020;30(1):135–47.
Melin A, Tornberg AB, Skouby S, et al. The LEAF questionnaire: a screening tool for the identification of female athletes at risk for the female athlete triad. Br J Sports Med. 2014;48(7):540–5.
Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;24(2):16014.
Camilleri M, Camilleri MJA. Diarrhea and constipation. In: Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson J, editors. Harrison’s principles of internal medicine. 20th ed. New York: McGraw-Hill; 2014.
Waterman JJ, Kapur R. Upper gastrointestinal issues in athletes. Curr Sports Med Rep. 2012;11(2):99–104.
Qamar MI, Read AE. Effects of exercise on mesenteric blood flow in man. Gut. 1987;28(5):583–7.
ter Steege RW, Geelkerken RH, Huisman AB, et al. Abdominal symptoms during physical exercise and the role of gastrointestinal ischaemia: a study in 12 symptomatic athletes. Br J Sports Med. 2012;46(13):931–5.
Gutekunst K, Kruger K, August C, et al. Acute exercises induce disorders of the gastrointestinal integrity in a murine model. Eur J Appl Physiol. 2014;114(3):609–17.
Holland AM, Hyatt HW, Smuder AJ, et al. Influence of endurance exercise training on antioxidant enzymes, tight junction proteins, and inflammatory markers in the rat ileum. BMC Res Notes. 2015;30(8):514.
Koon G, Atay O, Lapsia S. Gastrointestinal considerations related to youth sports and the young athlete. Transl Pediatr. 2017;6(3):129–36.
Walsh NP. Nutrition and athlete immune health: new perspectives on an old paradigm. Sports Med. 2019;49(Suppl 2):153–68.
Meydani SN, Das SK, Pieper CF, et al. Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: a randomized controlled trial in non-obese humans. Aging. 2016;8(7):1416–31.
Shimizu K, Aizawa K, Suzuki N, et al. Influences of weight loss on monocytes and T-cell subpopulations in male judo athletes. J Strength Cond Res. 2011;25(7):1943–50.
Tsai ML, Chou KM, Chang CK, et al. Changes of mucosal immunity and antioxidation activity in elite male Taiwanese taekwondo athletes associated with intensive training and rapid weight loss. Br J Sports Med. 2011;45(9):729–34.
Abedelmalek S, Chtourou H, Souissi N, et al. Caloric restriction effect on proinflammatory cytokines, growth hormone, and steroid hormone concentrations during exercise in judokas. Oxid Med Cell Longev. 2015;2015: 809492.
Imai T, Seki S, Dobashi H, et al. Effect of weight loss on T-cell receptor-mediated T-cell function in elite athletes. Med Sci Sports Exerc. 2002;34(2):245–50.
Tsai ML, Ko MH, Chang CK, et al. Impact of intense training and rapid weight changes on salivary parameters in elite female Taekwondo athletes. Scand J Med Sci Sports. 2011;21(6):758–64.
Moriyama M, Hugentobler WJ, Iwasaki A. Seasonality of respiratory viral infections. Annu Rev Virol. 2020;7(1):83–101.
Simancas-Racines D, Franco JV, Guerra CV, et al. Vaccines for the common cold. Cochrane Database Syst Rev. 2017;5: CD002190.
Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51–9.
Lei H, Li Y, Xiao S, Lin CH, et al. Routes of transmission of influenza A H1N1, SARS CoV, and norovirus in air cabin: comparative analyses. Indoor Air. 2018;28(3):394–403.
Lei H, Tang JW, Li Y. Transmission routes of influenza A(H1N1)pdm09: analyses of inflight outbreaks. Epidemiol Infect. 2018;146(13):1731–9.
Lantzouni E, Frank GR, Golden NH, et al. Reversibility of growth stunting in early onset anorexia nervosa: a prospective study. J Adolesc Health. 2002;31(2):162–5.
Modan-Moses D, Yaroslavsky A, Kochavi B, et al. Linear growth and final height characteristics in adolescent females with anorexia nervosa. PLoS One. 2012;7(9): e45504.
Modan-Moses D, Yaroslavsky A, Novikov I, et al. Stunting of growth as a major feature of anorexia nervosa in male adolescents. Pediatrics. 2003;111(2):270–6.
Fazeli PK, Klibanski A. Determinants of GH resistance in malnutrition. J Endocrinol. 2014;220(3):R57-65.
Laughlin GA, Yen SS. Nutritional and endocrine-metabolic aberrations in amenorrheic athletes. J Clin Endocrinol Metab. 1996;81(12):4301–9.
Waters DL, Qualls CR, Dorin R, et al. Increased pulsatility, process irregularity, and nocturnal trough concentrations of growth hormone in amenorrheic compared to eumenorrheic athletes. J Clin Endocrinol Metab. 2001;86(3):1013–9.
Slater J, McLay-Cooke R, Brown R, et al. Female recreational exercisers at risk for low energy availability. Int J Sport Nutr Exerc Metab. 2016;26(5):421–7.
Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Koehler K, Hoerner NR, Gibbs JC, et al. Low energy availability in exercising men is associated with reduced leptin and insulin but not with changes in other metabolic hormones. J Sports Sci. 2016;34(20):1921–9.
Papageorgiou M, Elliott-Sale KJ, Parsons A, et al. Effects of reduced energy availability on bone metabolism in women and men. Bone. 2017;105:191–9.
Papageorgiou M, Martin D, Colgan H, et al. Bone metabolic responses to low energy availability achieved by diet or exercise in active eumenorrheic women. Bone. 2018;114:181–8.
Loucks AB, Heath EM. Induction of low-T3 syndrome in exercising women occurs at a threshold of energy availability. Am J Physiol. 1994;266(3 Pt 2):R817–23.
Murphy MK, Black NA, Lamping DL, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess. 1998;2(3):i–iv, 1–88.
Dalkey NC, Helmer O. An experimental application of the Delphi method to the use of experts. Manag Sci. 1963;9:458–67.
Vanheest JL, Rodgers CD, Mahoney CE, et al. Ovarian suppression impairs sport performance in junior elite female swimmers. Med Sci Sports Exerc. 2014;46(1):156–66.
Stenqvist TB, Melin AK, Garthe I, Slater G, Paulsen G, Iraki J, et al. Prevalence of surrogate markers of relative energy deficiency in male Norwegian Olympic-level athletes. Int J Sport Nutr Exerc Metab. 2021. https://doi.org/10.1123/ijsnem.2020-0368Abstract.
Lane AR, Hackney AC, Smith-Ryan AE, Kucera K, Register-Mihalik JK, Ondrak K. Energy availability and RED-S risk factors in competitive, non-elite male endurance athletes. Transl Med Exerc Prescr. 2021;1(1):25–32.
Sharps FRJ, Wilson LJ, Graham CA, Curtis C. Prevalence of disordered eating, eating disorders and risk of low energy availability in professional, competitive and recreational female athletes based in the United Kingdom. Eur J Sports Sci. 2021. https://doi.org/10.1080/17461391.2021.1943712.
Heikura IA, Uusitalo ALT, Stellingwerff T, Bergland D, Mero AA, Burke LM. Low energy availability is difficult to assess but outcomes have large impact on bone injury rates in elite distance athletes. Int J Sport Nutr Exerc Metab. 2018;28(4):403–11.
Melin A, Tornberg AB, Skouby S, Moller SS, Sundgot-Borgen J, Faber J, et al. Energy availability and the female athlete triad in elite endurance athletes. Scand J Med Sci Sports. 2015;25(5):610–22.
Brook EM, Tenforde AS, Broad EM, Matzkin EG, Yang HY, Collins JE, et al. Low energy availability, menstrual dysfunction, and impaired bone health: a survey of elite para athletes. Scand J Med Sci Sports. 2019;29(5):678–85.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mary Jane De Souza, Nicole C. Strock, Emily A. Ricker, Kristen J. Koltun, Michelle Barrack, Elizabeth Joy, Aurelia Nattiv, Mark Hutchinson, Madhusmita Misra, and Nancy I. Williams declare that they have no conflicts of interest relevant to the content of this article.
The opinions and assertions expressed herein are those of the author(s) and do not necessarily reflect the official policy or position of the Uniformed Services University or the Department of Defense.
The contents of this publication are the sole responsibility of the author(s) and do not necessarily reflect the views, opinions or policies of The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
Funding
No sources of funding were used to assist in the preparation of this article.
Authorship contributions
MJD, NCAS, EAR, KJK, NIW wrote the first draft of the manuscript. MB, EJ, AN, MH, MM revised the original manuscript. All authors read and approved the final manuscript.
Rights and permissions
About this article
Cite this article
De Souza, M.J., Strock, N.C.A., Ricker, E.A. et al. The Path Towards Progress: A Critical Review to Advance the Science of the Female and Male Athlete Triad and Relative Energy Deficiency in Sport. Sports Med 52, 13–23 (2022). https://doi.org/10.1007/s40279-021-01568-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-021-01568-w