Abstract
Background
Vitamin D is essential for maintaining optimal bone health. The prevalence of vitamin D inadequacy in athletes is currently unclear.
Objective
The objective of this study is to determine the prevalence of vitamin D inadequacy in athletes.
Methods
We conducted a systematic review and meta-analysis. Multiple databases were searched and studies assessing serum 25-hydroxyvitamin D [25(OH)D] status in athletes were identified. Serum 25(OH)D is measured to clinically determine vitamin D status. Reviewers independently selected the eligible articles, assessed the methodological quality, and extracted data. Disagreements were resolved by consensus. Weighted proportions of vitamin D inadequacy [serum 25(OH)D <32 ng/mL] were calculated (DerSimonian–Laird random-effects model) and compared using Chi-squared (χ 2) test. Subgroup analyses were conducted and risk ratios (RRs) with 95 % confidence intervals (CIs) were reported.
Results
Twenty-three studies with 2,313 athletes [mean (standard deviation) age 22.5 (5.0) years, 76 % male] were included. Of 2,313 athletes, 56 % (44–67 %) had vitamin D inadequacy that significantly varied by geographical location (p < 0.001). It was significantly higher in the UK and in the Middle East. The risk significantly increased for winter and spring seasons (RR 1.85; 95 % CI 1.27–2.70), indoor sport activities (RR 1.19; 95 % CI 1.09–1.30), and mixed sport activities (RR 2.54; 95 % CI 1.03–6.26). The risk was slightly higher for >40°N latitude [RR 1.14 (95 % CI 0.91–1.44)] but it increased significantly [RR 1.85 (1.35–2.53)] after excluding the Middle East as an outlier. Seven studies with 359 athletes reported injuries. The prevalence of injuries in athletes was 43 % (95 % CI 20–68) [bone related = 19 % (95 % CI 7–36); muscle and soft-tissue = 37.5 % (95 % CI 11.5–68.5)].
Conclusion
Despite the limitations of the current evidence, the prevalence of vitamin D inadequacy in athletes is prominent. The risk significantly increases in higher latitudes, in winter and early spring seasons, and for indoor sport activities. Regular investigation of vitamin D status using reliable assays and supplementation is essential to ensure healthy athletes. The prevalence of injuries in athletes is notable but its association with vitamin D status is unclear. A well-designed longitudinal study is needed to answer this possible association.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Prevalence of vitamin D inadequacy in athletes is substantial. |
Prevalence of vitamin D inadequacy is significantly higher during the winter and early spring seasons, for indoor sport activities, and in higher latitudes. |
1 Introduction
Vitamin D is essential for the maintenance and promotion of musculoskeletal health. Musculoskeletal injury prevention and recovery are possibly affected by sufficient circulating of 25-hydroxyvitamin D [25(OH)D] [1, 2]. Measuring serum 25(OH)D is the clinical standard to assess vitamin D status as it reflects vitamin D intake from both ultraviolet (UV) B radiation exposure and diet [3]. 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) is the active form of vitamin D that stimulates intestinal absorption of calcium and phosphate for new bone formation [4]. There is currently no consensus on the 25(OH)D cut-off values for vitamin D deficiency or insufficiency [5]. However, the optimal range of serum 25(OH)D usually reflects when parathyroid hormone (PTH) levels are decreasing and calcium absorption is maximized [6]. Vitamin D deficiency is becoming a global problem in young, active, and healthy populations including athletes, and this may put them at increased risk of injury and prolonged recovery [7–11].
Several published reviews have discussed the association of vitamin D status with musculoskeletal health in athletes [1, 2, 12]. These articles have discussed the extent of vitamin D deficiency and insufficiency and its impact on increased risk of injury, poor health, and prolonged recovery in athletes. One meta-analysis examined the relationship of vitamin D values to bone strength and injuries in healthy adults and included four studies of athlete and military populations [13]. There is currently no published systematic review and meta-analysis that provides a comprehensive review of the literature and quantifies the prevalence of vitamin D inadequacy in athletes. This systematic review and meta-analysis aimed to primarily determine the prevalence of vitamin D inadequacy in athletes and secondarily to explore its association with injuries.
2 Methods
2.1 Design
This is a systematic review and meta-analysis based on a predefined protocol.
2.2 Study Eligibility
We considered studies eligible for inclusion if they examined vitamin D status in athletes. Our study population included both male and female athletes aged 10–40 years old. We included abstracts from conference proceedings if the eligibility was determined and enough data were provided. We only included studies published in the English language. We disregarded studies that studied military personnel because their demographics, environment, and nature of practice is likely different from athletes. We also excluded case reports, review articles, and basic science and non-human studies.
2.3 Search Strategy
We searched the following databases with the guidance of a professional librarian from their inception until the end of December 2013: MEDLINE (Ovid and PubMed), SPORTDiscus, and CINAHL. Our search used Boolean operators and the following MeSH (Medical Subject Headings) and key terms: “vitamin D”, “25-hydroxyvitamin D”, “vitamin D deficiency”, “vitamin D insufficiency”, “vitamin D inadequacy”, “vitamin D status”, “athletes”, and “sports”. We limited our search to human research and the English language. We merged the articles from different searches and removed the duplicate articles. We searched the references of relevant reviews to identify additional eligible articles. We performed a final search to identify the latest publications on 15 January 2014.
2.4 Study Selection and Quality Assessment
We applied an independent duplicate assessment process for the study selection and quality assessment to minimize bias. Multiple reviewers (RT, DD, and RH) independently applied the eligibility criteria to the titles and abstracts, and then to the full-text of the articles (DD and RT) that passed the title and abstract review. The level of agreement between the reviewers was measured using Kappa statistic. The discrepancies and disagreements were resolved with the help of a third reviewer (Nadia Latifi) and consensus was reached between all reviewers. Two reviewers independently assessed the methodological quality of the observational studies and randomized controlled trials (RCTs) using the MINORs (Methodological Index for Non-Randomized Studies) scale [14] and the Jadad scale [15], respectively. The MINORs scale yields a maximum score of 16 for non-comparative cohort studies and a maximum of 24 for comparative cohort studies. The Jadad scale yields a maximum score of 5 for RCTs.
2.5 Data Abstraction
To minimize errors in data entry, two reviewers independently extracted data using a data collection form developed a priori. The reviewers collected the following information from the text, figures, and tables: study design, study location, latitude (as an indicator of sunlight exposure), demographics, season or time of serum 25(OH)D measurements, mean and standard deviation (SD) of serum 25(OH)D with the unit of measurement, and cut-off values for vitamin D deficiency, insufficiency and sufficiency. The reviewers also extracted data on bone or non-bone injuries whenever reported. For RCTs that assessed the effect of vitamin D supplementation, we only extracted data on demographics and baseline mean serum 25(OH)D values. The approximate latitude for the geographical location was retrieved online (http://www.worldatlas.com) if not reported in the article. We contacted the first authors if more information was needed.
2.6 Definition of Vitamin D Inadequacy
The units of serum 25(OH)D measures were reported in either nmol/L or ng/mL. Some studies did not report the mean or median for serum 25(OH)D but all studies categorized the vitamin D status as sufficient, insufficient, and deficient. We converted and reported the 25(OH)D cut-off values in both units for consistency (1 ng/mL = 2.496 nmol/L) [16]. We used the cut-off values of vitamin D deficiency and insufficiency as defined in the articles. We combined the definitions of deficiency and insufficiency and called it ‘vitamin D inadequacy’ for consistency since the articles often used more varied cut-off values for deficiency but were more consistent in the cut-off values for insufficiency. The cut-off values used to define vitamin D insufficiency varied from ≤20 ng/mL (50 nmol/L) to <32 ng/mL (80 nmol/L). Therefore, for the purpose of this meta-analysis, vitamin D inadequacy is defined as serum 25(OH)D ≤32 ng/mL (<80 nmol/L).
2.7 Statistical Analysis
We calculated Kappa statistics to measure the level of agreement between the two reviewers for the study selection process. We reported the mean and SD of the methodological quality score for 23 articles. We used intra-class correlation coefficient (ICC) two-way mixed-effects analysis to calculate the level of agreement between the reviewers for quality assessment. We tested between-study heterogeneity using the Cochran’s Chi-squared (χ 2) Q test with a p value set at 0.1 for significance. The I 2 value representing the percentage of total variation across studies was reported as a measure of between-study heterogeneity. We planned a priori to use a random-effects model (DerSimonian–Laird) to calculate the weighted proportions of vitamin D inadequacy and injuries due to the inherent heterogeneity applied to the observational studies. The Chi-squared (χ 2) test was used to compare the between-group weighted proportions. The weighted proportions of vitamin D inadequacy were compared by season and latitude and the risk ratios (RRs) were calculated using an inverse variance random-effects model. We preferred reporting RRs as opposed to odds ratios since odds ratios tend to exaggerate the effect size in the presence of a common event [17]. We used the median latitude of the included studies as the cut-off point for stratification. Sensitivity analysis was conducted by excluding the Middle Eastern studies as an outlier (as indicated in the literature) [18, 19]. We reported weighted proportions and RRs with their corresponding 95 % confidence intervals (CIs). Weighted mean age with SD and the weighted proportion of male athletes were calculated. SD was calculated by dividing the range by six assuming normal distribution when SD was not reported. We considered a p value of 0.05 for statistical significance. StatsDirect 2.7 (StatsDirect Ltd, Altrincham, UK) and Review Manager 5.0 (Cochrane Collaboration, Oxford, UK) were used for the data analyses.
3 Results
3.1 Literature Search
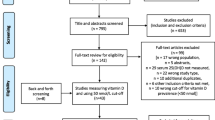
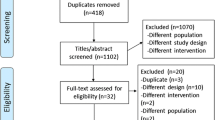
The flow chart highlights the search and review process (Fig. 1). The initial search yielded 1,478 articles. After excluding 232 duplicates, we reviewed 1,246 titles and abstracts for eligibility and excluded 1,194 articles that did not meet the eligibility criteria. Of the 52 articles that passed the title and abstract review, we excluded another 33 articles after the full-text review. We included three additional articles through screening the reference lists of the relevant reviews. The agreement between the two reviewers on selecting the studies was 0.78. We found one more article when we updated our search on 15 January 2014. Therefore, we included 23 articles [3, 20–41] with 2,313 athletes in this systematic review and meta-analysis.
3.2 Study Characteristics
The 23 included studies were published between 2008 and 2014. Seven were conducted in the UK or Ireland, three in Spain and France, six in the USA, three in Australia, and four in Qatar, Israel, and other Middle Eastern countries. The characteristics of the included studies are shown in Tables 1, 2, and 3. Of the 23 studies, four were RCTs, 18 were observational studies (cross-sectional or cohort studies), and one was a cross-sectional study with a nested RCT (Table 1). Sport activities were reported as indoor, outdoor, or a mixture of both. Serum 25(OH)D was measured using different units and at different seasons. To measure serum 25(OH)D levels, ten studies used chemiluminescent immunoassays (CLIA), three studies used radioimmunoassays (RIA), four studies used high-performance liquid chromatography (HPLC) tandem mass spectrometry, and one study used ELISA (Table 1). Five studies did not report the method of 25(OH)D assessment. Seven studies used the cut-off point of 20 ng/mL (50 nmol/L), ten used the cut-off point of 30 ng/mL (75 nmol/L), and six used the cut-off point of 32 ng/mL (80 nmol/L) to define vitamin D inadequacy. Two studies reported receiving Vitamin D External Quality Assessment Scheme (DEQAS) certification [25, 30] and one reported receiving the UK National External Quality Assessment Service (NEQAS) certification [40] for the measure of serum 25(OH)D. Study participants’ characteristics are shown in Table 2. The weighted mean (SD) age for the study population was 22.5 (5.0) years old and 76 % were male. The type of sport activities varied from team to solitary sport activities. All 23 studies reported the number of athletes with vitamin D inadequacy (vitamin D insufficiency or deficiency) (Table 2).
The methodological quality scores of articles are reported in Table 1. One article was an abstract from a conference proceeding and was not scored. The mean (SD) quality assessment scores were 11.9 (1.4) out of 16 for non-comparative studies, 19.8 (1.4) out of 24 for comparative studies, and 3.8 (1.30) out of five for RCTs. The ICC for consistent scoring of the methodological quality was 88.6 % (95 % CI 75–95) between the two reviewers.
3.3 Vitamin D Inadequacy in Athletes
The prevalence of vitamin D inadequacy for 2,313 athletes in 23 studies was 56 % (95 % CI 44–67) with large heterogeneity (I 2 = 96.3 %; 95 % CI 95.7–96.8). The vitamin D inadequacy in athletes significantly differed (p < 0.001) by geographical location: 39 % (95 % CI 17–65.0) for the USA (Fig. 2), 34 % (95 % CI 15–57) for Australia (Fig. 3), 32 % (95 % CI 8–64) for Spain/France (Fig. 4), 70 % (95 % CI 52.0–84) for the UK/Ireland (Fig. 5), and 84 % (95 % CI 72–92) for the Middle East (Fig. 6).
3.3.1 Seasonal Effects and Sport Activities
The prevalence of vitamin D inadequacy was significantly greater in winter and spring (weighted proportion: 65 %; 95 % CI 55–75) than in summer and fall/autumn (43 %; 95 % CI 15–74) with an RR of 1.85 (95 % CI 1.27–2.70, p = 0.001) (Fig. 7). The significant increased risk of vitamin D inadequacy in winter and spring was consistent for athletes from the USA (RR 4.21; 95 % CI 2.52–7.04), Australia (RR 2.37; 95 % CI 1.50–3.75), and Spain/France (RR 2.71; 95 % CI 1.19–6.17). The increased risk in winter and spring was not significant for British athletes (RR 1.18; 95 % CI 0.97–1.44). The decreased risk in winter and spring for the Middle Eastern athletes was also not significant (RR 0.88; 95 % CI 0.76–1.02). The increased risk of vitamin D inadequacy in winter and spring was significant for indoor (RR 1.19; 95 % CI 1.09–1.30) and mixed sport activities (RR 2.54; 95 % CI 1.03–6.26) but not for the outdoor sport activities (RR 1.97; 95 % CI 0.83–4.69) (Fig. 8). Heterogeneity (I2) varied from 76 to 97 % for these analyses. Two studies [26, 39] did not indicate the timing of the serum 25(OH)D measurement.
3.3.2 Latitude Effects and Sport Activities
The median latitude was 40°N (range: 25°S–53°N). All the studies from Australia [24, 32, 34] and the Middle East [3, 19, 23], one study from Spain [25], and two studies from the USA [31, 39] were conducted in regions of <40°N latitude (Table 1). The overall risk did not significantly increase for ≥40°N latitudes compared with <40°N latitudes and by season (Table 3). For athletes living in ≥40°N latitudes, the risk of vitamin D inadequacy significantly increased for indoor sport activities (RR 3.50; 95 % CI 2.44–5.01) and significantly decreased for outdoor sport activities (RR 0.76; 95 % CI 0.62–0.95).
Excluding studies from the Middle East as an outlier, the risk of vitamin D inadequacy significantly increased for ≥40°N latitudes (RR 1.85; 95 % CI 1.35–2.53) (Table 3). The increased risk was consistent for the winter and spring seasons (RR 1.34; 95 % CI 1.07–1.69), and for indoor (RR 3.50; 95 % CI 2.44–5.01) and mixed sport activities (RR 3.32; 95 % CI 2.15–5.11). For the summer and fall seasons, the increased risk (RR 1.39; 95 % CI 0.81–2.38) was not significant by latitude. For outdoor sport activities, the decreased risk of vitamin D inadequacy for ≥40°N latitudes was no longer significant (RR 0.93; 95 % CI 0.73–1.19).
3.4 Injuries in Athletes
Of the 23 included studies, seven including a total of 359 athletes reported data on injuries (Table 4). The weighted proportion of all injuries was 43 % (95 % CI 20–68). The weighted proportion for bone-related injuries was 19 % (95 % CI 7–36); it was 5.7 % (95 % CI 0.1–14.4) for fractures, 1 % (95 % CI 0.1–24) for stress fractures, and 10.7 % (95 % CI 1.0–30) for stress reactions. The weighted proportion of muscle and soft-tissue injuries was 37.5 % (95 % CI 11.5–68.5). The details of reported injuries are shown in Table 5. A retrospective Australian study [32] reported 12 stress reactions and one stress fracture in 18 female gymnasts during a 12-month period. Studies from Australia [24, 32, 34] examined injuries in gymnasts and ballet dancers during the summer and spring seasons. The proportion of muscle and soft-tissue injuries were 36 and 100 %, and the proportion of bone-related injuries were 19 and 44 %, respectively. A UK study by Wolman et al. [41] reported significantly more muscle and soft-tissue injuries in ballet dancers in winter (24 injuries in 19 athletes) than in summer (13 injuries in 19 athletes) seasons. Ducher et al. [24] studied 18 dancers and reported a mean of 1.9 injuries (range 0–5) by dancer and found no difference in the number of injuries between dancers with inadequate vitamin D (2.1 ± 0.6 injuries) and those with a normal vitamin D level (1.4 ± 0.6 injuries). Peeling et al. [34] found no significant differences in vitamin D values between injured and uninjured elite athletes. Shindle et al. [37] found a significant difference in serum 25(OH)D values between American professional football players with and without muscle injuries (mean 19.9, range 8–33 ng/mL vs. mean 24.7, range 9–46 ng/mL; p = 0.04). One cross-sectional study on Middle Eastern male athletes [28] showed 100 % vitamin D inadequacy and reported a history of 14 traumatic fractures.
4 Discussion
The prevalence of vitamin D deficiency and insufficiency is a global problem [1, 42] in both high [8, 43, 44] and low latitudes [19, 45–48]. Recent reviews have emphasized the active role of vitamin D in musculoskeletal health, immune function, protein synthesis, inflammatory response, and cell growth [1, 2, 31, 49]. These reviews highlighted the effects of vitamin D deficiency on musculoskeletal health on the adolescent population, and on athletic performance and related injuries. A Canadian survey of 5,306 adults and children living at latitudes between 43 and 52°N found that one-quarter of the participants did not meet the Recommended Dietary Allowance for vitamin D [8]. Similar findings in children and youth are reported from North America [7, 50, 51], Switzerland [43], Finland [52], Midland China [44] (31–40°N), Korea [47] (35–39°N), Israel [53], and Qatar [18, 19]. Redzic et al. [13] conducted a systematic review and examined the relationships between vitamin D status and muscle strength and injury incidence in healthy adults. They included two athlete and two military populations in association with injuries, but only two of the four studies provided sufficient data to calculate effect size. Badawi et al. [18] conducted a systematic review on the prevalence of vitamin D insufficiency in Qatar athletes only. Therefore, there is no published systematic review and meta-analysis to quantify the prevalence of vitamin D inadequacy in all athletes.
The findings from this meta-analysis showed a prevalence of 56 % (95 % CI 44–67) of vitamin D inadequacy in athletes. The prevalence of vitamin D inadequacy was high for the UK/Ireland (70 %; 95 % CI 52.0–84) (50–53°N) and the Middle East (84 %; 95 % CI 72–92) (25–38°N). It was 39 % (95 % CI 17–65.0) for the USA (30–47°N), 34 % (95 % CI 15–57) for Australia (25–35°S), and 32 % (95 % CI 8–64) for Spain (37–41°N) and France (46°N). The risk of increased vitamin D inadequacy was significantly higher during the winter and spring seasons than the summer and fall seasons for all geographical locations and for all sport activities, except for Middle Eastern athletes (Figs. 2, 3). The higher prevalence of vitamin D inadequacy (84 %) among Middle Eastern athletes for all seasons and sport activities compared with athletes from other lower-latitude countries such as Australia and Spain is not surprising. In fact, Racinais et al. [19] reported that 91 % of in Middle Eastern athletes had vitamin D deficiency during the summer and fall seasons. Similarly, a high prevalence of vitamin D deficiency is reported in the general population of Qatar and other Middle Eastern regions, particularly in young girls and women [18, 19, 45]. Numerous factors including sunlight exposure, insufficient dietary vitamin D intake, malabsorption, UV light insulation due to atmospheric dust particles, altered vitamin D metabolism, smaller body mass, and body concealment due to climate and ethnic beliefs have been related to vitamin D deficiency in the Middle East [18, 28, 29, 54]. Similarly, the high prevalence of vitamin D inadequacy in the UK and Ireland might be due to the poor sun exposure, particularly in winter and early spring seasons, and participation in indoor activities [33, 35, 41]. Our findings showed a significant decrease in vitamin D inadequacy in summer for both indoor [41] and mixed sport activities [35]. Research shows the use of sunscreen with a sun protection factor (SPF) of 15 and the degree of skin pigmentation could result in significant reduction in vitamin D absorption even if one spends ample time in the sun [2, 10, 11, 55].
The increased risk of vitamin D inadequacy was evident for ≥40°N latitude compared with <40°N latitude. When we excluded the Middle Eastern studies as an outlier, there was consistently higher risk of vitamin D inadequacy in ≥40°N latitude regions in all seasons and for indoor and mixed sports activities. The high prevalence of vitamin D inadequacy in the UK and Ireland is not novel as it is frequently highlighted by many reports from other countries located in the Northern Hemisphere, such as The Netherlands [56], North America [7, 8, 50, 51], Switzerland [43], and Finland [52]. This is likely attributed to the fact that individuals living in the Northern Hemisphere receives less UV radiation and thus likely less vitamin D intake than those living in the Southern Hemisphere [57]. The fact that latitude differences disappear when we include the Middle Eastern studies, as discussed above, is not surprising. The high prevalence of low vitamin D status from the Middle East is frequently reported for the general population, particularly for women [18, 19] and athletes [3, 28, 29]. Badawi et al. [18] noted that ethnic minorities from Arabic countries living in northern Europe, the USA, and Australia have a higher prevalence of poor vitamin D status [18, 56].
The high prevalence of vitamin D inadequacy is concerning for athletes of all ages since it may place them at an increased risk for bone and muscle/soft-tissue injuries. The prevalence of injuries from seven studies [24, 27, 28, 32, 34, 37, 41] was 43 % in athletes but we failed to examine its association with vitamin D status due to the lack of data. The rate of bone-related injuries was 18 %, while the muscle and soft-tissue injuries occurred in 37.5 %. In the UK, Wolman et al. [41] studied ballet dancers and reported a significantly higher number of muscle and soft-tissue injuries in the winter (100 %) than in the summer (68 %). The prevalence of injuries is likely underestimated. In the Wolman et al. [41] study, for example, the number of injuries exceeded the number of participating athletes because some participants had more than one injury. Studies from Australia [24, 32, 34] examined gymnasts and ballet dancers in the summer and spring and reported more injuries than the number of athletes despite the lower prevalence of vitamin D inadequacy in this latitude. Ducher et al. [24] reported a mean of 1.9 (SD 0.4) injuries per dancer. The type of injuries and their frequencies differed by sport activities. Peeling et al. [34] reported 14 bone-related injuries without reporting the specific type of injury. These studies from Australia found a higher number of injuries in dancers with inadequate vitamin D than in those with normal vitamin D values but the difference was not significant. Although the current evidence failed to study the association between athletes’ injuries and their vitamin D status, data from military personnel supports this association. Studies from the USA [58] and Finland [59] have shown a strong association between stress reactions and vitamin D deficiency in military personnel.
The impact of serum 25(OH)D on calcium metabolism and bone health, and its association with chronic health problems such as colonic cancer, arthritis, diabetes, cardiovascular disease, and depression is well-recognized [13, 60]. Its importance in athletes has also been reported by many authors [1, 2, 54]. A UK trial [21] found that vitamin D3 supplementation of 5,000 IU/day in athletes and healthy controls resulted in greater muscle performance [22]. They specifically found a significant increase in 10 m sprint times (p = 0.008) and vertical jump (p = 0.008) in the vitamin D group, whereas the placebo group showed no change (p = 0.587 and p = 0.204, respectively). Another trial from the same authors [21] randomized 30 club-level athletes into one of three groups receiving either a placebo or 20,000 or 40,000 IU/week oral vitamin D3 for 12 weeks. They found that both 20,000 and 40,000 IU vitamin D3 supplementation elevated serum 25(OH)D concentrations to above 50 nmol/L, but neither dose given for 12 weeks improved physical performance. The contradictory findings from these underpowered trials warrant further research of vitamin D supplementation in athletes. It is recommended that the body requires 3,000–5,000 IU of vitamin D per day [2, 9]. It has been reported that the increased physiological demands for vitamin D in athletes can be due to the high level of physical activities [2, 9]. Ogan and Pritchett [2] stated that the possibility of athletes requiring increased intake of vitamin D is due to the active use of vitamin D in many metabolic pathways. They recommend a minimum level of 40 ng/mL serum 25(OH)D in athletes. This might explain the negative findings from Close et al. [21] as they did not reach 40 ng/mL level in their experimental groups.
The current systematic review and meta-analysis has certain limitations due to the inherent biases of the included studies. Thus, readers should interpret the findings with caution. The studies were either cross-sectional studies or longitudinal cohort studies with diverse populations, which partly explains the large between-study heterogeneity. The other reasons for the large heterogeneity are likely due to geographical location, types of sport activities, timing of the serum 25(OH)D measurement, and different cut-off values of serum 25(OH)D for vitamin D deficiency and insufficiency. It was not feasible to base our analysis on the mean or median serum 25(OH)D values because some studies did not report this. All studies, however, reported the vitamin D status in the following categories: deficient, insufficient, and sufficient. The studies used different cut-off values for vitamin D deficiency and more consistent cut-off values for vitamin D insufficiency. There were two RCTs on vitamin D supplementation that we used as the baseline serum 25(OH)D data for analysis. Some studies reported the serum 25(OH)D values for a mixture of indoor and outdoor sport activities. We combined winter with early spring and summer with early fall due to limited available data. Some of the estimates of vitamin D inadequacy were based on one or two studies, thus limiting the generalizability. We were unable to find an association between injuries and vitamin D status in athletes due to the lack of adequate data. We included this information to save future investigators the time and effort of trying to answer this particular question from the current literature. It also outlines the need for more rigorous and stringent criteria in future studies. Despite these limitations, the strength of this evidence is its robust methodology. To minimize bias, multiple authors independently searched the literature, applied the eligibility criteria to the titles and the abstracts and subsequently to the full-text of the articles, assessed the methodological quality, and collected data.
5 Conclusion
Despite the limitations of the current evidence, the prevalence of vitamin D inadequacy in athletes is prominent. The risk significantly increases in higher latitudes, in winter and early spring seasons, and for indoor sport activities. Regular investigation of vitamin D status using reliable assays and supplementation is essential to ensure that athletes are healthy. The prevalence of injuries in athletes is notable but its association with vitamin D status is unclear. A well-designed longitudinal study is needed to answer this possible association.
References
Angeline ME, Gee AO, Shindle M, et al. The effects of vitamin D deficiency in athletes. Am J Sports Med. 2013;41(2):461–4.
Ogan D, Pritchett K. Vitamin D and the athlete: risks, recommendations, and benefits. Nutrients. 2013;5(6):1856–68.
Allison RJ, Close GL, Farooq A, et al. Severely vitamin D-deficient athletes present smaller hearts than sufficient athletes. Eur J Prev Cardiol. Epub 2014 Jan 7.
DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689S–96S.
Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–8.
Heaney RP. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr. 2004;80(6 Suppl):1706S–9S.
Calvo MS, Whiting SJ, Barton CN. Vitamin D fortification in the United States and Canada: current status and data needs. Am J Clin Nutr. 2004;80(6 Suppl):1710S–6S.
Whiting SJ, Langlois KA, Tanparast H, et al. The vitamin D status of Canadians relative to the 2011 dietary reference intakes: an examination in children and adults with and without supplement use. Am J Clin Nutr. 2011;94(1):128–35.
Holick MF. The vitamin D epidemic and its health consequences. J Nutr. 2005;135(11):2739S–48S.
Holick MF. The D-lightful vitamin D for child health. JPEN J Parenter Enteral Nutr. 2012;36(1 Suppl):9S–19S.
Holick MF. Vitamin D: a d-lightful solution for health. J Investig Med. 2011;59(6):872–80.
Shuler FD, Wingate MK, Moore GH, et al. Sports health benefits of vitamin D. Sports Health. 2012;4(6):496–501.
Redzic M, Lewis RM, Thomas DT. Relationship between 25-hydoxyvitamin D, muscle strength, and incidence of injury in healthy adults: a systematic review. Nutr Res. 2013;33(4):251–8.
Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–6.
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
Marcus R, Feldman D, Nelson D, et al. Fundamentals of osteoporosis. 4th ed. London: Academic Press; 2010.
Viera AJ. Odds ratios and risk ratios: what’s the difference and why does it matter? South Med J. 2008;101(7):730–4.
Badawi A, Arora P, Sadoun E, et al. Prevalence of vitamin D insufficiency in Qatar: a systematic review. J Public Health Res. 2012;1(3):229–35.
Racinais S, Hamilton B, Li CK, et al. Vitamin D and physical fitness in Qatari girls. Arch Dis Child. 2010;95(10):854–5.
Bescos Garcia R, Rodriguez Guisado FA. Low levels of vitamin D in professional basketball players after wintertime: relationship with dietary intake of vitamin D and calcium. Nutr Hosp. 2011;26(5):945–51.
Close GL, Leckey J, Patterson M, et al. The effects of vitamin D(3) supplementation on serum total 25(OH)D concentration and physical performance: a randomised dose-response study. Br J Sports Med. 2013;47(11):692–6.
Close GL, Russell J, Cobley JN, et al. Assessment of vitamin D concentration in non-supplemented professional athletes and healthy adults during the winter months in the UK: implications for skeletal muscle function. J Sports Sci. 2013;31(4):344–53.
Constantini NW, Arieli R, Chodick G, et al. High prevalence of vitamin D insufficiency in athletes and dancers. Clin J Sport Med. 2010;20(5):368–71.
Ducher G, Kukuljan S, Hill B, et al. Vitamin D status and musculoskeletal health in adolescent male ballet dancers a pilot study. J Dance Med Sci. 2011;15(3):99–107.
Galan F, Ribas J, Sanchez-Martinez PM, et al. Serum 25-hydroxyvitamin D in early autumn to ensure vitamin D sufficiency in mid-winter in professional football players. Clin Nutr. 2012;31(1):132–6.
Guillaume G, Chappard D, Audran M. Evaluation of the bone status in high-level cyclists. J Clin Densitom. 2012;15(1):103–7.
Halliday TM, Peterson NJ, Thomas JJ, et al. Vitamin D status relative to diet, lifestyle, injury, and illness in college athletes. Med Sci Sports Exerc. 2011;43(2):335–43.
Hamilton B, Grantham J, Racinais S, et al. Vitamin D deficiency is endemic in Middle Eastern sportsmen. Public Health Nutr. 2010;13(10):1528–34.
Hamilton B, Whiteley R, Farooq A, et al. Vitamin D concentration in 342 professional football players and association with lower limb isokinetic function. J Sci Med Sport. 2014;17(1):139–43.
He CS, Handzlik M, Fraser WD, et al. Influence of vitamin D status on respiratory infection incidence and immune function during 4 months of winter training in endurance sport athletes. Exerc Immunol Rev. 2013;19:86–101.
Lewis RM, Redzic M, Thomas DT. The effects of season-long vitamin d supplementation on collegiate swimmers and divers. Int J Sport Nutr Exerc Metab. 2013;23(5):431–40.
Lovell G. Vitamin D status of females in an elite gymnastics program. Clin J Sport Med. 2008;18(2):159–61.
Magee PJ, Pourshahidi LK, Wallace JM, et al. Vitamin D status and supplementation in elite irish athletes. Int J Sport Nutr Exerc Metab. 2013;23(5):441–8.
Peeling P, Fulton SK, Binnie M, et al. Training environment and Vitamin D status in athletes. Int J Sports Med. 2013;34(3):248–52.
Pollock N, Dijkstra P, Chakraverty R, et al. Low 25(OH) vitamin D concentrations in international UK track and field athletes. S Afr J Sports Med. 2012;24(2):55–9.
Shanely RA, Nieman DC, Knab AM, et al. Influence of vitamin D mushroom powder supplementation on exercise-induced muscle damage in vitamin D insufficient high school athletes. J Sports Sci. 2013;32(7):676–9.
Shindle MK, Voos J, Gulotta L, et al. Vitamin D status in a professional American Football team [abstract no. 46-9849]. AOSSM Annual Meeting; 7–10 Jul 2011; San Diego.
Storlie DM, Pritchett K, Pritchett R, et al. 12-Week vitamin D supplementation trial does not significantly influence seasonal 25(OH)D status in male collegiate athletes. Int J Health Nutr. 2011;2(2):8–13.
Willis KS, Smith DT, Broughton KS, et al. Vitamin D status and biomarkers of inflammation in runners. Open Access J Sports Med. 2012;3:35–42.
Wilson G, Fraser WD, Sharma A, et al. Markers of bone health, renal function, liver function, anthropometry and perception of mood: a comparison between flat and national hunt jockeys. Int J Sports Med. 2013;34(5):453–9.
Wolman R, Wyon MA, Koutedakis Y, et al. Vitamin D status in professional ballet dancers: winter vs. summer. J Sci Med Sport. 2013;16(5):388–91.
Wahl DA, Cooper C, Ebeling PR, et al. A global representation of vitamin D status in healthy populations. Arch Osteoporos. 2012;7(1–2):155–72.
Ceroni D, Anderson de la Llana R, Martin X, et al. Prevalence of vitamin D insufficiency in Swiss teenagers with appendicular fractures: a prospective study of 100 cases. J Child Orthop. 2012;6(6):497–503.
Zhang W, Stoecklin E, Eggersdorfer M. A glimpse of vitamin D status in Mainland China. Nutrition. 2013;29(7–8):953–7.
Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014;144PA:138–45.
Brito A, Cori H, Olivares M, et al. Less than adequate vitamin D status and intake in Latin America and the Caribbean: a problem of unknown magnitude. Food Nutr Bull. 2013;34(1):52–64.
Lee YA, Kim HY, Hong H, et al. Risk factors for low vitamin D status in Korean adolescents: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008–2009. Public Health Nutr. 2014;17(4):764–71.
Hirschler V, Maccallini G, Molinari C, et al. Low vitamin D concentrations among indigenous Argentinean children living at high altitudes. Pediatr Diabetes. 2013;14(3):203–10.
Lanteri P, Lombardi G, Colombini A, et al. Vitamin D in exercise: physiologic and analytical concerns. Clin Chim Acta. 2013;415:45–53.
Kumar J, Muntner P, Kaskel FJ, et al. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001-2004. Pediatrics. 2009;124(3):e362–70.
Turer CB, Lin H, Flores G. Prevalence of vitamin D deficiency among overweight and obese US children. Pediatrics. 2013;131(1):e152–61.
Lehtonen-Veromaa M, Mottonen T, Irjala K, et al. Vitamin D intake is low and hypovitaminosis D common in healthy 9- to 15-year-old Finnish girls. Eur J Clin Nutr. 1999;53(9):746–51.
Korchia G, Amitai Y, Moshe G, et al. Vitamin D deficiency in children in Jerusalem: the need for updating the recommendation for supplementation. Isr Med Assoc J. 2013;15(7):333–8.
Hamilton B. Vitamin D and athletic performance: the potential role of muscle. Asian J Sports Med. 2011;2(4):211–9.
Holick MF. Vitamin D: a D-lightful health perspective. Nutr Rev. 2008;66(10 Suppl 2):S182–94.
Prentice A. Vitamin D deficiency: a global perspective. Nutr Rev. 2008;66(10 Suppl 2):S153–64.
Godar DE. Worldwide increasing incidences of cutaneous malignant melanoma. J Skin Cancer. 2011;2011:858425.
Burgi AA, Gorham ED, Garland CF, et al. High serum 25-hydroxyvitamin D is associated with a low incidence of stress fractures. J Bone Miner Res. 2011;26(10):2371–7.
Ruohola JP, Laaksi I, Ylikomi T, et al. Association between serum 25(OH)D concentrations and bone stress fractures in Finnish young men. J Bone Miner Res. 2006;21(9):1483–8.
Ju SY, Lee YJ, Jeong SN. Serum 25-hydroxyvitamin D levels and the risk of depression: a systematic review and meta-analysis. J Nutr Health Aging. 2013;17(5):447–55.
Acknowledgments
No sources of funding were used to assist in the preparation of this review. The authors have no potential conflicts of interest that are directly relevant to the content of this review. The authors would like to thank Nadia Latifi for assisting with resolving discrepancies during the study selection process.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farrokhyar, F., Tabasinejad, R., Dao, D. et al. Prevalence of Vitamin D Inadequacy in Athletes: A Systematic-Review and Meta-Analysis. Sports Med 45, 365–378 (2015). https://doi.org/10.1007/s40279-014-0267-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-014-0267-6