Abstract
Objective
The aim was to conduct a systematic review and meta-analysis analyzing the impact of up to 24 h of prolonged sitting on postprandial glucose, insulin and triglyceride responses, blood pressure and vascular function, in comparison to sitting interrupted with light- to moderate-intensity physical activity.
Methods
To be included, studies had to examine the impact of prolonged sitting lasting < 24 h in apparently healthy males or females of any age. Studies were identified from searches of the MEDLINE, CINAHL and SportDISCUS databases on July 6, 2016. Study quality was assessed using the Downs and Black Checklist; publication bias was assessed via funnel plot.
Results
Forty-four studies met the inclusion criteria for the systematic review; of these, 20 were included in the meta-analysis, which compared prolonged sitting to the effects of interrupting sitting with regular activity breaks on postprandial glucose, insulin and triglycerides. When compared to prolonged sitting, regular activity breaks lowered postprandial glucose (d = − 0.36, 95% confidence interval [CI] − 0.50 to − 0.21) and insulin (d = − 0.37, 95% CI − 0.53 to − 0.20), but not triglyceride responses (d = 0.06, 95% CI − 0.15 to 0.26). Subgroup analyses indicated reductions in postprandial triglyceride responses only occurred 12–16 h after the intervention. The magnitude of the reductions in glucose, insulin or triglyceride response was not modified by the intensity of the activity breaks, the macronutrient composition of the test meal, or the age or body mass index of participants.
Conclusion
Prolonged sitting results in moderate elevations in postprandial glucose and insulin responses when compared to sitting interrupted with activity breaks.
PROSPERO ID
CRD42015020907.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Compared to prolonged sitting, breaking up sitting time with light- or moderate-intensity physical activity results in lower post-meal insulin and glucose levels. |
Breaks in sitting time may help reduce post-meal triglyceride levels, but this is not seen until the following day. |
1 Introduction
Evidence is emerging that sedentary behavior—that is any activity done while sitting, lying, or reclining, while expending no more than 1.5 metabolic equivalents [1]—is a distinct risk factor for chronic disease morbidity and mortality [2]. One biological explanation for this relationship is that sedentary behavior exerts an acute effect on markers of cardiometabolic risk [3,4,5]. In 2012, Saunders et al. [5] systematically reviewed the research examining changes in cardiometabolic indicators following exposure to ≤ 7 days of prolonged sedentary behavior, reporting evidence that prolonged periods of sedentary behavior consistently resulted in significant reductions in insulin sensitivity, glucose tolerance, and increased plasma triglyceride levels [5]. However, of the 25 studies identified at the time, only four examined the impact of sitting per se, as opposed to more unconventional and extreme forms of sedentary behavior, such as bed rest or immobility for medical reasons, which are uncommon in day-to-day life. Further, only five studies published at that time examined exposure to sedentary behavior lasting less than 24 h [5]. Thus, although these findings strongly suggested a physiological impact of several days of extreme and prolonged sedentary behavior, the effects of sedentary behaviors more typical of daily life, both in terms of duration and mode, were not addressed.
In recent years there has been a rapid increase in the volume of experimental research examining the acute impact of prolonged sitting on markers of cardiometabolic risk [3, 4]. A recent systematic review and meta-analysis of observational and experimental studies by Chastin et al. [4] examined the benefit of breaking up prolonged sitting with light- or moderate-intensity physical activity. They concluded that interruptions in sitting could help control postprandial glucose and insulin levels, although they found no effect on postprandial triglyceride levels [4]. However, their search identified just six eligible experimental studies, and did not include research in pediatric populations. To date, no review has examined the impact of the macronutrient composition of the test meal or its time of consumption in relation to postprandial metabolic response. This is an important omission as the timing of a meal challenge may greatly influence the postprandial triglyceride response [6] and could explain why Chastin et al. [4] observed no effect of sitting on postprandial triglyceride levels, despite significant reductions in postprandial glucose and insulin. Further, no existing review has investigated whether the impact of prolonged sitting is consistent across the age span. This is important, as initial studies in children and youth suggested that prolonged sitting may have had less cardiometabolic impact in pediatric populations, when compared with adults [7, 8].
Finally, at present no reviews have examined the impact of prolonged sitting on vascular function, which refers to the ability of the vasculature to appropriately adapt to the conditions and demands of the tissue it supplies. In particular, it is now well understood that the vasculature will adapt during conditions of increased metabolic demand from local vasodilatory signaling, and also as a result of changes in shear stress [9]. Persistent alterations in both the form and function of the vasculature are both associated with poorer cardiovascular health outcomes and directly linked to blood pressure. While the exercise physiology literature has clearly elucidated both the acute and chronic effects of increases in these stimuli for affecting blood pressure and vascular function, the effects of sedentary time are less clear, but early initial reports suggest inactivity may lead to decrements in function, which are similarly associated with poorer health outcomes [10, 11]. It is worth highlighting as well that an association has been noted between high blood glucose and poor vascular function, thus making this a logical area for investigation alongside the more traditional metabolic markers [12].
The purpose of the present study was therefore to systematically review and analyze the impact of up to 24 h of prolonged sitting on postprandial insulin, glucose and triglycerides, as well as blood pressure and vascular function, in comparison to sitting interrupted with light- or moderate-intensity physical activity. A secondary purpose was to determine whether the timing or macronutrient composition of the test meal(s), the intensity of activity breaks, or the age or body mass index (BMI) of study participants differentially influenced any effects of sitting on postprandial insulin, glucose, or triglyceride responses.
2 Methods
This systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for the transparent reporting of systematic reviews [13] (PROSPERO ID: CRD42015020907). Inclusion criteria for the studies included in the systematic review are detailed in Sects. 2.1, 2.2, and 2.3.
2.1 Population
Studies of apparently healthy males and females of all ages were eligible for review. In line with previous research, individuals were deemed to be apparently healthy if they had not received a positive disease diagnosis [14,15,16]. Those with risk factors for disease, but without a positive diagnosis (e.g., elevated fasting glucose or blood pressure below the threshold for the diagnosis of diabetes or hypertension) were therefore included in this review. In line with previous research, individuals with overweight/obesity, but without a positive diagnosis for other chronic disease, were considered apparently healthy [15, 16]. Studies in which participants were selected on the basis of existing disease (e.g., diabetes or cardiovascular disease) were excluded from this review to ensure that study participants would be sufficiently homogeneous for inclusion in meta-analyses. There were no restrictions on the age or sex of the participants.
2.2 Intervention and Comparator
To be included in this review, studies must have exposed participants to a period of uninterrupted sitting lasting no more than 24 h. Studies with longer periods of sitting were included if they measured the outcomes of interest within the first 24 h of sitting (all data beyond 24 h were excluded from this review). The comparator had to include some form of light- or moderate-intensity physical activity. In comparison to prolonged sitting, the benefits of vigorous-intensity exercise are well-established. Therefore, to limit the scope of the current manuscript, it was felt that focusing on light- and moderate-intensity activity breaks would provide the greatest contribution to the published literature. There were no specific criteria about the duration of the activity, which could be prolonged (e.g., several hours of standing or walking) or intermittent (e.g., a 2-min walk-break every 20 min). Activity was defined as light- (< 50% maximum oxygen uptake [VO2max]) or moderate-intensity exercise (> 50% VO2max to < 65% VO2max) following the Howley [17] guidelines for aerobic, leisure, and occupational physical activity.
2.3 Outcomes
To be included in the present review, all studies were required to report on the impact of prolonged sitting on one or more of the following health indicators: postprandial glucose, insulin, and triglyceride concentrations measured in plasma, serum, interstitial fluid or whole blood, blood pressure, arterial stiffness (collected via pulse wave velocity via tonometry, or beta-stiffness with ultrasound), or flow-mediated dilatation (a non-invasive measure of vascular reactivity to a shear stress).
2.4 Search Strategy and Study Selection
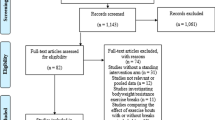
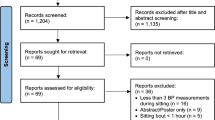
A systematic search was conducted using the databases MEDLINE, CINAHL, and SportDISCUS (all via EBSCOhost) on July 6, 2016. The search strategy was created with the help of a research librarian (Fig. 1). Only articles published in English were included in the review, and no limits were placed on the date of publication.
Using Covidence software (Covidence.org, Melbourne, Australia), two separate reviewers screened titles and abstracts of potentially relevant articles. Articles deemed potentially relevant by either reviewer were obtained for full-text review. The full-text review was also performed by two separate reviewers; at this stage any discrepancies between reviewers were decided via consensus. The reference list of each study identified in the initial database search and the library of each co-author were also reviewed to identify any potentially relevant studies.
2.5 Quality of Evidence
The risk of bias and strength of evidence was assessed by one reviewer (HA) using the Downs and Black Checklist [18]. This 27-point checklist assesses the strength of reporting, external validity, internal validity, and power. As some questions are worth more than 1 point, the maximum score that a study can receive is 32.
2.6 Data Extraction
Data extraction was conducted by one author and verified by another, with any disagreements resolved by consensus. Descriptive characteristics were extracted from each included study, including the BMI of participants and the intensity of the activity as well as mean and standard deviation (SD) for outcomes of interest. For studies that measured the postprandial insulin, glucose or triglyceride responses, incremental area under the curve (iAUC) or total area under the curve (AUC) were extracted in the form (e.g., net or positive iAUC) provided in the individual manuscripts as mean and SD for both the prolonged sitting and activity-based interventions. Where 95% confidence intervals (CIs) or standard errors were reported, SDs were calculated, assuming a t distribution if the sample size was less than n = 70. If an AUC (either incremental or total) was not reported, or only presented in a figure, the authors were contacted and asked to provide the relevant information. The amount of carbohydrate, fat and protein provided by the meal challenge(s) and the timing of the meal in relation to the activity were also extracted from relevant studies.
2.7 Meta-Analysis
Studies were included in the meta-analysis if they compared the effect of prolonged sitting to performing repeated short bouts of light to moderate activity, henceforth referred to as regular activity breaks, on postprandial glucose, insulin or triglyceride concentrations. To ensure that interventions were sufficiently homogeneous for comparison, only interventions with activity breaks less than 10 min in duration and a total exposure of < 24 h of uninterrupted sitting were included in meta-analyses. This was done as periods of activity lasting > 10 min are typically considered an activity “bout”, rather than simply a break in sedentary time. Thus, although studies with bouts of activity lasting > 10 min were included in the narrative review, they were not included in meta-analyses. Meta-analyses were used to calculate summary estimates for the effect of regular activity breaks compared to prolonged sitting on postprandial glucose, insulin and triglyceride responses. The overall summary estimate for each outcome was calculated as a standardized mean difference (95% CI) using the METAN command in STATA version 14.1 (StataCorp, College Station, USA); fixed effects were assumed and individual study results weighted by the inverse of their variance. Evidence of heterogeneity was assessed using a Cochran’s Q with a significance level of < 0.05, and I2 values used to judge the degree of heterogeneity, with values above 50% considered to be important. Some studies included more than one regular activity break intervention or measured postprandial responses at multiple time points. To facilitate appropriate weighting of studies in the meta-analysis, only one comparison from each study could be included. Where multiple arms involved different intensities of activity break, only the comparison between the intervention with the lower intensity activity break and prolonged sitting was included. Where the postprandial response was measured at multiple time points (e.g., concurrently with the intervention period and the day following the intervention period), only the response measured concurrently with the intervention was included. Publication bias was assessed through visual inspection of funnel plots. Meta-regression was used to examine the association between the carbohydrate or fat content (in grams) of the test meal and the standardized effect size, as well as the association between age of participants (in years) or BMI (kg/m2) and the standardized effect size.
Subgroup analyses were performed to assess the modifying effect of the intensity of the activity breaks (moderate, light or standing) and the timing of the meal challenge (measured concurrently with activity breaks or the following day). For studies in which more than one intensity of activity break was compared with prolonged sitting, all intensities were included in the forest plots. However, the test for differences between subgroups assumes independence between subgroups, so to facilitate a valid comparison, with similar n in each group, subgroup analyses were repeated using only the comparison between lowest intensity of activity break and prolonged sitting. Similarly a single study measured postprandial responses both concurrently with the activity breaks and the day following the activity. Both arms are presented in the forest plot; however, the statistical results of the subgroup analysis are confined to the responses measured on the following day.
3 Results
3.1 Narrative Synthesis
A total of 2668 individual studies were identified via the search process, after which 120 were selected for full-text review (Fig. 2). Of these, 76 were excluded because they did not meet the inclusion criteria: 60 studies employed an ineligible intervention or comparator (e.g., no sitting condition, activity breaks of vigorous intensity, etc.), 54 studies were excluded for having an ineligible study design (e.g., sitting for more than 1 day, ineligible population or lacking the outcomes of interest, etc.), five papers were reviews or conference abstracts, and one study was not available in English. Forty-four papers representing 42 separate interventions met all eligibility criteria and were included in this review (see Table 1 and Supplemental Dataset 1 in the electronic supplementary material). The mean (SD) score on the Downs and Black Checklist [18] was 26.5 (2.4) out of a possible 32 points.
Forty studies were designed to compare the effect of sitting with that of activity on postprandial glucose, all of which were of crossover design [7, 8, 19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. Of these, 23 found that sitting resulted in significantly higher levels of postprandial glucose and/or reduced glucose clearance in comparison to at least one of the activity conditions [20, 23, 26,27,28,29, 31,32,33,34, 39,40,41,42,43,44,45, 50, 52,53,54,55,56]. One study found a significant decrease in postprandial glucose levels in response to sitting [19], and 16 studies observed no significant difference between sitting and activity [7, 8, 21, 22, 24, 25, 28, 30, 36,37,38, 47,48,49, 51].
The impact of sitting compared to activity on postprandial insulin was assessed in 28 crossover studies [7, 8, 19, 22, 23, 25, 27, 30, 32, 34, 37,38,39,40,41,42, 44, 45, 47, 49,50,51,52, 54,55,56,57]. Fifteen studies found that sitting resulted in significantly increased postprandial insulin levels when compared to at least one activity condition [22, 23, 25, 27, 32, 37, 39, 41, 44, 45, 50, 54,55,56,57], two studies found that sitting significantly reduced insulin levels, while 11 studies found no significant difference between sitting and light or moderate activity [7, 8, 19, 34, 38, 40, 42, 47, 49, 51, 52]. Two studies reported in a single manuscript [30] observed a significant reduction in postprandial insulin following prolonged sitting, when compared to light-intensity walking.
Twenty-two crossover studies examined the impact of sitting compared to activity on postprandial triglyceride levels [7, 8, 19, 21, 22, 25, 28, 30, 35,36,37, 39,40,41,42,43, 45, 46, 49, 50, 57]. Eight studies found that sitting resulted in significantly higher postprandial triglyceride levels, in comparison to at least one light or moderate activity condition [21, 22, 25, 28, 35, 37, 40, 57]. Fourteen studies observed no difference between sitting and activity [7, 8, 19, 30, 36, 39, 41,42,43, 45, 46, 49, 50].
Six interventions examined the impact of sitting compared to activity on blood pressure or vascular function [10, 11, 33, 43, 51, 58]. Three studies found that blood pressure was higher during sitting than during light or moderate activity [33, 45, 58], and two studies observed no difference [47, 51]. One study found that flow-mediated dilation was significantly lower during prolonged sitting than during interrupted sitting [10].
3.2 Quantitative Synthesis
Due to the small number of studies and heterogeneity of methods examining blood pressure and vascular function, these outcomes were not examined via meta-analysis. Similarly, studies that compared prolonged sitting with prolonged light- or moderate-intensity activity, operationally defined as bouts of activity greater than 10 min in length, were insufficiently homogeneous for inclusion in meta-analyses. The 20 studies included in the meta-analysis were all of crossover design, and all compared the effects of regular activity breaks and prolonged sitting on postprandial glucose, insulin and/or triglyceride responses.
When compared to prolonged sitting, regular activity breaks lowered postprandial glucose (d = − 0.36, 95% CI − 0.50 to − 0.21) (Fig. 3) and insulin (d = − 0.37, 95% CI − 0.53 to − 0.20) (Fig. 4) responses, but did not change postprandial triglyceride responses (d = 0.06, 95% CI − 0.15 to 0.26) (Fig. 5). Neither the I2 statistic nor the chi-squared test indicated evidence of heterogeneity. Funnel plots (data not shown) did not indicate evidence of publication bias. All but one study included in the meta-analyses [46] employed a randomized design. Removing this study from the meta-analyses did not materially change the above findings (data not shown).
Effect (Cohen’s d) of regular activity breaks (< 10 min in duration) compared to prolonged sitting on postprandial glucose responses. Studies are sorted by carbohydrate content of the test meal. The diamond indicates the standardized mean difference (SMD) with associated 95% confidence interval for each subgroup. Shaded area represents weighting of individual studies
Effect (Cohen’s d) of regular activity breaks (< 10 min in duration) compared to prolonged sitting on postprandial insulin responses. Studies are sorted by carbohydrate content of the test meal. The diamond indicates the standardized mean difference (SMD) with associated 95% confidence interval for each subgroup. Shaded area represents weighting of individual studies
Effect (Cohen’s d) of regular activity breaks (< 10 min in duration) compared to prolonged sitting on postprandial triglyceride responses. Studies are sorted by fat content of the test meal. The diamond indicates the standardized mean difference (SMD) with associated 95% confidence interval for each subgroup. Shaded area represents weighting of individual studies
Subgroup analyses indicated that the intensity of the activity break did not modify the effect on postprandial glucose (p = 0.20), insulin (p = 0.38) or triglycerides (p = 0.72). The inclusion of all arms of the studies (n = 5) that reported comparisons for breaks of different intensities in this subgroup analysis did not noticeably change the results (glucose: p = 0.12; insulin: p = 0.51; triglycerides: p = 0.68). However, both moderate- and light-intensity activity breaks induced reductions in postprandial glucose (Supplemental Figure S1) and insulin response (Supplemental Figure S2), while standing breaks did not significantly affect glucose or insulin response. None of the intensities of activity break resulted in changes in postprandial triglyceride response compared to prolonged sitting (Supplemental Figure S3).
Subgroup analyses indicated that timing of the test meal did not modify the effect of regular activity breaks on postprandial glucose (p = 0.34) or insulin responses (p = 0.90), despite changes in glucose and insulin only being observed concomitantly with the period of activity breaks or prolonged sitting, and not the day following the activity intervention (Supplemental Figures S4 and S5). In contrast, subgroup analysis indicated that meal timing does influence the effect of regular activity breaks on postprandial triglyceride response (p = 0.01). A significantly lower postprandial triglyceride response with activity intervention was only observed 12–16 h after the activity (Supplemental Figure S6). These results did not change markedly when the one study that reported postprandial responses both during and the day following activity was included (glucose: p = 0.34; insulin: p = 0.89; triglycerides: p = 0.01)
Meta-regression indicated no evidence of a linear association between the amount of carbohydrate or fat provided in the test meal and the magnitude of the regular activity break-induced reductions in glucose, insulin or triglyceride response (Table 2). Similarly, meta-regression indicated no evidence of a linear association between the magnitude of the regular activity break-induced reductions in glucose, insulin or triglyceride response and either BMI (Table 3) or age of participants (data not shown; all p > 0.05).
4 Discussion
The purpose of the present systematic review and meta-analysis was to determine the impact of prolonged sitting lasting up to 1 day on important markers of cardiometabolic risk, in comparison to sitting interrupted with light- or moderate-intensity physical activity. Our results indicate that prolonged sitting results in elevated levels of postprandial glucose and insulin when compared to sitting interrupted with regularly performed light- or moderate-intensity activity breaks. The magnitude of the elevation is consistent with what could be considered a medium effect [59]. Given the effect of raised postprandial insulin and glucose concentrations on increasing the risk of chronic disease morbidity and mortality [60,61,62,63], these increases may be clinically relevant if experienced on a regular basis. Based on the Downs and Black Checklist scores, the quality of evidence was high, and we did not observe evidence of publication bias.
The impact of sitting on postprandial glucose and insulin levels does not appear to be influenced by participant age or the carbohydrate load of test meals. However, the total amount of carbohydrate provided in the test meal(s) in the interventions included in our meta-analyses ranged from 54.9 to 228.5 g. It is possible that larger carbohydrate loads may modify the effect of regular activity breaks on postprandial insulin and glucose. Although the mean BMI of study participants ranged from a low of 16.2 [44] to a high of 32.9 [50], we observed no impact on our findings. These results suggest that the relationship between activity breaks and postprandial insulin, glucose, and triglyceride levels may be consistent across a relatively large range of body weights.
Subgroup analyses indicated that the intensity of activity breaks did not modify their effect on postprandial insulin, glucose, or triglyceride responses. However, it is possible that the small number of studies eligible for this subgroup analysis limited the statistical power to detect small differences. Acknowledging this limitation, it is possible that standing breaks of less than 10 min in duration may be insufficient to reduce postprandial insulin or glucose levels in apparently healthy individuals. Given the small number of studies on this topic, future research should continue to investigate the impact of standing breaks to determine whether they offer similar benefit as other forms of light and moderate activity in both healthy individuals and in those with type 2 diabetes or impaired glucose tolerance.
We now have a convincing body of high-quality evidence from randomized controlled trials showing that light- to moderate-intensity activity breaks reduce postprandial glucose and insulin concentrations among apparently healthy individuals. Nevertheless, there are still many important questions to answer and knowledge gaps to be filled in this field of research. In particular, more work needs to be done to identify the timing, duration and mode of activity break that are likely to impart the most benefit to specific populations. Thinking more broadly about the implications of this knowledge for public health, we must begin to devise strategies that will enable individuals who habitually sit for long periods to perform activity breaks as part of their everyday routine.
Of the ten studies included in our meta-analysis that examined postprandial triglyceride levels, seven examined triglyceride levels during the intervention, while three examined triglycerides the day following the intervention. Differences in postprandial triglyceride responses were only observed when measured the day following the activity intervention. The small number of studies measuring triglyceride response and an even smaller number of studies measuring postprandial responses the day following the intervention may indicate these results should be interpreted with caution. However, the results are compatible with documented effects of more prolonged bouts of activity on postprandial responses, which occur the day following exposure to intermitted or sustained bouts of activity. The delayed response may be related to the upregulation of lipoprotein lipase activity, which peaks 8–16 h after a bout of activity [64]. Further research on this topic is clearly warranted.
At present there is insufficient evidence to draw strong conclusions on the impact of prolonged sitting on blood pressure or vascular form and function, suggesting that further research on this topic is clearly needed. Although not the focus of the current review, there has been relatively little experimental research on the relationship between prolonged sitting and numerous other important health outcomes, including coagulatory factors, regional blood flow, autonomic nervous system balance, stress hormone levels, and cognition, to name a few. Given that the impact of prolonged sitting on postprandial glucose and insulin is now relatively well-established, more research is needed to better understand the relationship between sitting and a wider range of health outcomes.
Several physiological mechanisms have been suggested which would link bouts of prolonged sitting with postprandial insulin, glucose and triglyceride levels [65, 66]. Periods of inactivity have been shown to result in insulin resistance in skeletal muscle [65], whereas light-intensity activity breaks have been shown to stimulate metabolic pathways related to glucose uptake [66, 67]. Less research has examined the mechanisms linking prolonged sitting with triglyceride levels. Although research in animal models suggests that muscle inactivity may result in reductions in lipoprotein lipase activity [68], work in humans has failed to detect changes in response to prolonged sitting [25]. Given that available evidence suggests a strong and consistent link between sitting and important markers of cardiometabolic risk, more research is needed to better elucidate the mechanisms underpinning these relationships.
4.1 Strengths and Limitations
The present study used a systematic search methodology and identified a large number of experimental studies (n = 44) and a wider age range than previous meta-analyses in this field. This allowed us to investigate the impact of age, meal composition, and the timing of the test-meal, which have not been examined in previous reviews on this topic. Our review also included studies measuring blood pressure and vascular parameters, although there was not sufficient evidence available on these topics to perform meta-analyses. This review was limited by the exclusion of non-English language papers and unpublished literature, all of which may have resulted in the omission of relevant findings. However, the funnel plots did not indicate evidence of publication bias in favor of large effect sizes in smaller studies. The meta-analysis performed in the current study included interventions which employed venous, capillary and interstitial blood samples. While it is recognized that these methods do not provide identical results, they are strongly correlated with each other [69, 70], and it was felt that combining the methods was therefore justified to maximize the available data. Further, our analyses were confined to apparently healthy individuals and therefore excluded studies on individuals with diabetes and cardiovascular disease, who are most likely to benefit from any reductions in cardiometabolic risk factors. It is possible that this may have diluted the observed relationship between activity breaks and reductions in postprandial insulin and glucose levels, thus making the reported effects more conservative. Given that research is now beginning to examine individuals with these chronic conditions [71, 72], future reviews should focus on these populations. Finally, the majority of the studies included in the current review exposed participants to > 3 h of uninterrupted sitting, and it is currently unclear if, or how frequently, adults in the general population accumulate sedentary bouts of this duration. Although this is a relatively long period of uninterrupted sitting, findings from the REGARDS study suggest that American adults accumulate 14% of their daily sedentary time in bouts of ≥ 90 min, a proportion which increases consistently with age [73]. Further research is therefore needed both to understand the frequency of prolonged sitting in the general population and to identify the minimum amount of uninterrupted sitting which results in clinically relevant changes in health outcomes.
5 Conclusions
Our findings suggest that acute periods of uninterrupted sitting result in significant increases in postprandial insulin and glucose levels, when compared to periods of sitting interrupted with light- or moderate-intensity physical activity. Breaking up sitting time may have benefits for postprandial triglyceride levels, but these are only seen the day following the intervention. There was insufficient data to quantitatively assess the effect of sitting with or without activity breaks on blood pressure or vascular function. It would be valuable for future research investigating the effects of sedentary behavior on cardiometabolic health to include blood pressure and vascular form and function measures as endpoints now that the weight of evidence clearly shows that regularly interrupting prolonged sitting with activity breaks produces marked and meaningful improvements in postprandial glucose metabolism.
References
Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE, et al. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project process and outcome. Int J Behav Nutr Phys Act. 2017;14:75.
Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med. 2015;162(2):123–32.
Benatti F, Ried-Larsen. The effects of breaking up prolonged sitting time: a review of experimental studies. Med Sci Sports Exerc. 2015;47(10):2053–61.
Chastin SF, Egerton T, Leask C, Stamatakis E. Meta-analysis of the relationship between breaks in sedentary behavior and cardiometabolic health. Obesity. 2015;23(9):1800–10.
Saunders TJ, Larouche R, Colley RC, Tremblay MS. Acute sedentary behaviour and markers of cardiometabolic risk: a systematic review of intervention studies. J Nutr Metab. 2012;2012:1–12.
Homer AR, Fenemor SP, Perry TL, Rehrer NJ, Cameron CM, Skeaff CM, et al. Regular activity breaks combined with physical activity improve postprandial plasma triglyceride, nonesterified fatty acid, and insulin responses in healthy, normal weight adults: a randomized crossover trial. J Clin Lipidol. 2017;11(5):1268–79.
Saunders TJ, Chaput J-P, Goldfield GS, Colley RC, Kenny GP, Doucet E, et al. Prolonged sitting and markers of cardiometabolic disease risk in children and youth: a randomized crossover study. Metab Clin Exp. 2013;62(10):1423–8.
Sisson SB, Anderson AE, Short KR, Gardner AW, Whited T, Robledo C, et al. Light activity following a meal and postprandial cardiometabolic risk in adolescents. Pediatr Exerc Sci. 2013;25(3):347–59.
Tomiyama H, Yamashina A. Non-invasive vascular function tests: their pathophysiological background and clinical application. Circ J Off J Jpn Circ Soc. 2010;74(1):24–33.
Thosar SS, Bielko SL, Wiggins CC, Wallace JP. Differences in brachial and femoral artery responses to prolonged sitting. Cardiovasc Ultrasound. 2014;12(1):50.
Larsen RN, Kingwell BA, Sethi P, Cerin E, Owen N, Dunstan DW. Breaking up prolonged sitting reduces resting blood pressure in overweight/obese adults. Nutr Metab Cardiovasc Dis. 2014;24(9):976–82.
Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349–54.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
Goodman JM, Thomas SG, Burr J. Evidence-based risk assessment and recommendations for exercise testing and physical activity clearance in apparently healthy individuals. Appl Physiol Nutr Metab. 2011;36(S1):S14–32.
Carson V, Hunter S, Kuzik N, Gray C, Poitras V, Chaput J-P, et al. Systematic review of the relationships between sedentary behaviour and health indicators in school-aged children and youth: an update. Appl Physiol Nutr Metab. 2016;6(Suppl. 3):S240–65.
Saunders T, Gray C, Poitras V, Chaput JP, Janssen I, Katzmarzyk P, et al. Combinations of physical activity, sedentary behaviour and sleep: relationships with health indicators in school-aged children and youth. Appl Physiol Nutr Metab. 2016;41(Suppl. 3):S283–93.
Howley ET. Type of activity: resistance, aerobic and leisure versus occupational physical activity. Med Sci Sports Exerc. 2001;33(6 Suppl):S364–9 (discussion S419–420).
Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–84.
Welle S. Metabolic responses to a meal during rest and low-intensity exercise. Am J Clin Nutr. 1984;40(5):990–4.
Mikines KJ, Sonne B, Farrell PA, Tronier B, Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol. 1988;254(3 Pt 1):E248–59.
Hardman AE, Aldred HE. Walking during the postprandial period decreases alimentary lipaemia. J Cardiovasc Risk. 1995;2(1):71–8.
Tsetsonis NV, Hardman AE. Reduction in postprandial lipemia after walking: influence of exercise intensity. Med Sci Sports Exerc. 1996;28(10):1235–42.
Marion-Latard F, Crampes F, Zakaroff-Girard A, De Glisezinski I, Harant I, Stich V, et al. Post-exercise increase of lipid oxidation after a moderate exercise bout in untrained healthy obese men. Horm Metab Res Horm Stoffwechselforschung Horm Metab. 2003;35(2):97–103.
Tsofliou F, Pitsiladis YP, Malkova D, Wallace AM, Lean MEJ. Moderate physical activity permits acute coupling between serum leptin and appetite-satiety measures in obese women. Int J Obes Relat Metab Disord J Int Assoc Study Obes. 2003;27(11):1332–9.
Katsanos CS, Grandjean PW, Moffatt RJ. Effects of low and moderate exercise intensity on postprandial lipemia and postheparin plasma lipoprotein lipase activity in physically active men. J Appl Physiol. 2004;96(1):181–8.
Høstmark AT, Ekeland GS, Beckstrøm AC, Meen HD. Postprandial light physical activity blunts the blood glucose increase. Prev Med. 2006;42(5):369–71.
Aadland E, Høstmark AT. Very light physical activity after a meal blunts the rise in blood glucose and insulin. Open Nutr J. 2008;2:94–9.
Tolfrey K, Doggett A, Boyd C, Pinner S, Sharples A, Barrett L. Postprandial triacylglycerol in adolescent boys: a case for moderate exercise. Med Sci Sports Exerc. 2008;40(6):1049–56.
Nygaard H, Tomten SE, Høstmark AT. Slow postmeal walking reduces postprandial glycemia in middle-aged women. Appl Physiol Nutr Metab. 2009;34(6):1087–92.
Hashimoto S, Ootani K, Hayashi S, Naito M. Acute effects of shortly pre- versus postprandial aerobic exercise on postprandial lipoprotein metabolism in healthy but sedentary young women. J Atheroscler Thromb. 2011;18(10):891–900.
Stephens BR, Granados K, Zderic TW, Hamilton MT, Braun B. Effects of 1 day of inactivity on insulin action in healthy men and women: interaction with energy intake. Metabolism. 2011;60(7):941–9.
Dunstan DW, Kingwell BA, Larsen R, Healy GN, Cerin E, Hamilton MT, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35(5):976–83.
Lunde MSH, Hjellset VT, Høstmark AT. Slow post meal walking reduces the blood glucose response: an exploratory study in female Pakistani immigrants. J Immigr Minor Health. 2012;14(5):816–22.
Takaishi T, Imaeda K, Tanaka T, Moritani T, Hayashi T. A short bout of stair climbing-descending exercise attenuates postprandial hyperglycemia in middle-aged males with impaired glucose tolerance. Appl Physiol Nutr Metab Physiol Appl Nutr Metab. 2012;37(1):193–6.
Tolfrey K, Bentley C, Goad M, Varley J, Willis S, Barrett L. Effect of energy expenditure on postprandial triacylglycerol in adolescent boys. Eur J Appl Physiol. 2012;112(1):23–31.
Altenburg TM, Rotteveel J, Dunstan DW, Salmon J, Chinapaw MJM. The effect of interrupting prolonged sitting time with short, hourly, moderate-intensity cycling bouts on cardiometabolic risk factors in healthy, young adults. J Appl Physiol. 2013;115(12):1751–6.
Farah NMF, Gill JMR. Effects of exercise before or after meal ingestion on fat balance and postprandial metabolism in overweight men. Br J Nutr. 2013;109(12):2297–307.
Gonzalez JT, Veasey RC, Rumbold PLS, Stevenson EJ. Breakfast and exercise contingently affect postprandial metabolism and energy balance in physically active males. Br J Nutr. 2013;110(4):721–32.
Hashimoto S, Hayashi S, Yoshida A, Naito M. Acute effects of postprandial aerobic exercise on glucose and lipoprotein metabolism in healthy young women. J Atheroscler Thromb. 2013;20(2):204–13.
Miyashita M, Park J-H, Takahashi M, Suzuki K, Stensel D, Nakamura Y. Postprandial lipaemia: effects of sitting, standing and walking in healthy normolipidaemic humans. Int J Sports Med. 2013;34(1):21–7.
Peddie MC, Bone JL, Rehrer NJ, Skeaff CM, Gray AR, Perry TL. Breaking prolonged sitting reduces postprandial glycemia in healthy, normal-weight adults: a randomized crossover trial. Am J Clin Nutr. 2013;98(2):358–66.
Thorp AA, Kingwell BA, Sethi P, Hammond L, Owen N, Dunstan DW. Alternating bouts of sitting and standing attenuate postprandial glucose responses. Med Sci Sports Exerc. 2014;46(11):2053–61.
Bailey DP, Locke CD. Breaking up prolonged sitting with light-intensity walking improves postprandial glycemia, but breaking up sitting with standing does not. J Sci Med Sport. 2015;18(3):294–8.
Belcher BR, Berrigan D, Papachristopoulou A, Brady SM, Bernstein SB, Brychta RJ, et al. Effects of interrupting children’s sedentary behaviors with activity on metabolic function: a randomized trial. J Clin Endocrinol Metab. 2015;100(10):3735–43.
Larsen RN, Kingwell BA, Robinson C, Hammond L, Cerin E, Shaw JE, et al. Breaking up of prolonged sitting over three days sustains, but does not enhance, lowering of postprandial plasma glucose and insulin in overweight and obese adults. Clin Sci Lond Engl 1979. 2015;129:117–27.
Ross K, Hinckson E, Zinn C. Effect of intermittent sitting time on acute postprandial lipemia in children. J Clin Transl Endocrinol. 2015;2(2):72–6.
Bailey DP, Broom DR, Chrismas BCR, Taylor L, Flynn E, Hough J. Breaking up prolonged sitting time with walking does not affect appetite or gut hormone concentrations but does induce an energy deficit and suppresses postprandial glycaemia in sedentary adults. Appl Physiol Nutr Metab Physiol Appl Nutr Metab. 2016;41(3):324–31.
Hansen RK, Andersen JB, Vinther AS, Pielmeier U, Larsen RG. Breaking up prolonged sitting does not alter postprandial glycemia in young, normal-weight men and women. Int J Sports Med. 2016;37(14):1097–102.
Hawari NSA, Al-Shayji I, Wilson J, Gill JMR. Frequency of breaks in sedentary time and postprandial metabolic responses. Med Sci Sports Exerc. 2016;48(12):2495–502.
Henson J, Davies MJ, Bodicoat DH, Edwardson CL, Gill JMR, Stensel DJ, et al. Breaking up prolonged sitting with standing or walking attenuates the postprandial metabolic response in postmenopausal women: a randomized acute study. Diabetes Care. 2016;39(1):130–8.
Wennberg P, Boraxbekk C-J, Wheeler M, Howard B, Dempsey PC, Lambert G, et al. Acute effects of breaking up prolonged sitting on fatigue and cognition: a pilot study. BMJ Open. 2016;6(2):e009630.
Bailey DP, Maylor BD, Orton CJ, Zakrzewski-Fruer JK. Effects of breaking up prolonged sitting following low and high glycaemic index breakfast consumption on glucose and insulin concentrations. Eur J Appl Physiol. 2017;117(7):1299–307.
Fletcher EA, Salmon J, McNaughton SA, Orellana L, Wadley GD, Bruce C, et al. Effects of breaking up sitting on adolescents’ postprandial glucose after consuming meals varying in energy: a cross-over randomised trial. J Sci Med Sport. 2017;21(3):280–5.
McCarthy M, Edwardson CL, Davies MJ, Henson J, Bodicoat DH, Khunti K, et al. Fitness moderates glycemic responses to sitting and light activity breaks. Med Sci Sports Exerc. 2017;49(11):2216–22.
McCarthy M, Edwardson CL, Davies MJ, Henson J, Rowlands A, King JA, et al. Breaking up sedentary time with seated upper body activity can regulate metabolic health in obese high-risk adults: a randomized crossover trial. Diabetes Obes Metab. 2017;19(12):1732–9.
Pulsford RM, Blackwell J, Hillsdon M, Kos K. Intermittent walking, but not standing, improves postprandial insulin and glucose relative to sustained sitting: a randomised cross-over study in inactive middle-aged men. J Sci Med Sport. 2017;20(3):278–83.
Schlierf G, Dinsenbacher A, Kather H, Kohlmeier M, Haberbosch W. Mitigation of alimentary lipemia by postprandial exercise—phenomena and mechanisms. Metabolism. 1987;36(8):726–30.
Zeigler ZS, Mullane SL, Crespo NC, Buman MP, Gaesser GA. Effects of standing and light-intensity activity on ambulatory blood pressure. Med Sci Sports Exerc. 2016;48(2):175–81.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. New York: Routledge; 1988.
Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119(10):812–9.
Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med. 2004;164(19):2147–55.
Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298(3):309–16.
Nordestgaard BG, Benn M, Schnohr P, Tybjærg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298(3):299–308.
Peddie MC, Rehrer NJ, Perry TL. Physical activity and postprandial lipidemia: are energy expenditure and lipoprotein lipase activity the real modulators of the positive effect? Prog Lipid Res. 2012;51(1):11–22.
Tremblay MS, Colley RC, Saunders TJ, Healy GN, Owen N. Physiological and health implications of a sedentary lifestyle. Appl Physiol Nutr Metab. 2010;35(6):725–40.
Bergouignan A, Latouche C, Heywood S, Grace MS, Reddy-Luthmoodoo M, Natoli AK, et al. Frequent interruptions of sedentary time modulates contraction- and insulin-stimulated glucose uptake pathways in muscle: ancillary analysis from randomized clinical trials. Sci Rep. 2016;24(6):32044.
Latouche C, Jowett JBM, Carey AL, Bertovic DA, Owen N, Dunstan DW, et al. Effects of breaking up prolonged sitting on skeletal muscle gene expression. J Appl Physiol. 2013;114(4):453–60.
Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56(11):2655.
Boyd R, Leigh B, Stuart P. Capillary versus venous bedside blood glucose estimations. Emerg Med J. 2005;22(3):177–9.
Thennadil SN, Rennert JL, Wenzel BJ, Hazen KH, Ruchti TL, Block MB. Comparison of glucose concentration in interstitial fluid, and capillary and venous blood during rapid changes in blood glucose levels. Diabetes Technol Ther. 2001;3(3):357–65.
Dempsey PC, Larsen RN, Sethi P, Sacre JW, Straznicky NE, Cohen ND, et al. Benefits for type 2 diabetes of interrupting prolonged sitting with brief bouts of light walking or simple resistance activities. Diabetes Care. 2016;39(6):964–72.
Dempsey PC, Owen N, Yates TE, Kingwell BA, Dunstan DW. Sitting less and moving more: improved glycaemic control for type 2 diabetes prevention and management. Curr Diabetes Rep. 2016;16(11):114.
Diaz KM, Howard VJ, Hutto B, Colabianchi N, Vena JE, Blair SN, et al. Patterns of sedentary behavior in US middle-age and older adults: the REGARDS study. Med Sci Sports Exerc. 2016;48(3):430–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Meredith Peddie’s work was supported by a Research Fellowship from the National Heart Foundation of New Zealand (Grant no. 1745). Hayden Atkinson’s work was supported by a Summer Undergraduate Research Award from the University of Prince Edward Island. Travis Saunders is supported by the Jeanne and J.-Louis Lévesque Research Professorship in Nutrisciences and Health. No other sources of funding were used to assist in the preparation of this article.
Conflict of interest
Travis Saunders has received research and/or in-kind support from Stepscount, Fitabase, and Ergotron. Hayden Atkinson, Jamie Burr, Brittany MacEwen, Murray Skeaff and Meredith Peddie declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
40279_2018_963_MOESM1_ESM.tiff
Figure S1 Effect (Cohen’s d) of regular activity breaks (< 10 min in duration) compared to prolonged sitting on postprandial glucose responses, stratified by intensity of activity breaks. Studies are sorted within each subgroup by age of participants. The diamond indicates the standardized mean difference (SMD) with associated 95% confidence interval for each subgroup (TIFF 929 kb)
40279_2018_963_MOESM2_ESM.tiff
Figure S2 Effect (Cohen’s d) of regular activity breaks (< 10 min in duration) compared to prolonged sitting on postprandial insulin responses, stratified by intensity of activity breaks. Studies are sorted within each subgroup by age of participants. The diamond indicates the standardized mean difference (SMD) with associated 95% confidence interval for each subgroup (TIFF 1019 kb)
40279_2018_963_MOESM3_ESM.tiff
Figure S3 Effect (Cohen’s d) of regular activity breaks (< 10 min in duration) compared to prolonged sitting on postprandial triglyceride responses, stratified by intensity of activity breaks. Studies are sorted within each subgroup by age of participants. The diamond indicates the standardized mean difference (SMD) with associated 95% confidence interval for each subgroup (TIFF 1092 kb)
40279_2018_963_MOESM4_ESM.tiff
Figure S4 Effect (Cohen’s d) of regular activity breaks (< 10 min in duration) compared to prolonged sitting on postprandial glucose responses, stratified by timing of the test meal. The diamond indicates the standardized mean difference (SMD) with associated 95% confidence interval for each subgroup (TIFF 1006 kb)
40279_2018_963_MOESM5_ESM.tiff
Figure S5 Effect (Cohen’s d) of regular activity breaks (< 10 min in duration) compared to prolonged sitting on postprandial insulin responses, stratified by timing of the test meal. The diamond indicates the standardized mean difference (SMD) with associated 95% confidence interval for each subgroup (TIFF 1105 kb)
40279_2018_963_MOESM6_ESM.tiff
Figure S6 Effect (Cohen’s d) of regular activity breaks (< 10 min in duration) compared to prolonged sitting on postprandial triglyceride responses, stratified by timing of the test meal. The diamond indicates the standardized mean difference (SMD) with associated 95% confidence interval for each subgroup (TIFF 1156 kb)
Rights and permissions
About this article
Cite this article
Saunders, T.J., Atkinson, H.F., Burr, J. et al. The Acute Metabolic and Vascular Impact of Interrupting Prolonged Sitting: A Systematic Review and Meta-Analysis. Sports Med 48, 2347–2366 (2018). https://doi.org/10.1007/s40279-018-0963-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-018-0963-8