Abstract
Ketone bodies (KB) provide an alternative energy source and uniquely modulate substrate metabolism during endurance exercise. Nutritional ketosis (blood KBs > 0.5 mM) can be achieved within minutes via exogenous ketone supplementation or days-to-weeks via conforming to a very low-carbohydrate, ketogenic diet (KD). In contrast to short-term (< 2 weeks) KD ingestion, chronic adherence (> 3 weeks) leads to a state of keto-adaptation. However, despite elevating blood KBs to similar concentrations, exogenous ketone supplementation and keto-adaptation are not similar metabolic states as they elicit diverse and distinct effects on substrate availability and metabolism during exercise; meaning that their influence on endurance exercise performance is different. In contrast to contemporary, high(er)-carbohydrate fuelling strategies, inducing nutritional ketosis is rarely ergogenic irrespective of origin and, in fact, can impair endurance performance. Nonetheless, exogenous ketone supplementation and keto-adaptation possess utility for select endurance events and individuals, thus warranting further research into their performance effects and potential strategies for their optimisation. It is critical, however, that future research considers the limitations of measuring blood KB concentrations and their utilisation, and assess the effect of nutritional ketosis on performance using exercise protocols reflective of real-world competition. Furthermore, to reliably assess the effects of keto-adaptation, rigorous dietary-training controls of sufficient duration should be prioritised.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Endurance exercise requires high rates of sustained energy production, primarily via the oxidation of carbohydrate (CHO) and fat [1]; with exercise intensity positively associated with CHO utilisation [1]. Increasing blood ketone body (KB) concentration modulates substrate metabolism and oxidation during exercise, most notably by reducing CHO utilisation and free fatty-acid (FFA) availability, whilst providing a minor direct contribution of substrate into the tricarboxylic acid (TCA) cycle [2, 3]. Ketogenic diets (KD) and exogenous ketone supplements (EKS) increase blood KB concentration and may influence endurance performance; however, these two strategies are not equivalent as they exert two distinct metabolic states. Therefore, understanding how KD and EKS ingestion impact substrate metabolism is prudent to elucidate their application for endurance athletes.
2 Ketone Bodies and Nutritional Ketosis
Ketone bodies refers to acetoacetate (AcAc), acetone and β-hydroxybutyrate (βHB). However, only AcAc and acetone are ketones as they contain a carboxyl group with two hydrocarbons; βHB is technically a KB as the hydrocarbon atom is replaced by a hydroxyl group on the third carbon (Fig. 1). Compared with AcAc and βHB, acetone is largely excreted in the urine and breath and of negligible physiological importance during exercise [2]. Postprandial blood KB concentration is ~ 0.1–0.2 mM and varies depending on CHO availability [2]. Blood KB concentration > 0.5 mM is a commonly used threshold qualifying a state of nutritional ketosis or hyperketonaemia [4]; however, concentrations > 0.2 mM have also been proposed [2]. Ketone bodies can bypass the blood–brain barrier via passive diffusion [5] and enter extrahepatic tissues (e.g., brain, heart and skeletal muscle) via monocarboxylate transporters (MCTs) [6] to provide an alternative oxidative fuel source during periods of low CHO availability, such as starvation, fasting, prolonged exercise, or conformity to a ketogenic diet (KD) [2].
3 Ketogenic Diets and Endogenous Ketogenesis
Conforming to a KD increases blood KB concentrations to > 0.5 mM within days [2, 7] and can be sustained for weeks [7,8,9] to months [10, 11]. Ketogenesis occurs predominantly in hepatic mitochondria following β-oxidation of fatty acids to produce AcAc, the central KB in energy metabolism. The majority of AcAc is reduced to D-βHB, the primary circulating KB, thus shifting the blood AcAc:βHB ratio from 1:1 to–1:4 [2]. The ability for a fat-derived fuel to be used by the brain during periods of CHO insufficiency is critical to meet the brain’s energy demand, thus negating the requirement for gluconeogenic protein catabolism [12, 13]. A KD is typically defined as < 50 g CHO day−1 or < 5% of energy intake (EI) from CHO, 15–20% of EI from protein and 75–80% of EI from fat [7,8,9, 14, 15]. The defining feature demarcating a KD from low(er)-CHO, high(er)-fat (LCHF) diets (~ 2.5 g CHO kg−1 day−1 or < 25% EI from CHO) is hyperketonaemia [4].
4 Defining Keto-Adaptation for Endurance Athletes
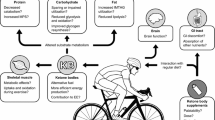
Keto-adaptation refers to the multi-organ and multi-system (physiological) adaptations exerted by ingesting a KD over several weeks-to-months [16]. There is no clear definition of what constitutes keto-adaptation or strategies for its optimisation, except adherence for at least 3–4 weeks and supplementation with sodium and potassium [17]. However, it is likely population-specific and exist on a continuum depending on the duration of dietary conformity. Studies investigating keto-adaptation in endurance athletes for at least 3 weeks demonstrate alterations to substrate availability and metabolism (Table 1), faecal [18] and oral [19] microbiome, and iron regulation [20, 21]; whereas, acid–base balance [22] and mucosal immunity [23] remain unaltered.
Depleted skeletal muscle [24] and hepatic [25] glycogen stores and an inability to maintain CHO oxidation rates [24, 26] may precede metabolic exhaustion during exercise. Therefore, the primary rationale for keto-adaptation is to reduce CHO-utilisation rates, whilst sustaining energy production, for a given exercise intensity. To achieve this, numerous adaptations occur that are synonymous with fat-adaptation (reviewed elsewhere [27,28,29,30] and are summarised in Table 1), which include: (1) increased fat oxidation [7–10]; (2) reduced blood glucose utilisation [8]; and 3) reduced skeletal muscle [8, 11] and hepatic [11] glycogen utilisation. Intermittent or continuous exposure to hyperketonaemia demarcates keto- from fat-adaptation; however, it remains uncertain—and contentious—what specific adaptations constitute keto-adaptation, their impact on endurance performance, and how these evolve with chronic KD ingestion (i.e., weeks vs. months vs. years). Furthermore, keto-adaptation is not a binary physiological state categorised by blood KB concentrations greater or less than 0.5 mM; rather, it is the ability to rapidly and efficiently increase ketogenesis and ketolysis relative to (lowering) CHO availability, whilst maintaining total substrate oxidation rates sufficient for energy requirements.
The obligate duration of conforming to a KD to optimise keto-adaptation is uncertain. A minimum of 3–4 weeks appears necessary for performance [7,8,9]; however, whether ergogenic or ergolytic adaptations occur beyond this time-frame is unknown as studies of this duration either do not examine performance [10, 11], fail to rigorously monitor dietary intake and training load [14], or do not employ dietary standardisation prior to performance testing [15]. Therefore, to improve quantifying keto-adaptation in endurance athletes, the following variables should be reported: (1) dietary intake (refer to Mirtschin et al. [31] and Shaw et al. [7] for examples); (2) daily (morning) pre-exercise blood and/or urinary KB concentrations (to confirm dietary adherence); and (3) training load. Thereafter, cardiorespiratory parameters, ratings of perceived exertion (RPE), blood metabolites, and substrate oxidation during metabolic tests and/or performance tests can determine the utility of keto-adaptation and, potentially, time-course adaptations to KD ingestion. Noteworthy, steady-state whole-body carbohydrate and fat (not KB) oxidation can be calculated using indirect calorimetry without adjusting stoichiometric equations as fatty-acid-derived KBs are an intermediate metabolite of fat metabolism and contribute to total fat oxidation [32]—assuming insensible KB losses via the breath and urine and non-respiratory excretion of CO2 are negligible, which may otherwise corrupt stoichiometric equations [33].
5 Exogenous Ketone Supplementation
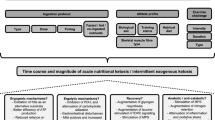
Exogenous ketone and ketogenic supplements induce nutritional ketosis within minutes without necessitating CHO restriction (reviewed elsewhere [3, 34, 35]). Their influence on performance is an area of interest [36] as they can elicit unique metabolic responses during exercise (Table 1). Ketone salts (KB + sodium, potassium, calcium, or magnesium), typically sold as a racemic mixture, increase blood D-βHB to ~ 0.3–1 mM [37,38,39,40,41]; however, they provide an undesirable salt load that may result in cation overload, acidosis, and gastrointestinal distress [42, 43]. Similarly, the racemic R,S-1,3-butanaediol (BD) increases blood D-βHB to ~ 1 mM [44, 45]; however, as a ketogenic supplement, the resulting R-BD and S-BD moieties require subsequent conversion to the isotopic enantiomers, D-βHB and L-βHB [46]. BD metabolism is rate-limited by the hepatic enzymes alcohol dehydrogenase and aldehyde dehydrogenase [47,48,49,50] (Fig. 2); therefore, blood BD accumulates when ingested in quantities beyond enzymatic saturation [51].
A simplified schematic of the cleavage and major metabolic pathways of 1,3-butanediol-based ketone esters and ketone salts prior to oxidation in the tricarboxylic acid cycle within extrahepatic tissues. βHB β-hydroxybutyrate, AcAc acetoacetate, HMG-CoA 3-hydroxy-3-methylglutaryl-CoA, AcAc-CoA acetoacetyl-CoA, Ac-CoA acetyl-CoA, TCA tricarboxylic acid. Following oral ingestion, ketone esters and salts are cleaved and absorbed in the gut. R,S-1,3-butanediol is metabolised to R,S-3-hydroxybutanal prior to the ketone bodies acetoacetate, βHB, and acetone. Acetone is formed by the decarboxylation of AcAc and is not shown in the schematic as it does not contribute to energy production. Ketone bodies entering circulation are transported to extrahepatic tissues (e.g., skeletal muscle, kidney, brain, and heart), which enter via monocarboxylate transporters to be oxidised in the TCA cycle for energy production. Excretion of non-metabolised ketone bodies occurs via the faeces, exhalation by the lungs as acetone, or kidneys as AcAc and βHB
Ketone esters include the R,S-BD AcAc diester [52, 53] and R-BD D-βHB monoester [54, 55]. In humans, R-BD D-βHB monoester ingestion has attracted the most attention, with studies investigating its safety and tolerability [43, 54, 56], and pharmacokinetics [40, 54, 57], as well as its regulatory effects on blood glucose [58], appetite [59], inflammation [60], endurance performance [55, 61], RPE [62], acid–base balance [63], recovery [64, 65], and overreaching [66]. R,S-BD AcAc esters and R-BD D-βHB monoester are catabolised by carboxylesterases and esterases predominantly situated in the gastrointestinal tract [67], with the liberated BD metabolised as previously described. R,S-BD AcAc diesters increase blood D-βHB concentration to similar concentrations as racemic ketone salts and BD, whereas there is a significantly greater rise in blood AcAc concentration (~ 0.4 mM) [53]. In contrast, the R-BD D-βHB monoester increases blood D-βHB concentrations to 2–5 mM [40, 55, 57], which is attenuated when coingested with food [40], indicating that the contents of the gut may affect the digestion and absorption of EKSs.
6 Chirality of Exogenous Ketone Supplements
Multiple pathways exist for the absorption and metabolism of EKSs, particularly due to chiral differences of βHB (R- and S- can be interchanged with D- and L-, respectively (Fig. 2). The D-βHB derived from R,S-BD metabolism is identical to D-βHB produced via endogenous ketogenesis, whereas L-βHB is a by-product of fat metabolism present in trace and low amounts in blood [68] and extrahepatic tissue [69], respectively. In rats, the administration of R-BD and S-BD contributed to 86–98% and 47–75% of total ketogenesis (D-βHB + AcAc), respectively, indicating that S-BD has a significantly lower rate of conversion to D-βHB. D-βHB is rapidly catabolised to acetyl-CoA and adenosine triphosphate (ATP) via the TCA cycle in peripheral tissues, whereas L-βHB does not enter the TCA cycle and is converted by the liver to FFAs and sterols or acetyl-CoA prior to D-βHB or CO2 (see Fig. 2) [70, 71]. The slow conversion and excretion of L-βHB means that it can remain present in the blood for up to 24 h, increasing the potential of blood L-βHB accumulation [40]. Speculatively, lowering of the D-βHB/L-βHB ratio may negate the D-βHB-induced suppression of glucose metabolism in muscle, as previously demonstrated within cardiac tissue in rats [69], thus preserving CHO-oxidation rates and performance during high-intensity exercise. However, further research is required to investigate the interaction of D- and L-βHB on substrate metabolism in exercising humans and how this changes relative to CHO availability.
7 Quantifying Ketosis
Various methods measure biological KB concentrations [72]. Dipsticks qualitatively or semi-quantitatively measure the presence of urinary AcAc and are a cost-effective option to confirm conformity to a KD [73]; however, they are unable to detect D-βHB and have a low sensitivity to acute shifts in blood KB concentration [72]. Whereas, point-of-care, handheld monitors measure capillary whole-blood D-βHB concentration. Several point-of-care devices are available and widely used in healthcare settings for individuals with diabetes [72]. These devices specifically measure D-βHB, not L-βHB, by using D-βHB dehydrogenase coupled with electrochemical detection. However, compared with laboratory measures, point-of-care devices tend to have a higher coefficient of variation [74] and can overestimate blood D-βHB concentration by two-to-threefold [53, 75]; which makes it difficult to identify the optimal range of blood D-βHB concentration for endurance performance.
8 Limitations of Measuring Ketone Body Utilisation
The uptake and oxidation of KBs within skeletal muscle during exercise has been reviewed elsewhere [2, 3, 34]. Well-trained athletes have a greater capacity to oxidise KBs due to a higher abundance of MCTs [76] and ketolytic enzymes [77]. However, measuring KB oxidation is difficult as stoichiometric equations have only been validated for CHO and fat [78]. To estimate KB oxidation using indirect methods, further estimates are required for KB volume distribution values (i.e., total amount of KBs in the body divided by KB plasma concentration) and KB uptake into skeletal muscle [79]. For KB uptake, differences between the rates of KB appearance (i.e., the rate of hepatic ketogenesis during keto-adaptation or the rate of gastrointestinal absorption and/or BD conversion following exogneous ketone or ketogenic supplementation) and KB uptake must be known. Furthermore, not all KBs extracted from the blood are oxidised, as they can be stored in the form of D-3-hydroxybutyrylcarnitine (keto-carnitine), for which its role in energy production is uncertain [55, 80]. Insensible losses may also occur via the breath and urine [2].
A method proposed for estimating KB uptake into skeletal muscle following EKS ingestion uses incremental area-under-the-curve of blood D-βHB concentration between resting and exercising conditions [55, 79]; however, during keto-adaptation, this strategy cannot be employed as rates of endogenous ketogenesis are unknown. In a study involving highly trained cyclists ingesting 0.573 g kg−1 of the R-BD D-βHB monoester, the contribution of D-βHB to energy expenditure was estimated to be 0.35 and 0.5 g min−1, or 16 and 18% of oxygen uptake, at 45 and 75% Wmax, respectively [55]. Blood D-βHB concentrations in the high-intensity trial were ~ 3 and 1 mM lower than the resting and low-intensity trials, respectively [55], demonstrating an exercise intensity-dependent effect on KB utilisation. Nevertheless, the accuracy of these calculations have not been confirmed using tracer techniques and are grossly higher than previous estimates using sodium AcAc infusion during moderate-intensity exercise [81]; therefore, indirect methods may overestimate KB oxidation and should be interpreted cautiously until validated.
9 Nutritional Ketosis and Endurance Performance
High-intensity (> 80%VO2max) endurance performance up to ~ 3 h is CHO-dependent [82], but not necessarily limited by CHO availability [83]. Maximising energy production per unit of oxygen is important to performance; therefore, a shift towards fat oxidation may be ergolytic as the oxidation of fat compared with CHO results in a ~ 5% reduction in efficiency [84]. Whereas, at moderate intensities, depleting endogenous CHO availability can result in fatigue [85]; therefore, strategies to reduce the rate of endogenous CHO utilisation, whilst maintaining exercise intensity, are desirable. Ketone bodies downregulate skeletal muscle [55] and hepatic [86] glycogen utilisation and, as demonstrated in rodent skeletal and cardiac muscle models [87, 88], reduce pyruvate dehydrogenase complex (PDHc) activity; however, for the latter, evidence in humans following dietary intervention is currently unavailable. They also self-regulate their own production by suppressing adipocyte lipolysis [2], thus reducing FFA availability to exercising muscle. Ketone bodies may also be a more efficient fuel source than CHO and fat, as demonstrated in isolated rodent tissue models [89,90,91], with increased energy yield per C2 unit [90] and greater Gibbs free energy of ATP hydrolysis [42]. Therefore, nutritional ketosis is frequently implicated in fuelling strategies for endurance performance; however, potential performance benefits and detriments exist at both high- and moderate-exercise intensities.
9.1 Exogenous Ketone Supplementation and High-Intensity Endurance Performance
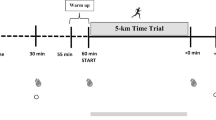
No beneficial performance effects during high-intensity exercise have been demonstrated following the ingestion of EKSs that increase blood D-βHB concentrations up to ~ 1 mM. Racemic ketone salts [38, 39] and BD [44, 45] exert no effect on oxygen uptake, blood glucose concentration, lactate accumulation, or RPE at exercise intensities > 70% VO2max, and demonstrate no [39, 44, 45] or negative [38] effects on performance (Table 2). Whereas, the racemic R,S-BD AcAc diester, in conjunction with recommended CHO fuelling strategies, was shown to increase capillary blood D-βHB concentrations to 0.8–1.3 mM (serum D-βHB ~ 0.4 mM) and serum AcAc concentration to ~ 0.5 mM during a ~ 50 min cycling time-trial (TT) [53]. Blood glucose, free fatty acid, and lactate concentrations were lower than placebo [81], which was potentially due to the higher increase in serum AcAc concentration compared to the ingestion of βHB salts or BD alone, due to their cleavage from BD and immediate entry into circulation. In this case, however, performance was significantly impaired by ~ 2% (~ 3.7% reduction in average power output [339 ± 37 vs. 352 ± 35 W]), which was attributed to severe gastrointestinal distress [53] (Table 2). Therefore, it seems EKSs attaining blood D-βHB concentrations up to ~ 1 mM should be avoided for high-intensity endurance performance, particularly if exercise duration does not deplete CHO availability.
In contrast, ingesting the non-racemic R-BD D-βHB monoester increases blood D-βHB concentration to ~ 1–2.5 mM during high-intensity exercise [55, 61, 92]. In response, blood glucose, FFA and lactate concentration, and skeletal muscle glycogen utilisation decline, and intramuscular triglyceride utilisation appear to increase (Table 1). The coingestion of R-BD D-βHB monoester and CHO after an overnight fast increased distance cycled by ~ 2% during a 30 min TT preceded by 60 min at 75% of the maximal power achieved during the incremental test [55] (Table 2). However, R-BD D-βHB monoester ingestion with CHO fuelling strategies during the prior 24 h and exercise has failed to alter high-intensity exercise capacity in a run-to-exhaustion protocol preceded by 75 min of high-intensity intermittent running [61] and a 10 km time-trial running performance preceded by 60 min at 65% VO2max [92] (Table 2). Similarly, maximal power output did not change following R-BD D-βHB monoester ingestion compared with placebo prior to an incremental exercise test, despite increased leg discomfort and the anxiety of breathing and leg discomfort [62] and increased acid–base balance [63] (Table 2). Therefore, ingesting the R-BD D-βHB monoester may not be ergogenic during high-intensity exercise.
9.2 Keto-Adaptation and High-Intensity Endurance Performance
Keto-adaptation tends to impair high-intensity endurance performance (reviewed elsewhere [27, 30]). However, after keto-adaptation periods of > 2–3 months, results are equivocal, as both negative [93] and no effects [15] were reported in recreational endurance athletes; albiet there is no published research in well-trained or elite athletes. The primary concern is lowering of CHO availability and oxidation via suppression of glycolysis and PDHc activity, which can manifest during metabolic testing as reduced respiratory exchange ratio (RER) at VO2max (i.e. < 1.0) [7,8,9]. Keto-adaptation also increases energy expenditure and oxygen uptake beyond what can be explained by reductions in RER from pre- to post-adaptation (i.e., accounted oxygen uptake) when exercising at > 70% VO2max [7]; suggesting increased mitochondrial uncoupling. In concordance, 31-day keto-adaptation reduced running speed at VO2max in trained runners [7] and 3 weeks of keto-adaptation during intensified training in elite race walkers negated improvements in a 10-km TT performance compared with a high-CHO diet [9]. Therefore, increased fat and KB oxidation does not seem to compensate for attenuated carbohydrate metabolism following keto-adaptation, thus appearing to compromise high-intensity endurance performance.
9.3 Exogenous Ketone Supplementation and Prolonged Moderate-Intensity Endurance Performance
Exogenous ketone supplementation has been tentatively linked with performance effects for endurance competition lasting several hours [94, 95]; however, no studies have examined their effect. Despite the potential for increased IMTG utilisation following R-BD D-βHB monoester ingestion [55], IMTG stores are depleted by ~ 50–70% during the initial 2–3 h of (fasted) exercise, which places greater reliance on adipose tissue lipolysis to maintain fat oxidation [96,97,98,99]. Since ketone ester ingestion suppresses adipose tissue lipolysis [53, 55], an important fuel source for prolonged events [100], CHO-oxidation rates could increase, thus accelerating fatigue. Moreover, endurance competition lasting several hours is highly influenced by gastrointestinal symptoms, which can be exacerbated by exogenous ketone supplementation [42, 45, 53, 61], thus preventing sufficient CHO and energy intake. Clearly, further research investigating the effects of EKSs on prolonged, moderate-intensity endurance performance is required.
9.4 Keto-Adaptation and Prolonged Moderate-Intensity Endurance Performance
Keto-adaptation may be ergogenic for prolonged, moderate-intensity endurance events limited by endogenous CHO availability; however, only a single study has investigated the effect of keto-adaptation on endurance performance or capacity lasting > 3 h [7] (Table 3). Arguably, the ergogenic effect of keto-adaptation may be greatest when CHO ingestion is restricted, potentially due to limited fuelling opportunities or in individuals who cannot tolerate CHO ingestion during competition [4, 30]. Following several weeks-to-months of ingesting a KD, fat oxidation (including fatty-acid-derived KBs) can contribute to > 70% of energy expenditure at 70–80% VO2max [9, 11] and up to ~ 90% at 65% VO2max [10], with rates invariably > 1 g min−1 and extending up to ~ 1.9 g min−1 [9]. However, when exercising ≥ 2 h without CHO ingestion, the rate of ketogenesis likely exceeds ketolysis as blood D-βHB concentrations rise > 1.5 mM [7, 9,10,11]. It is uncertain if increases in endogenous KB production result in corresponding direct increases in KB oxidation or, more likely, greater modulation of endogenous substrate metabolism, such as via effects on adipose tissue lipolysis, glycolysis, and/or PDHc activity.
In a seminal study, 4 weeks of keto-adaptation preserved mean time-to-exhaustion (TTE) at 62–64% VO2max in five trained cyclists [8] (Table 3). However, the study design favoured keto-adaptation due to: (1) a single-arm design; (2) failure to implement performance nutrition strategies in the CHO trial; and (3) a large improvement in a single participant in the post- (keto-adapted) trial, thus distorting the results. A recent study aimed to address these limitations by investigating 31 days of keto-adaptation in eight trained runners using a randomised, repeated-measures, counter-balanced, crossover design [7] (Table 3). Mean TTE was preserved at 70% VO2max, despite an increase in the rate of exercising energy expenditure [7], suggesting impaired exercise efficiency. Therefore, the ergogenic benefits of elevated fat and KB oxidation and the regulatory role of increased blood KB concentration on substrate metabolism are unlikely to outweigh high-CHO fuelling strategies; however, future research examining keto-adaptation, particularly for exercise durations > 4–6 h and in conjunction CHO ingestion before and/or during exercise, remains warranted.
10 Is There an Optimal Blood Ketone Body Concentration for Endurance Performance?
Optimal implies an ergogenic effect of KBs either via their regulation of or contribution to substrate provision for energy production; however, there is currently no clear benefit of nutritional ketosis for endurance performance following either exogenous ketone supplementation (Table 1) or keto-adaptation (Table 2). Published studies also contain small sample sizes (~ 8–12) and lack rigor to identify positive and negative responders due to the underlying noise of measurement error [101]. Blood KB concentrations also reflect the rates of ketogenesis (or KB appearance following EKS ingestion) and KB uptake by peripheral tissues, thus making interpretation difficult. Considering blood KB concentrations during keto-adaptation are metabolically regulated depending on energy demand and CHO availability [2], it may not be possible to identify an optimal range for endurance performance. In contrast, exogenous ketone supplementation can acutely manipulate blood KB concentration, which has prompted suggestion of an optimal blood D-βHB range of 1–3 mM [3]; however, 1 mM appears too low (Table 2). Therefore, for EKSs, further research exploring specific dosing strategies should consider the following: (1) blood KB concentration measurement technique; (2) blood D-βHB/L-βHB/AcAc concentration and ratio; (3) substrate availability and metabolism (e.g., fasted vs. fed vs. acute CHO fuelling strategies); (4) previous exposure to hyperketonaemia; (5) athlete training status; (6) physiological demands of competition; and (7) tolerability.
11 Conclusion
Exogenous ketone supplementation and keto-adaptation induce nutritional ketosis; however, they are not similar physiological states. Underlying alterations to substrate availability and metabolism differentiate the ergogenic potential for each and despite decades of research, it is only recently that their performance effects within ecologically valid contexts are being examined. Therefore, current recommendations to induce nutritional ketosis for endurance performance are unsubstantiated, but are an exciting area of future research.
References
Romijn JA, Coyle EF, Sidossis LS, et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993;265:E380–91.
Robinson AM, Williamson DH. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev. 1980;60:143–87.
Evans M, Cogan KE, Egan B. Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. J Physiol. 2016;595:2857–71. https://doi.org/10.1113/JP273185.
Volek JS, Noakes T, Phinney SD. Rethinking fat as a fuel for endurance exercise. Eur J Sport Sci. 2015;15:13–20. https://doi.org/10.1080/17461391.2014.959564.
Pardridge WM (1991) Blood–brain barrier transport of glucose, free fatty acids, and ketone bodies. In: Fuel homeostasis and the nervous system. Advances in experimental medicine and biology. Oxygen Transport to Tissue Xxxiv, pp 43–53.
Halestrap AP, Wilson MC. The monocarboxylate transporter family—role and regulation. IUBMB Life. 2012;64:109–19. https://doi.org/10.1002/iub.572.
Shaw DM, Merien F, Braakhuis A, et al. Effect of a ketogenic diet on submaximal exercise capacity and efficiency in runners. Med Sci Sport Exerc. 2019. https://doi.org/10.1249/MSS.0000000000002008.
Phinney SD, Bistrian BR, Evans WJ, et al. The human metabolic response to chronic ketosis without caloric restriction: preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism. 1983;32:769–76.
Burke LM, Ross ML, Garvican-Lewis LA, et al. Low Carbohydrate, High Fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J Physiol. 2016;595:1–23. https://doi.org/10.1113/JP273230.
Volek JS, Freidenreich DJ, Saenz C, Kunces LJ. Metabolic characteristics of keto-adapted ultra-endurance runners. Metabolism. 2015;65:100–10.
Webster CC, Noakes TD, Chacko SK, et al. Gluconeogenesis during endurance exercise in cyclists habituated to a long-term low carbohydrate high fat diet. J Physiol. 2016;594:4389–405. https://doi.org/10.1113/JP271934.
Cahill GF. Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. https://doi.org/10.1146/annurev.nutr.26.061505.111258.
Owen OE, Morgan AP, Kemp HG, et al. Brain metabolism during fasting. J Clin Invest. 1967;46:1589–95. https://doi.org/10.1172/JCI105650.
McSwiney FT, Wardrop B, Hyde PN, et al. Keto-adaptation enhances exercise performance and body composition responses to training in endurance athletes. Metab Clin Exp. 2018;81:25–34. https://doi.org/10.1016/j.metabol.2017.10.010.
Dostal T, Plews DJ, Hofmann P, et al. Effects of a 12-week very-low carbohydrate high-fat diet on maximal aerobic capacity, high-intensity intermittent exercise, and cardiac autonomic regulation: non-randomized parallel-group study. Front Physiol. 2019;10:1–12. https://doi.org/10.3389/fphys.2019.00912.
Sherrier M, Li H. The impact of keto-adaptation on exercise performance and the role of metabolic-regulating cytokines. Am J Clin Nutr. 2019;67:789-12. https://doi.org/10.1093/ajcn/nqz145.
Phinney SD (2004) Ketogenic diets and physical performance. Nutr Metab.
Murtaza N, Burke L, Vlahovich N, et al. The effects of dietary pattern during intensified training on stool microbiota of elite race walkers. Nutrients. 2019;11:261-14. https://doi.org/10.3390/nu11020261.
Murtaza N, Burke LM, Vlahovich N, et al. Analysis of the effects of dietary pattern on the oral microbiome of elite endurance athletes. Nutrients. 2019;11:614. https://doi.org/10.3390/nu11030614.
McKay AKA, Peeling P, Pyne DB, et al. Chronic adherence to a ketogenic diet modifies iron metabolism in elite athletes. Med Sci Sport Exerc. 2019;51:548–55. https://doi.org/10.1249/MSS.0000000000001816.
McKay AKA, Peeling P, Pyne DB, et al. Acute carbohydrate ingestion does not influence the post-exercise iron-regulatory response in elite keto-adapted race walkers. J Sci Med Sport. 2019. https://doi.org/10.1016/j.jsams.2018.12.015.
Carr A, Sharma AP, Ross ML, et al. Chronic ketogenic low carbohydrate high fat diet has minimal effects on acid-base status in elite athletes. Nutrients. 2018;10:1–13. https://doi.org/10.3390/nu10020236.
McKay AKA, Pyne DB, Peeling P, et al. The impact of chronic carbohydrate manipulation on mucosal immunity in elite endurance athletes. J Sports Sci. 2018;37:553–9. https://doi.org/10.1080/02640414.2018.1521712.
Coggan AR, Coyle EF. Reversal of fatigue during prolonged exercise by carbohydrate infusion or ingestion. J Appl Physiol. 1987;63:2388–95.
Gonzalez JT, Fuchs CJ, Smith FE, et al. Ingestion of glucose or sucrose prevents liver but not muscle glycogen depletion during prolonged endurance-type exercise in trained cyclists. Am J Physiol Endocrinol Metab. 2015;309:E1032–9. https://doi.org/10.1152/ajpendo.00376.2015.
Coyle EF, Coggan AR, Hemmert MK, Ivy JL. Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. J Appl Physiol. 1986;61:165–72.
Burke LM. “Fat adaptation” for athletic performance: the nail in the coffin? J Appl Physiol. 2006;100:7–8. https://doi.org/10.1152/japplphysiol.01238.2005.
Yeo WK, Carey AL, Burke L, et al. Fat adaptation in well-trained athletes: effects on cell metabolism. Appl Physiol Nutr Metab. 2011;36:12–22. https://doi.org/10.1139/H10-089.
Spriet LL. New insights into the interaction of carbohydrate and fat metabolism during exercise. Sports Med. 2014;44:87–96. https://doi.org/10.1007/s40279-014-0154-1.
Burke LM. Re-examining high-fat diets for sports performance: did we call the “Nail in the Coffin” too soon? Sports Med. 2015;45(Suppl 1):S33–49. https://doi.org/10.1007/s40279-015-0393-9.
Mirtschin JG, Forbes SF, Cato LE, et al. Organisation of dietary control for nutrition-training intervention involving periodized carbohydrate (CHO) availability and ketogenic low CHO high fat (LCHF) diet. Int J Sport Nutr Exerc Metab. 2018;28:480–9. https://doi.org/10.1123/ijsnem.2017-0249.
Jeukendrup AE, Wallis GA. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int J Sports Med. 2005;26:S28–37. https://doi.org/10.1055/s-2004-830512.
Rowlands DS. Model for the behaviour of compartmental CO2 stores during incremental exercise. Eur J Appl Physiol. 2005;93:555–68. https://doi.org/10.1007/s00421-004-1217-z.
Pinckaers PJM, Churchward-Venne TA, Bailey D, van Loon LJC. Ketone bodies and exercise performance: the next magic bullet or merely hype? Sports Med. 2016;47:383–91. https://doi.org/10.1007/s40279-016-0577-y.
Cox PJ, Clarke K. Acute nutritional ketosis: implications for exercise performance and metabolism. Extrem Physiol Med. 2014;3:17.
Margolis LM, O’Fallon KS. Utility of ketone supplementation to enhance physical performance: a systematic review. Adv Nutr. 2019;31:834–8. https://doi.org/10.1093/advances/nmz104.
Evans M, Patchett E, Nally R, et al. Effect of acute ingestion of β-hydroxybutyrate salts on the response to graded exercise in trained cyclists. Eur J Sport Sci. 2018;45:1–11. https://doi.org/10.1080/17461391.2017.1421711.
O’Malley T, Myette-Cote E, Durrer C, Little JP. Nutritional ketone salts increase fat oxidation but impair high-intensity exercise performance in healthy adult males. Appl Physiol Nutr Metab. 2017;42:1031–5. https://doi.org/10.1139/apnm-2016-0641.
Rodger S, Plews D, Laursen P, Driller M. Oral β-hydroxybutyrate salt fails to improve 4-minute cycling performance following submaximal exercise. J Sci Cycling. 2017;6:26–31.
Stubbs BJ, Cox PJ, Evans R, et al. On the metabolism of exogenous ketones in humans. Front Physiol. 2017;8:848. https://doi.org/10.3389/fphys.2017.00848.
Waldman HS, Basham SA, Price FG, et al. Exogenous ketone salts do not improve cognitive responses after a high-intensity exercise protocol in healthy college-aged males. Appl Physiol Nutr Metab. 2018;43:711–7. https://doi.org/10.1139/apnm-2017-0724.
Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2004;70:309–19. https://doi.org/10.1016/j.plefa.2003.09.007.
Stubbs BJ, Cox PJ, Kirk T, et al. Gastrointestinal effects of exogenous ketone drinks are infrequent, mild and vary according to ketone compound and dose. Int J Sport Nutr Exerc Metab. 2019. https://doi.org/10.1123/ijsnem.2019-0014.
Scott BE, Laursen PB, James LJ, et al. The effect of 1,3-butanediol and carbohydrate supplementation on running performance. J Sci Med Sport. 2018. https://doi.org/10.1016/j.jsams.2018.11.027.
Shaw DM, Merien F, Braakhuis A, et al. The effect of 1,3-butanediol on cycling time-trial performance. Int J Sport Nutr Exerc Metab. 2019. https://doi.org/10.1123/ijsnem.2018-0284.
Puchowicz MA, Smith CL, Bomont C, et al. Dog model of therapeutic, ketosis induced by oral administration of R, S-1,3-butanediol diacetoacetate. J Nutr Biochem. 2000;11:281–7. https://doi.org/10.1016/S0955-2863(00)00079-6.
Desrochers S, David F, Garneau M, et al. Metabolism of R- and S-1,3-butanediol in perfused livers from meal-fed and starved rats. Biochem J. 1992;285:647–53.
Desrochers S, Quinze K, Dugas H, et al. R, S-1,3-butanediol acetoacetate esters, potential alternates to lipid emulsions for total parenteral nutrition. J Nutr Biochem. 1995;6:111–8. https://doi.org/10.1016/0955-2863(94)00011-A.
Mehlman MA, Tobin RB, Hahn HK, et al. Metabolic fate of 1,3-butanediol in the rat: liver tissue slices metabolism. J Nutr. 1971;101:1711–8.
Tate RL, Mehlman MA, Tobin RB. Metabolic fate of 1,3-butanediol in rat—conversion to beta-hydroxybutyrate. J Nutr. 1971;101:1719–26.
Desrochers S, Dubreuil P, Brunet J, et al. Metabolism of (R, S)-1,3-butanediol acetoacetate esters, potential parenteral and enteral nutrients in conscious pigs. Am J Physiol. 1995;31:E660–7.
D’agostino DP, Pilla R, Held HE, et al. Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats. Am J Physiol Regul Integr Comp Physiol. 2013;304:R829–36. https://doi.org/10.1152/ajpregu.00506.2012.
Leckey JJ, Ross ML, Quod M, et al. Ketone diester ingestion impairs time-trial performance in professional cyclists. Front Physiol. 2017;8:806. https://doi.org/10.3389/fphys.2017.00806.
Clarke K, Tchabanenko K, Pawlosky R, et al. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul Toxicol Pharmacol. 2012;63:401–8. https://doi.org/10.1016/j.yrtph.2012.04.008.
Cox PJ, Kirk T, Ashmore T, et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 2016;24:256–68. https://doi.org/10.1016/j.cmet.2016.07.010.
Clarke K, Tchabanenko K, Pawlosky R, et al. Oral 28-day and developmental toxicity studies of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate. Regul Toxicol Pharmacol. 2012;63:196–208. https://doi.org/10.1016/j.yrtph.2012.04.001.
Shivva V, Cox PJ, Clarke K, et al. The population pharmacokinetics of d-β-hydroxybutyrate following administration of (R)-3-Hydroxybutyl (R)-3-hydroxybutyrate. AAPS J. 2016;18:678–88. https://doi.org/10.1208/s12248-016-9879-0.
Myette-Cote E, Neudorf H, Rafiei H, et al. Prior ingestion of exogenous ketone monoester attenuates the glycemic response to an oral glucose tolerance test in healthy young individuals. J Physiol. 2018;596:1385–95. https://doi.org/10.1113/JP275709.
Stubbs BJ, Cox PJ, Evans RD, et al. A ketone ester drink lowers human ghrelin and appetite. Obesity. 2017;26:269–73. https://doi.org/10.1002/oby.22051.
Neudorf H, Durrer C, Myette-Cote E, et al. Oral ketone supplementation acutely increases markers of NLRP3 inflammasome activation in human monocytes. Mol Nutr Food Res. 2019. https://doi.org/10.1002/mnfr.201801171.
Evans M, Egan B. Intermittent running and cognitive performance after ketone ester ingestion. Med Sci Sport Exerc. 2018;50:2330–8. https://doi.org/10.1249/MSS.0000000000001700.
Faull OK. Beyond RPE: the perception of exercise under normal and ketotic conditions. Front Physiol. 2019;10:229. https://doi.org/10.3389/fphys.2019.00229.
Dearlove DJ, Faull OK, Rolls E, et al. Nutritional ketoacidosis during incremental exercise in healthy athletes. Front Physiol. 2019;10:290. https://doi.org/10.3389/fphys.2019.00290.
Vandoorne T, De Smet S, Ramaekers M, et al. Intake of a ketone ester drink during recovery from exercise promotes mTORC1 signalling but not glycogen resynthesis in human muscle. Front Physiol. 2017;8:310. https://doi.org/10.3389/fphys.2017.00310/full.
Holdsworth DA, Cox PJ, Kirk T, et al. A ketone ester drink increases postexercise muscle glycogen synthesis in humans. Med Sci Sport Exerc. 2017;49:1789–95. https://doi.org/10.1249/MSS.0000000000001292.
Poffé C, Ramaekers M, Van Thienen R, Hespel P. Ketone ester supplementation blunts overreaching symptoms during endurance training overload. J Physiol. 2019. https://doi.org/10.1113/JP277831.
Van Gelder J, Shafiee M, De Clercq E, et al. Species-dependent and site-specific intestinal metabolism of ester prodrugs. Int J Pharm. 2000;205:93–100. https://doi.org/10.1016/S0378-5173(00)00507-X.
Tsai Y-C, Liao T-H, Lee J-A. Identification of L-3-hydroxybutyrate as an original ketone body in rat serum by column-switching high-performance liquid chromatography and fluorescence derivatization. Analytical Biochemistry. 2003;319:34–41. https://doi.org/10.1016/S0003-2697(03)00283-5.
Tsai Y-C, Chou Y-C, Wu A-B, et al. Stereoselective effects of 3-hydroxybutyrate on glucose utilization of rat cardiomyocytes. Life Sci. 2006;78:1385–91. https://doi.org/10.1016/j.lfs.2005.07.013.
Lincoln BC, Rosiers CD, Brunengraber H. Metabolism of S-3-hydroxybutyrate in the perfused rat liver. Arch Biochem Biophys. 1987;259:149–56. https://doi.org/10.1016/0003-9861(87)90480-2.
Webber RJ. Utilization of L(+)-3-hydroxybutyrate, D(−)-3-hydroxybutyrate, acetoacetate, and glucose for respiration and lipid synthesis in the 18-day-old rat. J Biol Chem. 1977;252:5222–6.
Misra S, Oliver NS. Utility of ketone measurement in the prevention, diagnosis and management of diabetic ketoacidosis. Diabet Med. 2015;32:14–23. https://doi.org/10.1111/dme.12604.
Urbain P, Bertz H. Monitoring for compliance with a ketogenic diet: what is the best time of day to test for urinary ketosis? Nutr Metab. 2016;13:77. https://doi.org/10.1186/s12986-016-0136-4.
Ceriotti F, Kaczmarek E, Guerra E, et al. Comparative performance assessment of point-of-care testing devices for measuring glucose and ketones at the patient bedside. J Diabetes Sci Technol. 2015;9:268–77. https://doi.org/10.1177/1932296814563351.
Guimont M-C, Desjobert H, Fonfrède M, et al. Multicentric evaluation of eight glucose and four ketone blood meters. Clin Biochem. 2015;48:1310–6. https://doi.org/10.1016/j.clinbiochem.2015.07.032.
Thomas C, Bishop DJ, Lambert K, et al. Effects of acute and chronic exercise on sarcolemmal MCT1 and MCT4 contents in human skeletal muscles: current status. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1–14. https://doi.org/10.1152/ajpregu.00250.2011.
Winder WW, Holloszy JO, Baldwin KM. Enzymes involved in ketone utilization in different types of muscle: adaptation to exercise. Eur J Biochem. 1974;47:461–7. https://doi.org/10.1111/j.1432-1033.1974.tb03713.x.
Romijn JA, Coyle EF, Hibbert J, Wolfe RR. Comparison of indirect calorimetry and a new breath 13C/12C ratio method during strenuous exercise. Am J Physiol. 1992;263:E64–71.
Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:628–34.
Soeters MR, Serlie MJ, Sauerwein HP, et al. Characterization of D-3-hydroxybutyrylcarnitine (ketocarnitine): an identified ketosis-induced metabolite. Metab Clin Exp. 2012;61:966–73. https://doi.org/10.1016/j.metabol.2011.11.009.
Féry F, Balasse EO. Effect of exercise on the disposal of infused ketone bodies in humans. J Clin Endocrinol Metab. 1988;67:245–50. https://doi.org/10.1210/jcem-67-2-245.
Hawley JA, Leckey JJ. Carbohydrate dependence during prolonged, intense endurance exercise. Sports Med. 2015;45(Suppl 1):S5–12. https://doi.org/10.1007/s40279-015-0400-1.
Gejl KD, Thams L, Hansen M, et al. No superior adaptations to carbohydrate periodization in elite endurance athletes. Med Sci Sport Exerc. 2017;49:2486–97. https://doi.org/10.1249/MSS.0000000000001377.
Krogh A, Lindhard J. The relative value of fat and carbohydrate as sources of muscular energy: with appendices on the correlation between standard metabolism and the respiratory quotient during rest and work. Biochem J. 1920;14:290–363.
Ørtenblad N, Nielsen J. Muscle glycogen and cell function—location, location, location. Scand J Med Sci Sports. 2015;25:34–40. https://doi.org/10.1111/sms.12599.
Mikkelsen KH, Seifert T, Secher NH, et al. Systemic, cerebral and skeletal muscle ketone body and energy metabolism during acute hyper-D-β-hydroxybutyratemia in post-absorptive healthy males. J Clin Endocrinol Metab. 2015;100:636–43. https://doi.org/10.1210/jc.2014-2608.
Olson MS, Dennis SC, DeBuysere MS, Padma A. The regulation of pyruvate dehydrogenase in the isolated perfused rat heart. J Biol Chem. 1978;253:7369–75.
Ashour B, Hansford RG. Effect of fatty acids and ketone on the activity of pyruvate dehydrogenase in skeletal-muscle mitochondria. Biochem J. 1983;214:725–36.
Sato K, Kashiwaya Y, Keon CA, et al. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995;9:651–8.
Burgess SC, Iizuka K, Jeoung NH, et al. Carbohydrate-response element-binding protein deletion alters substrate utilization producing an energy-deficient liver. J Biol Chem. 2008;283:1670–8. https://doi.org/10.1074/jbc.M706540200.
Murray AJ, Knight NS, Cole MA, et al. Novel ketone diet enhances physical and cognitive performance. FASEB J. 2016;30:4021–32. https://doi.org/10.1096/fj.201600773R.
Evans M, McSwiney FT, Brady AJ, Egan B. No benefit of ingestion of a ketone monoester supplement on 10-km running performance. Med Sci Sport Exerc. 2019;51:2506–15. https://doi.org/10.1249/MSS.0000000000002065.
Zinn C, Wood M, Williden M, et al. Ketogenic diet benefits body composition and well-being but not performance in a pilot case study of New Zealand endurance athletes. J Int Soc Sports Nutr. 2017;14:22. https://doi.org/10.1186/s12970-017-0180-0.
Maunder E, Kilding AE, Plews DJ. Substrate metabolism during ironman triathlon: different horses on the same courses. Sports Med. 2018;48:2219–26. https://doi.org/10.1007/s40279-018-0938-9.
Tiller NB, Roberts JD, Beasley L, et al. International Society of Sports Nutrition Position Stand: nutritional considerations for single-stage ultra-marathon training and racing. J Int Soc Sports Nutr. 2019;16:1–23. https://doi.org/10.1186/s12970-019-0312-9.
Stellingwerff T, Boon H, Gijsen AP, et al. Carbohydrate supplementation during prolonged cycling exercise spares muscle glycogen but does not affect intramyocellular lipid use. Pflugers Arch. 2007;454:635–47. https://doi.org/10.1007/s00424-007-0236-0.
Stellingwerff T, Boon H, Jonkers RAM, et al. Significant intramyocellular lipid use during prolonged cycling in endurance-trained males as assessed by three different methodologies. Am J Physiol Endocrinol Metab. 2007;292:E1715–23. https://doi.org/10.1152/ajpendo.00678.2006.
van Loon LJC, Koopman R, Stegen JHCH, et al. Intramyocellular lipids form an important substrate source during moderate intensity exercise in endurance-trained males in a fasted state. J Physiol. 2004;553:611–25. https://doi.org/10.1113/jphysiol.2003.052431.
De Bock K, Richter EA, Russell AP, et al. Exercise in the fasted state facilitates fibre type-specific intramyocellular lipid breakdown and stimulates glycogen resynthesis in humans. J Physiol. 2005;564:649–60. https://doi.org/10.1113/jphysiol.2005.083170.
Frayn KN. Fat as a fuel: emerging understanding of the adipose tissue-skeletal muscle axis. Acta Physiol (Oxf). 2010;199:509–18. https://doi.org/10.1111/j.1748-1716.2010.02128.x.
Chrzanowski-Smith OJ, Piatrikova E, Betts JA, et al. Variability in exercise physiology: can capturing intra-individual variation help better understand true inter-individual responses? Eur J Sport Sci. 2019. https://doi.org/10.1080/17461391.2019.1655100.
Helge JW, Watt PW, Richter EA, et al. Fat utilization during exercise: adaptation to a fat-rich diet increases utilization of plasma fatty acids and very low density lipoprotein-triacylglycerol in humans. J Physiol. 2001;537:1009–20.
Braakhuis AJ, Hopkins WG. Impact of dietary antioxidants on sport performance: a review. Sports Med. 2015;45:939–55. https://doi.org/10.1007/s40279-015-0323-x.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this article.
Conflict of Interest
Shaw DM, Merien F, Braakhuis A, Maunder E, and Dulson D declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Shaw, D.M., Merien, F., Braakhuis, A. et al. Exogenous Ketone Supplementation and Keto-Adaptation for Endurance Performance: Disentangling the Effects of Two Distinct Metabolic States. Sports Med 50, 641–656 (2020). https://doi.org/10.1007/s40279-019-01246-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-019-01246-y