Abstract
It is well known that prolonged passive muscle stretch reduces maximal muscle force production. There is a growing body of evidence suggesting that adaptations occurring within the nervous system play a major role in this stretch-induced force reduction. This article reviews the existing literature, and some new evidence, regarding acute neurophysiological changes in response to passive muscle stretching. We discuss the possible contribution of supra-spinal and spinal structures to the force reduction after passive muscle stretch. In summary, based on the recent evidence reviewed we propose a new hypothesis that a disfacilitation occurring at the motoneuronal level after passive muscle stretch is a major factor affecting the neural efferent drive to the muscle and, subsequently, its ability to produce maximal force.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Prolonged muscle stretching causes a reduction of maximal force production. |
A major factor causing this stretch-induced force loss is a reduction in the motor command from the central nervous system to the muscle. |
A reduced ability to amplify the motor command at the spinal level seems to be a major factor reducing muscle activation after passive stretch. |
1 Introduction and Overview
Static muscle stretching exercises are commonly utilised in pre-exercise preparatory routines [1]. It is purported that stretching exercises could increase range of motion and decrease injury incidence, especially in high-intensity stretch-shortening cycle activities, by increasing the compliance of the muscle-tendon unit [2]. Several randomised controlled studies [3–5] and reviews [6, 7] have reported a positive effect of pre-exercise stretching specifically on soft tissue muscle injury risk. Also, it has been reported that pre-exercise muscle stretching is still common practice amongst athletes, and that coaches usually recommend, on average, 13 min of stretching prior to exercise [8–11] with a typical duration of approximately 15 s per stretch [9–11]. Additionally, longer durations of muscle stretching are commonly utilised prior to exercise in clinical rehabilitation especially in patients with a range of motion deficits [12, 13]. Indeed, in order to promote a transient increase in muscle-tendon unit compliance some evidence indicates that it may be necessary to stretch for at least 4 min per muscle group [14].
However, the use of stretching exercises before physical activity for the purpose of performance enhancement or injury prevention has been criticised [14–18]. Importantly, it has often been shown that acute static muscle stretching reduces maximal contractile force and power production, especially when it lasts longer than 60 s (for review see Behm et al. [6]). For example, static muscle stretching performed with total (i.e. accumulated) duration of 60 s or longer was found to elicit an average force reduction of 7.5% when testing was performed within a few minutes of the stretching [19], and longer-duration stretching has been shown to reduce force for up to 1 h [20] or more [21]. This decrease in contractile capacity may compromise functional performance that immediately follows static muscle stretching and thus influence performance in sports or rehabilitation exercises. Thus, there is a current need to understand the factors influencing, and mechanisms underpinning, this effect so that strategies that mitigate against it can be developed.
1.1 Peripheral Hypothesis
An earlier hypothesis offered to explain force loss [22] was that static stretch could change the mechanical characteristics of the muscle-tendon unit itself, resulting in a decrease in its stiffness or shifting the muscle’s operating length to a less optimal point on its force–length relationship [20, 23]. For instance, an increase in tendon compliance after static muscle stretching might negatively affect force transmission and/or cause the muscle to operate at a shorter length, which could ultimately affect maximal force production. This idea was based on observations that a rightward shift in the torque-angle relationship during maximal contractions occurs after stretch [14]. However, changes in the torque-angle relationship cannot be taken as evidence of changes in the muscle’s mechanical properties as it can also be affected by neural and intrinsic muscle properties (i.e. calcium release). Indeed, it is well known that the whole muscle-tendon unit’s passive stiffness decreases after static stretch [14]. Moreover, it has been consistently shown that acute static stretching has little or no effect on tendon stiffness and active (during maximal contraction) muscle length, especially when a warm-up is performed prior to the stretching (i.e. the muscle-tendon unit has been pre-conditioned) [24–27]. This suggests that the muscle (but not the tendon) might be more compliant at rest but not during maximal contractions, when the active muscle force makes the muscle stiffer.
Another hypothesis related to peripheral (muscle based) mechanisms is that the mechanical stress caused by static stretch can affect the excitation–contraction (E–C) coupling process, reducing contractile force capability [28]. Evidence for this hypothesis comes from a study of isolated soleus muscles from rats showing that static stretch increased myofibrillar Ca2+ concentration, and this was associated with a reduction in contractile force (twitch tension) [29]. To date, there is no direct evidence of this in experiments on intact human muscle. For instance, this hypothesis was tested by Trajano et al. [30, 31], who compared the ratio of the torque elicited by low- vs. high-frequency electrical muscle stimulation after stretch. Disproportional reductions in the low-frequency stimulation in comparison with the high-frequency stimulation (shifting the force–frequency relationship) could be taken as evidence of disruption in the E–C coupling process, possibly due to an increase in myofibrillar Ca2+ concentration caused by stretch [32, 33]. However, no change in the low- vs. high-frequency tetanic torque ratio was observed after stretch and the individual variations on this ratio were not correlated with changes in muscle force after stretch. Thus, based on the available evidence it is unlikely that impairments of the E–C coupling process play an important role in the force loss caused by stretch.
1.2 Neural Hypothesis
An alternative hypothesis is that contractile force decrements result from a reduced efferent neural drive (i.e. reduced central drive) during voluntary contraction. Efferent drive has typically been measured as a decrease in muscle electrical activity recorded using electromyography following acute static muscle stretching [20, 25, 34–36]. Nonetheless, it is critical to note that the reduction in electromyogram (EMG) amplitude may more broadly indicate either (or both) a decrease in efferent neural drive to the muscle or changes in post-synaptic potentials within the muscle, which typically result in a reduced force production [37]. Thus, mechanisms associated with the transmission of potentials, ultimately influencing E–C coupling, may be implicated in addition to changes in efferent neural drive based on the available EMG data. Another important consideration is that these EMG data do not provide information regarding the specific site at which neuromuscular activation might be compromised (e.g. supra-spinal, spinal or muscular). Of final note is that post-stretch decreases in EMG amplitude are not always observed [21, 38, 39], and it is not known whether this results from sensitivity and reliability issues associated with EMG measurements or rather indicates that other mechanisms must be at least as influential on the EMG signal as neural drive modification [40–43]. Thus, a clear understanding of the possible effect of muscle stretching on descending neural drive is important in the greater context of understanding the effects of static stretching on muscle force production and movement performance and thus on the likelihood of developing strategies that mitigate against the force loss. The purpose of this review, therefore, is to critically review the evidence for neural changes in response to acute static muscle stretching and determine their importance for the reduction of muscle force observed subsequent to stretching.
2 Reduction in Supra-Spinal Drive
2.1 Evidence of Exercise-Induced Acute Changes in Supra-Spinal Centres
Alterations in supra-spinal drive can noticeably affect muscular force production. From an exercise-related perspective, reductions in muscular force during and after continued activation of a muscle (i.e. muscle fatigue) have been shown to result from an inability of the descending supra-spinal drive to maximally activate the muscle’s motor neurone pool [37]. These reductions in supra-spinal drive have been demonstrated during and after exercise leading to muscle fatigue through repeated [44] and sustained [45, 46] maximal contractions, submaximal sustained contractions [47] and long-duration (endurance) efforts [48]. For instance, an 18% decrease in maximal voluntary force was reported after running a marathon [48], with this force loss attributed at least in part to a reduction in corticospinal excitability measured as the amplitude of the motor-evoked potential (MEP), and possibly a suboptimal motor cortical output, measured as a reduction in voluntary activation during cortical transcranial magnetic stimulation (TMS) [48]. Nevertheless, it is important to note that although it is commonly used as an indicator of cortical excitability [49], changes in MEP amplitude or area do not reflect only the excitability of the motor cortex and can also be influenced by changes in motor neuronal excitability [44]. Moreover, reductions in MEP amplitude can be taken as evidence of a reduced efficiency of transmission along the corticospinal pathway for 12 min after a 2-min maximal isometric elbow flexor contraction [45]. Taken together, these results suggest that acute reductions in muscular force output can occur through alterations in supra-spinal drive and that these can be observed in humans in response to exercise. The mechanisms underpinning this sub-optimal input from the motor cortex to the motor neurone are still unclear and need further investigation. However, changes in the behaviour of cortical neurones and/or the influence of afferent fibres inhibiting the descending volley should be considered as potential mechanisms [50].
2.2 Evidence of Stretch-Related Mechanisms Acting on the Motor Cortex
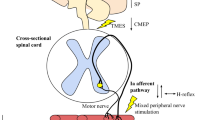
Although there is considerable evidence supporting the existence of a sub-optimal supra-spinal output to motor neurones after fatiguing muscle contractions, very little is known about how muscle stretch might affect supra-spinal drive to the muscle. Thus, at this time there is no clear evidence as to whether a supra-spinal depression might influence muscular force production subsequent to a bout of muscle stretching. It is well known that motor cortical outflow may be influenced by sensory inflow [51], and it is interesting to note that changes in limb position, for example, can acutely influence the organisation of the primary motor cortex [52, 53]. In 1953, Gellhorn and Hyde demonstrated that changes in muscle length could affect the extent of the cortical area from which a specific muscle could be activated via surface electrical stimulation. Moreover, evidence from animal and human experiments provides convincing evidence that stretch-sensitive afferent fibres project to the cerebral cortex. Studies using animal (primate) models have shown that muscle spindle (i.e. stretch-activated) type I and II afferent fibres project to cortical areas 3a (somato-sensory cortex) and 4 (motor cortex), which provides evidence for the possibility that muscle stretch could influence cortical activity especially in those areas [54, 55]. In particular, area 3a is purported to be involved in somato-motor-vestibular integration [56]. The neurones in this cortical region can project both mono- and poly-synaptically (via inter-neurones) to the spinal motor neurones of the stretched muscles [51, 57] as well as the primary motor cortex [58–60], suggesting a possible contribution to the control of motor output. Another interesting possibility is that inputs from joint and skin receptors, which can project to the motor cortex via the somato-sensory cortex and thalamus, could also inhibit motor cortical outflow [61]. Human experiments have consistently demonstrated the possible involvement of cortical structures in response to the stimulation of stretch-sensitive afferents. For instance, it has been shown that muscle stretch could evoke cortical potentials in humans [62, 63]. Additionally, prolonged muscle vibration (which preferentially activates Ia afferent fibres) [64] and changes in muscle length (i.e. towards longer muscle length) [65] have been shown to reduce corticospinal excitability as assessed using TMS, suggesting that input from stretch-sensitive afferents might modulate corticospinal excitability. In general, there is compelling evidence that prolonged periods of sensory stimulation can modify corticospinal excitability and motor function (for review see Veldman et al. [66]). In light of the above-mentioned evidence, it seems reasonable to speculate that passive muscle stretch could acutely and directly affect motor cortical outflow during maximal contractions. Nonetheless, based on the fact that this assumption has not been explicitly examined through the study of changes in supra-spinal structures after stretch, its contribution can currently be considered ‘possible’ (Fig. 1). Future studies using a combination of TMS over the motor cortex to elicit MEPs together with electrical stimulation of the spinal tracts over the cervicomedullary junction [to evoke a cervicomedullary MEP (CMEP) and obtain responses in the arms] or the thoracic spine [to evoke a thoracic MEP (TMEP) and obtain responses in the legs] could help to determine the contribution of cortical and sub-cortical mechanisms [67, 68]. Additionally, studies using imaging techniques (e.g. fMRI) and other electrophysiological techniques (paired-pulse TMS, high-density EMG motor unit decomposition, EMG-EEG coherence) might also help to shed light on the contribution of supra-spinal mechanisms.
2.3 Limitations of Previous Research Measuring Neural (Central) Drive after Muscle Stretch
It is commonly argued that prolonged muscle stretches (e.g. >60 s) result in a reduced muscle activity as measured by EMG [23, 25, 28]. Specifically, a strong correlation has been observed between the reduction in muscle force after acute plantar flexor muscle stretching and the reductions in EMG amplitude measured during maximal voluntary contraction [25]. The EMG signal is considered to be largely reflective of the number of active motor units as well as the discharge frequency of its action potentials [41]; however, a reduction in EMG amplitude does not unquestionably indicate a reduced supra-spinal (motor cortical) drive, as changes in spinal reflex loops, motor neurone intrinsic properties and muscle sarcolemmal action potential propagation can affect the EMG amplitude [40, 41]. Moreover, caution should be exercised when inferring changes in neural input (i.e. supra-spinal and spinal) to the muscle through EMG measurements because changes in EMG amplitude can occur in response to factors other than changes in neural drive, such as amplitude cancellation [43, 69–71] and motor unit synchronisation [41, 72], changes in muscle length [72–74] and alterations in intracellular action potential amplitude and velocity [40, 42]. Thus, reductions in EMG amplitude per se cannot be taken as evidence for reductions in neural drive to the muscle.
In addition to EMG amplitude alterations, researchers have also reported decreases in voluntary muscle activation (%VA; as measured using the interpolated twitch technique; ITT) after acute passive stretch [20, 36], possibly indicating a reduction in neural drive to the muscle. However, these changes are not always seen [21, 39]. The principle of the ITT is to apply an electrical stimulus to the muscle or its nerve simultaneously with a maximal voluntary contraction (MVC) in order to increase the firing frequency of the fibres above that which is obtained volitionally, theoretically allowing for maximal muscle contractile capacity to be achieved [75]. The force produced during ‘maximal’ muscle activation is then compared to the force produced by an electrical twitch immediately after the MVC, producing a ratio that reflects the extent of voluntary muscle activation [76]. Importantly, in healthy individuals all the motor units should be recruited between 70–80% of a MVC in the vast majority of muscle groups during slow contractions (reviewed by de Luca and Kline [77]). Moreover, when contractions are performed with the intention to produce fast rates of force development (as commonly done in research studies to date), the motor unit recruitment threshold is reduced considerably [78–82]. Therefore, all motor units should be recruited during explosive maximal efforts and any increase in force observed when electrical stimulation is applied during MVCs must result from an increase in firing frequency rather than the recruitment of additional motor units. However, this measurement has been shown to be influenced by supra-spinal, spinal and/or peripheral structures [83–87] and cannot therefore be considered reflective only of neural drive. For instance, measures of %VA obtained using ITT have been reported to be affected by several factors such as: muscle length [88], force transmission through series elastic components [89], synchronisation of motor unit firing causing summation of twitch forces [87] and changes in intracellular calcium concentration [86, 90]. Another problem affecting the interpretation of previous data is that different muscles were targeted and different stretch protocols were used between studies, so it is not possible to reconcile the inconsistent findings. Thus, data obtained using EMG and ITT data have been inconsistent, and the use of these techniques has not allowed for accurate delineation of the specific site(s) at which muscle activation might be modified by stretching.
Alternatively, other methods have been used to assess changes in neural drive. Firstly, when the muscle or its motor nerve is electrically stimulated the excitability of the muscle membrane can be non-invasively assessed by measuring the amplitude of the compound muscle action potential (M-wave). Normalising the surface EMG (root-mean-squared; RMS) signal to the M-wave maximal amplitude (i.e. EMG:M) eliminates the effect of peripheral changes in membrane excitability and indicates if there is a change in central drive to the muscle [85]. Although the rectified EMG signal is also typically used, the utilisation of the RMS filter is recommended in order to minimise amplitude cancelation in the signal [41], including when the signal is normalized to the M-wave [40]. However, this measurement can still be affected by factors such as changes in motor unit synchronisation and amplitude cancellation. Secondly, the first volitional wave (V-wave), which is an electrophysiological variant of the H-reflex elicited by a supra-maximal stimulus intensity during voluntary maximal contraction [91], can be used to assess changes in neural drive. The H-reflex is a monosynaptic reflex evoked when the axons of the homonymous Ia afferent are electrically stimulated at a sub-maximal intensity on a mixed nerve [92]. When the nerve is stimulated, a descending action potential (M-wave) causes muscle contraction and the Ia afferent fibres projecting back to the spinal cord excite the α-motor neurone pool to create another action potential in the innervated skeletal muscle (H-reflex). Its amplitude is usually utilised as a measure of spinal excitability, although it also relies on the axonal excitability of the Ia afferent fibres, the efficiency of Ia afferent synapses (e.g. pre-synaptic inhibition) and the net excitability of the alpha motor neurones [92–95]. However, when supra-maximal nerve stimulation is applied during a maximal voluntary contraction, together with the direct M-wave, the H-reflex reappears (i.e. V-wave) as the antidromic impulses (i.e. opposite direction of normal impulse) in the motor neurones collide with the efferent nerve impulses caused by the voluntary contraction [96–100]. The supra-maximal intensity used during nerve stimulation to evoke the V-wave promotes massive excitation of all Ia afferent axons in the peripheral nerve, subsequently recruiting both large and small motor neurones [101]. The V-wave amplitude during MVCs is purported to be indicative of changes in motor unit firing frequency (for the same reasons mentioned previously in relation to the ITT) and may be considered a useful measure of descending drive obtained during maximal efforts. Nonetheless, it can be directly affected by activity in descending pathways and possibly pre-synaptic inhibition at the spinal level. Also, it may not be reliable under some experimental conditions, and the averaging of multiple measures is recommended when possible [97]. Despite this caveat, V-wave measurements could provide substantial evidence for/against central drive modifications and can be used to more clearly determine whether acute static stretching influences central drive. In fact, based on the information presented above, the concurrent measurement of V-wave amplitude, EMG:M and %VA might provide good evidence of central changes in neural drive after muscle stretching. A consistent change in all three tests could be taken as excellent evidence for an influence of muscle stretch on efferent neural drive, and motivate more detailed examinations of the neuromuscular pathway to identify the site of change.
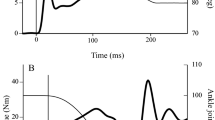
Recently two studies have investigated the effect of passive stretch on neural drive performing these three measurements simultaneously [30, 31]. Both studies reported that the magnitudes of force reduction after stretch as well as the subsequent force recovery were strongly correlated with the depression and recovery of these three measures (see Fig. 2). It is also interesting to note that stretches performed intermittently (i.e. with a 15-s interval) seemed to reduce both peak muscular force and the measures of neural drive (EMG:M and %VA) more than continuous stretch (i.e. a single 5-min stretch) [30]. These two studies, in addition to others that have shown a strong correlation between the reduction in force after stretch and reductions in EMG amplitude [25, 102], provide strong evidence that a reduction in neural drive is an important mechanism affecting force loss after passive muscle stretching. Importantly, all these studies presented a large variability in the magnitude of the force loss and the measured reductions in neural drive were able to explain most of this variation (r 2 = 0.85–0.26) [25, 30, 31, 102]. However, based on these results it is not possible to determine the specific site(s) affected by muscle stretching (i.e. supra-spinal vs. spinal).
Example of data obtained from a subject before (pre-stretch), immediately after (post-stretch) and 15 min post-stretch. a The soleus V-wave amplitude reduction after stretch followed by recovery 15 min later. The same behaviour can be observed for voluntary activation measured by the interpolated twitch technique (ITT) (b), EMG amplitude (c) and plantarflexor torque trace (d), suggesting that both the reduction and recovery of the plantarflexor torque occurred with a concomitant reduction and recovery of central drive. EMG electromyogram, RMS root-mean-squared
3 Inhibition or Disfacilitation at the Spinal Level and the Influence of Afferent Information
The spinal circuitry is a complex network of sensory neurones, inter-neurones and motor neurones that can inhibit or facilitate descending volitional signals, and it is possible that muscle stretching might cause inhibition or disfacilitation of different components in this circuitry via input from afferent fibres. Some peripheral proprioceptive structures are known to detect muscle stretch and could be involved in the process of the muscle’s force control. For instance, structures such as muscle spindles, Golgi tendon organs and free nerve endings have been suggested as the most likely candidates to mediate the neural, and thus force, inhibition caused by passive stretching [20, 103–105]. The possible contribution of each of these structures will thus be discussed.
3.1 Muscle Spindles
Muscle spindles are positioned in parallel with the muscle fibres [106] and are innervated by large group Ia (primary endings) and smaller group II (secondary endings) afferent fibres [107]. The primary and secondary muscle spindle endings can sense both dynamic and static changes in muscle length [107]. Group Ia afferent fibres are known to strongly influence motor neuronal facilitation and, more specifically, the development of persistent inward currents (PICs) [108]. PICs are a voltage-dependent characteristic of motor neurones that amplify and prolong synaptic inputs, changing the input-output relationship and producing sustained membrane depolarisation [109]. In fact, in order to achieve maximal discharge frequency, and thus produce maximal force, motor neurones rely on the gain in synaptic input caused by PICs [110]. Also, it has been known for some time that prolonged (1-h) fast repetitive passive stretches can negatively affect the efficiency of Ia afferent pathway to excite α-motor neurones, measured as the H-reflex amplitude [106]. However, shorter durations of static passive stretch have been shown to reduce the EMG responses elicited mechanically by tendon taps [111] and stretch reflexes [112] without reducing the amplitude of electrically elicited H-reflexes [112, 113] when measured at rest. Hence, it is possible that stretch-mediated reductions in the efficiency of the Ia afferent pathway to elicit excitatory postsynaptical potentials might arise from desensitisation of muscle spindles rather than pre-synaptic inhibition of Ia terminals and could impair the ability to develop PICs in spinal motor neurones and ultimately reduce the ability to produce maximal force. The possibility that motor neurone disfacilitation is an important contributor to the force loss has been suggested previously [114]; however, these authors argued that the disfacilitation would occur due to an inability of volitional fusimotor drive (beta and gamma intra-fusal motor neurones) to increase muscle spindle discharge after stretch without considering, and testing, possible PICs contribution. Recently, new insights into this hypothesis have stemmed from a study where it was observed that motor neuronal facilitation (possibly PICs) was inhibited after 5 min of passive stretch [115]. In this study the motor neurone’s ability to facilitate synaptic input from muscle spindles was tested using a protocol where electrical stimulation was superimposed onto tendon vibration (without the presence of volitional motor command). This protocol was used because it elicited muscle contractions through a reflex arc (Ia pathway) and also demonstrated features compatible with the presence of PICs (length-dependency, self-sustained motor unit firing, and wind-up effect) [109]. Interestingly, the ability to elicit contractions through the reflex arc was reduced after passive stretch, while contractions elicited by direct activation of motor axon branches were not, suggesting that the ability to use sensory input to amplify motor output was reduced. Also, the temporal profile of the recovery of the motor neurone facilitation was similar to the recovery of muscle force and central drive shown in other studies [20, 25, 30, 31, 105]. This finding strongly suggests that motor neuronal disfacilitation, possibly mediated by an inhibition of the Ia pathway, could be an important factor affecting the stretch-induced force loss. However, it is still not clear whether pre- or post-synaptic mechanisms contribute to this phenomenon. Future studies should focus on testing: (1) the motor neurone excitability by using CMEPs (or TMEPs) and F-waves (for review see McNeil et al. [116]); and (2) other well-established spinal circuits using paired H-reflexes techniques (e.g. pre-synaptic inhibition and reciprocal inhibition) (for review see Pierrot-Deseilligny and Burke [95]).
On a final note, it is important to consider the possible influence of neuromodulators (e.g. monoamines) on the motor neurone disfacilitation caused by stretch. Monoamines [i.e. norepinephrine (NE) and serotonin (5-HT)] play an important role in PIC facilitation in the spinal cord, by hyperpolarizing its activation voltage [109]. Also, an increase in monoamine concentration can exert facilitatory effects on the motor neurone itself such as: depolarization of the resting membrane potential, hyperpolarisation of the spike threshold and reducing the spike afterhyperpolarisation amplitude and duration (for review see Heckman and Enoka [117]). For instance, 5-HT reuptake-inhibitor drugs (i.e. citalopram and escitalopram) have been shown to increase stretch reflex responses [118, 119] and to increase the input-output gain in human motor neurones [119]. Thus, experiments with pharmacological interventions targeting the increase in monoaminergic drive at the motor neurone level might help to shed light on whether an increase in motoneuronal facilitation could mitigate or abolish the stretch-induced force loss.
3.2 Golgi Tendon Organs
Golgi tendon organs are located predominantly at the myotendinous junction and are innervated by group Ib afferent fibres [120]. These afferent fibres sample the force produced by the muscle fibres attached in series with the receptor [121–124]. It was hypothesised that Golgi tendon organs could play a role in the stretch-induced force loss because Ib fibres are known to cause autogenic inhibition, decreasing the excitability of the homonymous motor neurone [20, 36]. However, one key problem with this hypothesis is that Golgi tendon organs seem to respond to stretch typically under some level of muscle activity (i.e. active stretch) [125]. Also, Golgi tendon organs have been reported to be a poor passive stretch-sensitive receptor when whole muscles are tested in physiological conditions [123, 126, 127]. A second problem is that tendon organs could cause facilitation rather than inhibition during muscle stretch in some cases (e.g. Ib excitation during the stance phase of locomotion [128–132]). A third problem is that Ib inhibition of homonymous motor neurones seems to be depressed during higher force contractions [133] and higher levels of volitional descending drive [134], probably through pre-synaptic control exerted by the supraspinal centres [135]. This suggests that Ib inhibition might have little, if any, influence during maximal voluntary contractions. Also, it is interesting to note that the Ib pathway receives many other sources of input (i.e. descending pathways, Ia, cutaneous and joint receptors) that could control both facilitation and/or inhibition of the Ib interneurone [120]. In addition, in humans it has been demonstrated that Ib-mediated autogenic inhibition has a duration of approximately 60 ms which would be insufficient to explain the longer duration inhibitory effects caused by passive stretch [125, 136]. Thus, presently there is a lack of evidence for the assumption of a significant influence of Golgi tendon organs on the stretch-induced force loss, and further work needs to be done to define its possible contribution. Studies investigating the inhibitory area on the ongoing EMG caused by electrical stimulation over the triceps surae muscle-tendon junction [125, 137] or with paired H-reflexes [138] could help to improve our understanding of the effects of stretch on Ib-inhibition.
3.3 Free Nerve Endings
Another interesting possibility is the involvement of free nerve endings, located at the muscle or connective tissue, in the process of muscle tension control. For instance, it has been shown that free nerve endings innervated largely by group II and III afferent fibres are sensitive to stretch [139]. Moreover, it is thought that these free nerve endings are responsible for the clasp-knife reflex [140], which is a typical response in humans with spasticity, which promotes a fast reduction in the muscle’s passive tension in response to stretch [120]. Interestingly, this inhibition lasts beyond the termination of stretch [141]. It is therefore reasonable to speculate the involvement of free nerve endings in the force inhibition caused by passive muscle stretching; however, this assumption has not been verified and further studies are necessary to test this hypothesis.
4 Conclusion
Current research has provided evidence that neural mechanisms might contribute to the loss of muscular force capacity within minutes of prolonged (e.g. >1 min) muscle stretching. Reductions in neural drive to the muscle have been extensively reported in the literature with the utilisation of different techniques, however it is not clear where in the neuraxis these reductions occur. It is reasonable to speculate the possible involvement of supra-spinal structures on stretch-induced force loss, but there is still a lack of experimental data to support this hypothesis. Future studies using both cortical and spinal cord stimulation techniques may shed light on the possible contribution of supra-spinal mechanisms to the stretch-induced force loss. However, reduced spinal excitability and impaired motor neurone facilitation processes are currently seen as important potential candidates to explain force loss (Fig. 1). There is still a lack of data investigating whether other peripheral sensory structures (Golgi tendon organs and free nerve endings) might contribute to stretch-induced force losses. An understanding of these will be essential if methods of mitigating the loss of force after muscle stretching are to be developed.
References
Simenz CJ, Dugan CA, Ebben WP. Strength and conditioning practices of National Basketball Association strength and conditioning coaches. J Strength Cond Res. 2005;19(3):495–504.
Witvrouw E, Mahieu N, Danneels L, et al. Stretching and injury prevention. Sports Med. 2004;34(7):443–9.
Amako M, Oda T, Masuoka K, et al. Effect of static stretching on prevention of injuries for military recruits. Mil Med. 2003;168(6):442.
Bixler B, Jones RL. High-school football injuries: effects of a post-halftime warm-up and stretching routine. Fam Pract Res J. 1992;12(2):131–9.
Ekstrand J, Gillquist J, Möller M, et al. Incidence of soccer injuries and their relation to training and team success. Am J Sports Med. 1983;11(2):63–7.
Behm DG, Blazevich AJ, Kay AD, et al. Acute effects of muscle stretching on physical performance, range of motion, and injury incidence in healthy active individuals: a systematic review. Appl Physiol Nutr Metab. 2016;41(999):1–11.
Small K, Mc Naughton L, Matthews M. A systematic review into the efficacy of static stretching as part of a warm-up for the prevention of exercise-related injury. Res Sports Med. 2008;16(3):213–31.
Shehab R, Mirabelli M, Gorenflo D, et al. Pre-exercise stretching and sports related injuries: knowledge, attitudes and practices. Clin J Sport Med. 2006;16(3):228–31.
Ebben WP, Blackard DO. Strength and conditioning practices of National Football League strength and conditioning coaches. J Strength Cond Res. 2001;15(1):48–58.
Ebben WP, Carroll RM, Simenz CJ. Strength and conditioning practices of National Hockey League strength and conditioning coaches. J Strength Cond Res. 2004;18(4):889–97.
Ebben WP, Hintz MJ, Simenz CJ. Strength and conditioning practices of Major League Baseball strength and conditioning coaches. J Strength Cond Res. 2005;19(3):538–46.
Wiart L, Darrah J, Kembhavi G. Stretching with children with cerebral palsy: what do we know and where are we going? Pediatr Phys Ther. 2008;20(2):173–8.
Committee ACP. Exercise prescription for older adults with osteoarthritis pain: Consensus practice recommendations. J Am Geriatr Soc. 2001;49(6):808–23.
McHugh MP, Cosgrave CH. To stretch or not to stretch: the role of stretching in injury prevention and performance. Scand J Med Sci Sports. 2010;20(2):169–81.
Pope RP, Herbert RD, Kirwan JD, et al. A randomized trial of preexercise stretching for prevention of lower-limb injury. Med Sci Sports Exerc. 2000;32(2):271–7.
Shrier I. Meta-analysis on preexercise stretching. Med Sci Sports Exerc. 2004;36(10):1832.
Thacker SB, Gilchrist J, Stroup DF, et al. The impact of stretching on sports injury risk: a systematic review of the literature. Med Sci Sports Exerc. 2004;36(3):371–8.
Rubini EC, Costa AL, Gomes PS. The effects of stretching on strength performance. Sports Med. 2007;37(3):213–24.
Kay AD, Blazevich AJ. Effect of acute static stretch on maximal muscle performance: a systematic review. Med Sci Sports Exerc. 2012;44(1):154–64.
Fowles JR, Sale DG, MacDougall JD. Reduced strength after passive stretch of the human plantarflexors. J Appl Physiol. 2000;89(3):1179–88.
Power K, Behm D, Cahill F, et al. An acute bout of static stretching: effects on force and jumping performance. Med Sci Sports Exerc. 2004;36(8):1389–96.
Kokkonen J, Nelson AG, Cornwell A. Acute muscle stretching inhibits maximal strength performance. Res Q Exerc Sport. 1998;69(4):411–5.
Cramer JT, Beck TW, Housh TJ, et al. Acute effects of static stretching on characteristics of the isokinetic angle–torque relationship, surface electromyography, and mechanomyography. J Sports Sci. 2007;25(6):687–98.
Kay AD, Blazevich AJ. Isometric contractions reduce plantar flexor moment, Achilles tendon stiffness, and neuromuscular activity but remove the subsequent effects of stretch. J Appl Physiol. 2009;107(4):1181–9.
Kay AD, Blazevich AJ. Moderate-duration static stretch reduces active and passive plantar flexor moment but not Achilles tendon stiffness or active muscle length. J Appl Physiol. 2009;106(4):1249–56.
Kay AD, Blazevich AJ. Concentric muscle contractions before static stretching minimize, but do not remove, stretch-induced force deficits. J Appl Physiol. 2010;108(3):637–45.
Morse CI, Degens H, Seynnes OR, et al. The acute effect of stretching on the passive stiffness of the human gastrocnemius muscle tendon unit. J Physiol. 2008;586(1):97–106.
Avela J, Kyrolainen H, Komi PV. Altered reflex sensitivity after repeated and prolonged passive muscle stretching. J Appl Physiol. 1999;86(4):1283–91.
Armstrong RB, Duan C, Delp MD, et al. Elevations in rat soleus muscle [Ca2+] with passive stretch. J Appl Physiol. 1993;74(6):2990–7.
Trajano GS, Nosaka K, Seitz L, et al. Intermittent stretch reduces force and central drive more than continuous stretch. Med Sci Sports Exerc. 2014;46(5):902–10.
Trajano GS, Seitz L, Nosaka K, et al. Contribution of central vs. peripheral factors to the force loss induced by passive stretch of the human plantar flexors. J Appl Physiol. 2013;115(2):212–8.
Jones D. High-and low-frequency fatigue revisited. Acta Physiol Scand. 1996;156(3):265–70.
Martin V, Millet GY, Martin A, et al. Assessment of low-frequency fatigue with two methods of electrical stimulation. J Appl Physiol. 2004;97(5):1923–9.
Cornwell A, Nelson AG, Sidaway B. Acute effects of stretching on the neuromechanical properties of the triceps surae muscle complex. Eur J Appl Physiol. 2002;86(5):428–34.
Cramer JT, Housh TJ, Weir JP, et al. The acute effects of static stretching on peak torque, mean power output, electromyography, and mechanomyography. Eur J Appl Physiol. 2005;93(5–6):530–9.
Behm DG, Button DC, Butt JC. Factors affecting force loss with prolonged stretching. Can J Appl Physiol. 2001;26(3):261–72.
Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81(4):1725–89.
Herda TJ, Costa PB, Walter AA, et al. The effects of two modes of static stretching on muscle strength and stiffness. Med Sci Sports Exerc. 2011;43(9):1777–84.
Ryan ED, Beck TW, Herda TJ, et al. Do practical durations of stretching alter muscle strength? A dose-response study. Med Sci Sports Exerc. 2008;40(8):1529–37.
Arabadzhiev TI, Dimitrov VG, Dimitrova NA, et al. Interpretation of EMG integral or RMS and estimates of “neuromuscular efficiency” can be misleading in fatiguing contraction. J Electromyogr Kinesiol. 2010;20(2):223–32.
Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96(4):1486–95.
Dimitrova NA, Dimitrov GV. Interpretation of EMG changes with fatigue: facts, pitfalls, and fallacies. J Electromyogr Kinesiol. 2003;13(1):13–36.
Christie A, Inglis JG, Kamen G, Gabriel DA. Relationships between surface EMG variables and motor unit firing rates. Eur J Appl Physiol. 2009;107(2):177–85.
Taylor JL, Allen GM, Butler JE, et al. Supraspinal fatigue during intermittent maximal voluntary contractions of the human elbow flexors. J Appl Physiol. 2000;89(1):305–13.
Gandevia S, Petersen N, Butler J, et al. Impaired response of human motoneurones to corticospinal stimulation after voluntary exercise. J Physiol. 1999;521(3):749–59.
Gandevia S, Allen GM, Butler JE, et al. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol. 1996;490(Pt 2):529.
Søgaard K, Gandevia SC, Todd G, et al. The effect of sustained low-intensity contractions on supraspinal fatigue in human elbow flexor muscles. J Physiol. 2006;573(2):511–23.
Ross EZ, Middleton N, Shave R, et al. Corticomotor excitability contributes to neuromuscular fatigue following marathon running in man. Exp Physiol. 2007;92(2):417–26.
Nordlund MM, Thorstensson A, Cresswell AG. Central and peripheral contributions to fatigue in relation to level of activation during repeated maximal voluntary isometric plantar flexions. J Appl Physiol. 2004;96(1):218–25.
Taylor JL, Gandevia SC. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J Appl Physiol. 2008;104(2):542–50.
Matthews PB. The human stretch reflex and the motor cortex. Trends Neurosci. 1991;14(3):87–91.
Gellhorn E, Hyde J. Influence of proprioception on map of cortical responses. J Physiol. 1953;122(2):371–85.
Scott SH, Sergio LE, Kalaska JF. Reaching movements with similar hand paths but different arm orientations. II. Activity of individual cells in dorsal premotor cortex and parietal area 5. J Neurophysiol. 1997;78(5):2413–26.
Phillips C, Powell T, Wiesendanger M. Projection from low-threshold muscle afferents of hand and forearm to area 3a of baboon’s cortex. J Physiol. 1971;217(2):419–46.
Hore J, Preston J, Cheney P. Responses of cortical neurons (areas 3a and 4) to ramp stretch of hindlimb muscles in the baboon. J Neurophysiol. 1976;39(3):484–500.
Huffman KJ, Krubitzer L. Area 3a: topographic organization and cortical connections in marmoset monkeys. Cereb Cortex. 2001;11(9):849–67.
Rathelot J-A, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci. 2009;106(3):918–23.
Avendaño C, Isla AJ, Rausell E. Area 3a in the cat II. Projections to the motor cortex and their relations to other corticocortical connections. J Comp Neurol. 1992;321(3):373–86.
Murray EA, Coulter JD. Organization of corticospinal neurons in the monkey. J Comp Neurol. 1981;195(2):339–65.
Huerta M, Pons T. Primary motor cortex receives input from area 3a in macaques. Brain Res. 1990;537(1):367–71.
Canedo A. Primary motor cortex influences on the descending and ascending systems. Progr Neurobiol. 1997;51(3):287–335.
Starr A, McKeon B, Skuse N, et al. Cerebral potentials evoked by muscle stretch in man. Brain. 1981;104(Pt 1):149–66.
Cohen LG, Starr A, Pratt H. Cerebral somatosensory potentials evoked by muscle stretch, cutaneous taps and electrical stimulation of peripheral nerves in the lower limbs in man. Brain. 1985;108(1):103–21.
Marconi B, Filippi GM, Koch G, et al. Long-term effects on motor cortical excitability induced by repeated muscle vibration during contraction in healthy subjects. J Neurol Sci. 2008;275(1):51–9.
Coxon JP, Stinear JW, Byblow WD. Amplitude of muscle stretch modulates corticomotor gain during passive movement. Brain Res. 2005;1031(1):109–17.
Veldman M, Maffiuletti N, Hallett M, et al. Direct and crossed effects of somatosensory stimulation on neuronal excitability and motor performance in humans. Neurosci Biobehav Rev. 2014;47:22–35.
Martin PG, Butler JE, Gandevia SC, et al. Noninvasive stimulation of human corticospinal axons innervating leg muscles. J Neurophysiol. 2008;100(2):1080–6.
Taylor JL. Stimulation at the cervicomedullary junction in human subjects. J Electromyogr Kinesiol. 2006;16(3):215–23.
Keenan KG, Farina D, Merletti R, et al. Amplitude cancellation reduces the size of motor unit potentials averaged from the surface EMG. J Appl Physiol. 2006;100(6):1928–37.
Keenan KG, Farina D, Maluf KS, et al. Influence of amplitude cancellation on the simulated surface electromyogram. J Appl Physiol. 2005;98(1):120–31.
Farina D, Cescon C, Negro F, et al. Amplitude cancellation of motor-unit action potentials in the surface electromyogram can be estimated with spike-triggered averaging. J Neurophysiol. 2008;100(1):431.
Yao W, Fuglevand RJ, Enoka RM. Motor-unit synchronization increases EMG amplitude and decreases force steadiness of simulated contractions. J Neurophysiol. 2000;83(1):441–52.
Farina D, Merletti R, Nazzaro M, et al. Effect of joint angle on EMG variables in leg and thigh muscles. IEEE Eng Med Biol Mag. 2001;20(6):62–71.
Frigon A, Carroll TJ, Jones KE, et al. Ankle position and voluntary contraction alter maximal M waves in soleus and tibialis anterior. Muscle Nerve. 2007;35(6):756–66.
Merton PA. Voluntary strength and fatigue. J Physiol. 1954;123(3):553–64.
Shield A, Zhou S. Assessing voluntary muscle activation with the twitch interpolation technique. Sports Med. 2004;34(4):253–67.
De Luca C, Kline J. Influence of proprioceptive feedback on the firing rate and recruitment of motoneurons. J Neural Eng. 2011;9(1):016007.
Desmedt J, Godaux E. Ballistic contractions in man: characteristic recruitment pattern of single motor units of the tibialis anterior muscle. J Physiol. 1977;264(3):673–93.
Desmedt JE, Godaux E. Ballistic contractions in fast or slow human muscles; discharge patterns of single motor units. J Physiol. 1978;285(1):185–96.
Freund H-J, Büdingen H-J. The relationship between speed and amplitude of the fastest voluntary contractions of human arm muscles. Exp Brain Res. 1978;31(1):1–12.
Harwood B, Rice CL. Changes in motor unit recruitment thresholds of the human anconeus muscle during torque development preceding shortening elbow extensions. J Neurophysiol. 2012;107(10):2876–84.
Yoneda T, Oishi K, Fujikura S, et al. A. Recruitment threshold force and its changing type of motor units during voluntary contraction at various speeds in man. Brain Res. 1986;372(1):89–94.
Taylor JL. Last word on point: counterpoint: the interpolated twitch does/does not provide a valid measure of the voluntary activation of muscle. J Appl Physiol. 2009;107(1):367.
De Haan A, Gerrits K, de Ruiter C. Counterpoint: the interpolated twitch does not provide a valid measure of the voluntary activation of muscle. J Appl Physiol. 2009;107(1):355.
Millet GY, Lepers R. Alterations of neuromuscular function after prolonged running, cycling and skiing exercises. Sports Med. 2004;34(2):105–16.
Neyroud D, Cheng AJ, Bourdillon N, et al. Muscle fatigue affects the interpolated twitch technique when assessed using electrically-induced contractions in human and rat muscles. Front Physiol. 2016;7.
Contessa P, Puleo A, De Luca CJ. Is the notion of central fatigue based on a solid foundation? J Neurophysiol. 2016;115(2):967–77.
Arampatzis A, Mademli L, De Monte G, et al. Changes in fascicle length from rest to maximal voluntary contraction affect the assessment of voluntary activation. J Biomech. 2007;40(14):3193–200.
Taylor JL. Point: counterpoint: the interpolated twitch does/does not provide a valid measure of the voluntary activation of muscle. J Appl Physiol. 2009;107(1):354–5.
Place N, Yamada T, Bruton JD, et al. Interpolated twitches in fatiguing single mouse muscle fibres: implications for the assessment of central fatigue. J Physiol. 2008;586(11):2799–805.
Upton AR, McComas AJ, Sica RE. Potentiation of “late” responses evoked in muscles during effort. J Neurol Neurosurg Psychiatry. 1971;34(6):699–711.
Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of Ia afferents during movement in humans. J Neurosci Methods. 1997;74(2):189–99.
Aagaard P, Simonsen EB, Andersen JL, et al. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol. 2002;93(4):1318–26.
Knikou M. The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods. 2008;171(1):1–12.
Pierrot-Deseilligny E, Burke D. The circuitry of the human spinal cord: its role in motor control and movement disorders. Cambridge: Cambridge University Press; 2005.
Aagaard P. Training-induced changes in neural function. Exerc Sport Sci Rev. 2003;31(2):61–7.
Solstad GM, Fimland MS, Helgerud J, et al. Test-retest reliability of V-wave responses in the soleus and gastrocnemius medialis. J Clin Neurophysiol. 2011;28(2):217–21.
Gondin J, Duclay J, Martin A. Soleus- and gastrocnemii-evoked V-wave responses increase after neuromuscular electrical stimulation training. J Neurophysiol. 2006;95(6):3328–35.
Duclay J, Martin A. Evoked H-reflex and V-wave responses during maximal isometric, concentric, and eccentric muscle contraction. J Neurophysiol. 2005;94(5):3555–62.
Pensini M, Martin A. Effect of voluntary contraction intensity on the H-reflex and V-wave responses. Neurosci Lett. 2004;367(3):369–74.
Aagaard P, Simonsen EB, Andersen JL, et al. Neural adaptation to resistance training: changes in evoked V-wave and H-reflex responses. J Appl Physiol. 2002;92(6):2309–18.
Ryan ED, Herda TJ, Costa PB, et al. Acute effects of passive stretching of the plantarflexor muscles on neuromuscular function: the influence of age. Age. 2014;36(4):1–12.
Avela J, Finni T, Liikavainio T, et al. Neural and mechanical responses of the triceps surae muscle group after 1 h of repeated fast passive stretches. J Appl Physiol. 2004;96(6):2325.
Nelson AG, Guillory IK, Cornwell C, et al. Inhibition of maximal voluntary isokinetic torque production following stretching is velocity-specific. J Strength Cond Res. 2001;15(2):241–6.
Avela J, Kyrolainen H, Komi PV, et al. Reduced reflex sensitivity persists several days after long-lasting stretch-shortening cycle exercise. J Appl Physiol. 1999;86(4):1292–300.
Matthews BH. The response of a muscle spindle during active contraction of a muscle. J Physiol. 1931;72(2):153–74.
Prochazka A, Ellaway P. Sensory systems in the control of movement. Compr Physiol. 2012;2:2615–27.
Heckman C, Binder MD. Analysis of effective synaptic currents generated by homonymous Ia afferent fibers in motoneurons of the cat. J Neurophysiol. 1988;60(6):1946–66.
Heckmann CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve. 2005;31(2):135–56.
Hultborn H, Denton ME, Wienecke J, et al. Variable amplification of synaptic input to cat spinal motoneurones by dendritic persistent inward current. J Physiol. 2003;552(Pt 3):945–52.
Rosenbaum D, Hennig EM. The influence of stretching and warm-up exercises on Achilles tendon reflex activity. J Sports Sci. 1995;13(6):481–90.
Weir DE, Tingley J, Elder GC. Acute passive stretching alters the mechanical properties of human plantar flexors and the optimal angle for maximal voluntary contraction. Eur J Appl Physiol. 2005;93(5–6):614–23.
Opplert J, Genty J-B, Babault N. Do stretch durations affect muscle mechanical and neurophysiological properties? Int J Sports Med. 2016;37(9):673–9.
Herda TJ, Ryan ED, Smith AE, et al. Acute effects of passive stretching vs vibration on the neuromuscular function of the plantar flexors. Scand J Med Sci Sports. 2009;19(5):703–13.
Trajano GS, Seitz LB, Nosaka K, et al. Can passive stretch inhibit motoneuron facilitation in the human plantar flexors? J Appl Physiol. 2014;117(12):1486–92.
McNeil CJ, Butler JE, Taylor JL, et al. Testing the excitability of human motoneurons. Front Hum Neurosci. 2013;7.
Heckman CJ, Enoka RM. Motor unit. Compr Physiol. 2012;2:2629–82.
D’Amico JM, Murray KC, Li Y, et al. Constitutively active 5-HT2/α1 receptors facilitate muscle spasms after human spinal cord injury. J Neurophysiol. 2013;109(6):1473–84.
Wei K, Glaser JI, Deng L, et al. Serotonin affects movement gain control in the spinal cord. J Neurosci. 2014;34(38):12690–700.
Jami L. Golgi tendon organs in mammalian skeletal muscle: functional properties and central actions. Phys Rev. 1992;72(3):623–66.
Petit J, Scott J, Reynolds K. Tendon organ sensitivity to steady-state isotonic contraction of in-series motor units in feline peroneus tertius muscle. J Physiol. 1997;500(Pt 1):227–33.
Gregory J, Proske U. The responses of Golgi tendon organs to stimulation of different combinations of motor units. J Physiol. 1979;295(1):251–62.
Stuart D, Mosher C, Gerlach R, et al. Mechanical arrangement and transducing properties of Golgi tendon organs. Exp Brain Res. 1972;14(3):274–92.
Houk J, Henneman E. Responses of Golgi tendon organs to active contractions of the soleus muscle of the cat. J Neurophysiol. 1967;30(3):466–81.
Khan SI, Burne JA. Afferents contributing to autogenic inhibition of gastrocnemius following electrical stimulation of its tendon. Brain Res. 2009;1282:28–37.
Stephens JA, Reinking RM, Stuart DG. Tendon organs of cat medial gastrocnemius: responses to active and passive forces as a function of muscle length. J Neurophysiol. 1975;38(12):17–123.
Houk J. A viscoelastic interaction which produces one component of adaptation in responses of Golgi tendon organs. J Neurophysiol. 1967;30:1482–93.
Hagbarth K-E, Vallbo Å. Discharge characteristics of human muscle afferents during muscle stretch and contraction. Exp Neurol. 1968;22(4):674–94.
Granit R. Reflex self-regulation of muscle contraction and autogenetic inhibition. J Neurophysiol. 1950;13(5):351–72.
Gossard J-P, Brownstone R, Barajon I, et al. Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Exp Brain Res. 1994;98(2):213–28.
Hultborn H. State-dependent modulation of sensory feedback. J Physiol. 2001;533(1):5–13.
Quevedo J, Fedirchuk B, Gosgnach S, et al. Group I disynaptic excitation of cat hindlimb flexor and bifunctional motoneurones during fictive locomotion. J Physiol. 2000;525(2):549–64.
Zytnicki D, Lafleur J, Horcholle-Bossavit G, et al. Reduction of Ib autogenetic inhibition in motoneurons during contractions of an ankle extensor muscle in the cat. J Neurophysiol. 1990;64(5):1380–9.
Khan SI, Burne JA. Reflex inhibition of normal cramp following electrical stimulation of the muscle tendon. J Neurophysiol. 2007;98(3):1102–7.
Fournier E, Karz R, Pierrot-Deseilligny E. Descending control of reflex pathways in the production of voluntary isolated movements in man. Brain Res. 1983;288(1):375–7.
Rogasch NC, Burne JA, Binboğa E, et al. Synaptic potentials contributing to reflex inhibition in gastrocnemius following tendon electrical stimulation. Clin Neurophysiol. 2011;122(6):1190–6.
Khan SI, Burne JA. Inhibitory mechanisms following electrical stimulation of tendon and cutaneous afferents in the lower limb. Brain Res. 2010;1308:47–57.
Pierrot-Deseilligny E, Katz R, Morin C. Evidence for lb inhibition in human subjects. Brain Res. 1979;166(1):176–9.
Cleland CL, Hayward L, Rymer W. Neural mechanisms underlying the clasp-knife reflex in the cat. II. Stretch-sensitive muscular-free nerve endings. J Neurophysiol. 1990;64(4):1319–30.
Cleland CL, Rymer W. Functional properties of spinal interneurons activated by muscular free nerve endings and their potential contributions to the clasp-knife reflex. J Neurophysiol. 1993;69(4):1181–91.
Cleland CL, Rymer W. Neural mechanisms underlying the clasp-knife reflex in the cat. I. Characteristics of the reflex. J Neurophysiol. 1990;64(4):1303–18.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this manuscript.
Conflict of interest
Gabriel Trajano, Kazunori Nosaka and Anthony Blazevich declare that they have no conflicts of interest relevant to the content of this review.
Rights and permissions
About this article
Cite this article
Trajano, G.S., Nosaka, K. & Blazevich, A.J. Neurophysiological Mechanisms Underpinning Stretch-Induced Force Loss. Sports Med 47, 1531–1541 (2017). https://doi.org/10.1007/s40279-017-0682-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-017-0682-6