Abstract
Background
Although physical exercise is recommended for asthmatics, evidence on the effects of exercise on clinical key factors is still missing.
Objectives
We performed a systematic review and meta-analysis to determine the effect of exercise training (EXT) on quality of life (QoL), bronchial hyperresponsiveness (BHR), exercise-induced bronchoconstriction (EIB), lung function and exercise capacity, plus the factors affecting changes in QoL and exercise capacity in asthmatics after a period of EXT.
Data Sources
A computerized search was conducted in MEDLINE, EMBASE, and CINAHL (last search on 15 November 2012), without language restriction, and references of original studies and reviews were searched for further relevant studies.
Study Selection
Two independent investigators screened full-text studies with asthmatic subjects undertaking EXT (defined as training for ≥7 days, ≥2 times per week, ≥5 training sessions in total) that assessed at least one of the following outcomes: QoL, airway hyperreactivity, forced expiratory volume in one second (FEV1), peak expiratory flow (PEF), inflammatory parameters, exercise capacity, or exercise endurance. Potentially relevant studies were excluded if only respiratory muscle training, breathing exercises or yoga was performed, if asthmatic subjects with co-morbidities were investigated, if only data of mixed patient groups without separate results for asthmatics were presented, if training regimens were not sufficiently specified, if no numerical outcome data were presented, and if new long-term medication was introduced in addition to physical training. Of 500 potentially relevant articles, 13.4 % (67 studies including 2,059 subjects) met the eligibility criteria and were included for further analyses.
Study Appraisal and Synthesis Methods
Data extraction and risk of bias assessment was performed according to the Cochrane Handbook for Systematic Reviews of Interventions. A meta-analysis of all randomized controlled trials (RCTs) was performed to determine the effect of EXT on asthma symptoms, BHR, EIB, FEV1, exercise capacity and exercise endurance compared with control training. In addition, relative pre/post changes were analysed in all RCTs and controlled trials. Finally, multiple linear regression models were used to identify effects of relative changes in airway hyperreactivity (BHR or EIB), lung function (FEV1 or PEF) and training hours on QoL and exercise performance.
Results
In a total of 17 studies including 599 subjects, meta-analyses showed a significant improvement in days without asthma symptoms, FEV1 and exercise capacity while BHR only tended to improve. The analysis of relative within-group changes after EXT showed, however, significant improvements in QoL (17 %), BHR (53 %), EIB (9 %), and FEV1 (3 %) compared with control conditions. Multiple linear regression models revealed that changes in airway hyperreactivity and lung function significantly contributed to the change in QoL, while mainly the changes in airway hyperreactivity contributed to the change in exercise capacity.
Conclusion
EXT was shown to improve asthma symptoms, QoL, exercise capacity, BHR, EIB, and FEV1 in asthmatics and improvements in BHR explained part of the improvement in QoL and exercise capacity. Thus, physical activity should be recommended as a supplementary therapy to medication. However, more well controlled studies should be performed assessing the relationship of physical activity, QoL, airway hyperreactivity, lung function and especially airway inflammation as well as medication intake.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Asthma is a chronic inflammatory airway disease associated with expiratory airflow limitation and bronchial hyperresponsiveness (BHR) accompanied by symptoms such as cough, wheezing, increased sputum production, but also sleep disturbance, limitation of daily activity and the use of rescue medication [1]. All of these factors can contribute to a reduced quality of life (QoL) in people with asthma.
Exercise training (EXT) is known to reduce low-grade systemic inflammation [2, 3] and therefore has the potential to reduce the severity of asthma. However, about 90 % of asthmatic subjects suffer from exercise-induced bronchoconstriction (EIB), i.e. airway narrowing and increased airway resistance during or after exercise, likely resulting from the airways drying out, causing a hyperosmolar environment which leads to mast cell mediator release [4]. These exercise-related adverse events might prevent asthmatics from performing regular physical exercise, though results are conflicting as to whether asthmatics, in particular asthmatic children, are typically less fit and perform less physical activity compared with non-asthmatic peers (summarized by Welsh et al. [5]). However, these effects can often be prevented by pre-exercise medication, e.g., use of short acting β2-agonists [4, 6]. Therefore, it is not surprising that EXT was found to improve maximal as well as endurance exercise performance similarly in asthmatics and healthy subjects [7–9].
In a recent meta-analysis, Chandratilleke et al. [10] not only investigated improvements in variables related to exercise performance after EXT, but also assessed changes in asthma-specific variables such as lung function in response to training. However, they found no significant change in forced vital capacity, forced expiratory volume in one second (FEV1) or peak expiratory flow rate (PEF). The meta-analysis of asthma symptoms was deemed inappropriate due to heterogeneity of the outcome variables. However, this meta-analysis [10] and a further review [11] found that the majority of the included studies reported improvements in QoL. Since EXT is well known to improve performance and QoL in healthy subjects [12], training-associated improvements in performance and QoL of asthmatics are not unexpected. We asked ourselves whether evidence exists that BHR and EIB also improve with EXT, whether these improvements are associated with improvements in asthma-related symptoms and QoL, and whether these changes might allow a reduction in the use of asthma medication. The reduced need for medication might per se have a positive effect on QoL.
Therefore, we performed a systematic review for all studies that included EXT interventions in patients with asthma. With selected studies we conducted meta-analyses to assess training-induced effects on QoL, airway hyperresponsiveness, lung function, exercise capacity and endurance. In addition, we evaluated changes of all EXT groups relative to changes in control groups. Finally, we applied a multiple linear regression model to assess asthma-related factors affecting the improvement in exercise performance and QoL.
2 Methods
2.1 Search
A computerized search for all physical training studies in asthmatic subjects was performed in MEDLINE, EMBASE and CINAHL containing the following search terms (last search performed on 15 November 2012): (asthma* OR ((airway OR respiratory OR bronchial) AND (bronchoconstrict* OR bronchospas* OR hyperreact* OR hyperresponsiv*))) AND (training OR conditioning) AND (endurance OR strength OR exercise OR sport* OR “physical activity” OR run* OR cycl* OR swim* OR row* OR gymnastic OR resistive OR (hyperpnoea OR hyperpnea) OR ventilatory OR threshold OR inspiratory OR expiratory OR respiratory). No restrictions were applied regarding language of publication or age of the subjects.
2.2 Study Selection
All titles and abstracts of the computerized search were independently screened by two investigators (SND, PE) for potential relevance. The following studies were excluded: animal studies, studies without asthmatic subjects, reviews, guidelines, letters, commentaries, studies not including EXT (defined as training for ≥7 days, ≥2 times per week, ≥5 training sessions in total). Studies where subjects performed only respiratory muscle training, breathing exercises or yoga were also excluded. References of reviews were searched manually for additional relevant publications.
Relevant publications were excluded if they were published only as an abstract, poster or short communication. In addition, studies were excluded if investigating asthmatic subjects with co-morbidities; if investigating mixed groups (asthmatic subjects and subjects with other diseases) and if the results of asthmatic subjects were not reported separately; if subjects were in a training camp; if training regimens were not sufficiently specified; if no numerical outcome data were presented; and if new long-term medication was introduced in addition to physical training. However, studies including any form of education or breathing exercises (clearly distinct from respiratory muscle training) were included, since we assumed that all asthmatic patients have received some ‘patient education’ and demonstration of ‘breathing exercises’ by their physician at the time of diagnosis or at some point thereafter.
Studies included in this review had to contain at least one of the following outcomes: QoL (including asthma symptoms), airway hyperreactivity assessed either as BHR in response to an inhalation challenge or as EIB in response to an exercise challenge, FEV1, PEF, respiratory resistance or reactance (measured with forced oscillation technique), parameters on airway inflammation, maximal oxygen consumption \({(\dot{V}{\text{O}}_{2,\rm{max}} )}\), maximal working capacity (W max), or exercise endurance (measuring change in test duration or distance).
2.3 Data Collection
Data were collected by two investigators (SND, PE) independently. In case of inconsistency, results were discussed with a third investigator (CMS). Publications in a language not spoken by the investigators (i.e., not published in English, German, French, Spanish, Italian or Dutch) were discussed with native speakers. In studies with an asthmatic EXT group (with or without a control group) that also included a non-asthmatic EXT and/or control group, only data from the asthmatic group(s) were included in the analyses. Moreira et al. [13], Varray et al. [14] and Wang and Hung [15] did not give single data on lung function in their original publication. However, Chandratilleke et al. [10] published their data which we also included in our analysis.
2.4 Meta-Analysis
Meta-analyses were performed on the main outcomes of randomized controlled trials (RCTs) using the Review Manager 5.1 software (Copenhagen, Denmark). Since only a few studies reported the relative changes and corresponding standard deviations (SDs), the analysis was performed using post-intervention values, if baseline values did not significantly differ from each other. Missing SDs were calculated if possible. Heterogeneity was assessed by I 2- and Chi-squared (χ2) statistics and considered as such with I 2 > 60 % and p < 0.1 in the χ2 statistics. In general, a fixed-effects model and mean differences (MD) with a 95 % confidence interval (CI) was chosen. However, when large heterogeneity was expected, we decided to use subgroup analyses (W max, Endurance) and/or to incorporate heterogeneity in the analysis using a random-effects model (PEF, Endurance) [16]. In case of different scales for the same outcome measure, the standardized mean difference (SMD) was chosen instead of the MD [16]. In case there was more than one test for one parameter (e.g., exercise performance), the result with (i) the largest level of significance, (ii) the greatest percent change (calculated from mean values pre- and post-intervention) or (iii) the same scale compared with other studies in the same meta-analysis was chosen.
To assess the risk of bias across studies, the standard error of each study was plotted against the MD or SMD, respectively. This funnel plot was only used if there were ten or more studies for a variable, because of the high risk of achieving an asymmetry by chance [16].
The risk of bias in individual studies was assessed according to the Cochrane Handbook [16] by two investigators individually (SND, PE). The categories judged were the following: random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessors, incomplete outcome data, selective reporting and other bias. If a second or control group was available but was not relevant or appropriate for our review, a non-controlled design was assumed and hence random sequence generation, allocation concealment, blinding of participants and outcome assessors were considered at high risk of bias, although this might not be true for the original study design. Furthermore, only potential bias that could have affected our previously defined outcome variables was considered.
2.5 Additional Analyses
For RCTs and controlled trials (CTs), mean percent changes of the EXT group were calculated and corrected for potential placebo effects; i.e., they were corrected by the mean percent changes of the control group (EXT − control). Then, they were weighted according to the study size or, if possible, according to the number of participants in whom the respective parameter was measured, and finally, these relative changes of the EXT group were statistically compared with baseline (zero) using a Wilcoxon Signed-Rank Test. Statistical analysis was performed with IBM SPSS Statistics, Version 20 (Armonk, USA) and presented in graphical form (Fig. 4). Values are given as mean ± SD. The effects of non-controlled trials (NCTs) are only shown graphically but they were not taken into account for weighted group mean changes or any other statistical analyses.
The software environment R (R Foundation for Statistical Computing, Vienna, Austria) was used to apply multiple linear regression analyses (Generalized Estimating Equations models), taking into account all included studies irrespective of the presence of a control group since control groups do not contribute to this model. If the same parameter was evaluated by different tests within one study, the test with (i) the largest level of significance and (ii) the highest percentage change (calculated from mean values pre- and post-intervention) was chosen in order to avoid collinearity. If both FEV1 and PEF were measured, FEV1 was chosen due to its superiority in terms of clinical validity to estimate airway obstruction compared with PEF [17]. Additionally, the following parameters were taken together to prevent collinearity: general QoL, asthma-related QoL (AQoL), and asthma symptoms (ASymp) to assess the overall QoL; EIB measured with the percentage fall in FEV1 or PEF and BHR to evaluate airway hyperreactivity; and finally \({\dot{V}{\text{O}}_{2,\rm{max}}}\), W max, and exercise endurance to rate an overall change in exercise capacity. If several variables were assessed in one study, again the one with (i) the largest level of significance and (ii) the highest percentage change was chosen.
2.6 Combined Data
If studies presented average results of men and women [18] or of different age groups separately [19], results were combined and weighted according to the number of participants in each subgroup. If data from questionnaires were given as subcategories, the data were combined to give a total score as specified for the respective questionnaire.
For data analyses the significance level was set at p ≤ 0.05.
3 Results
3.1 Study Selection and Characteristics
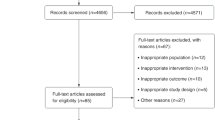
The search revealed 3,982 citations, of which 500 potentially relevant articles were identified, whereof 138 studies were retrieved for a more detailed evaluation (see Fig. 1 for details). After the selection process, 67 studies [7–9, 13–15, 18–78] were finally included. Detailed characteristics of the 67 studies included are given in the Electronic Supplementary Material, Table S1. Randomization was present in 23 studies (34 %), 17 studies were CTs (25 %) and 27 were NCTs (40 %). In total, the studies included 1,363 subjects in the EXT group, while 696 served as controls. A list of the detailed reasons for exclusion of the 71 potential studies later excluded can be found in the Electronic Supplementary Material, Table S2.
Flow chart of studies excluded from and included in the meta-analysis and/or % change analysis and/or linear regression analysis. EIB exercise-induced bronchoconstriction, FEV 1 forced expiratory volume in one second, IMT inspiratory muscle training, RCT randomized controlled trial, RMT respiratory muscle training, SD standard deviations
3.2 Risk of Bias Within Studies
An overview of the risk of bias within studies is given in the Electronic Supplementary Material, Table S3. The assessment was difficult mainly due to poor reporting of methods, protocols and/or data. In RCTs, 20 % of all evaluated domains were judged to be at low risk, 70 % unclear and 10 % at high risk of bias. In CTs, these were 7, 51 and 42 % and in NCTs, 8, 32 and 60 %, respectively. No study addressed all of the domains adequately.
3.3 Meta-Analysis
For meta-analyses of ASymp, lung function and \({\dot{V}{\text{O}}_{2,\rm{max}}}\), the study by Mendes et al. [54] was excluded because we could not clarify whether groups were different before the intervention phase in the reduced set of subjects analysed here. To minimize bias, the two training groups (morning and afternoon) of Silva et al. [66] were taken together since only one control group was available. In the study of Ahmaidi et al. [21], to be consistent with other studies, only measured \({\dot{V}{\text{O}}_{2,\rm{max}}}\) was included while the calculated \({\dot{V}{\text{O}}_{2,\rm{max}}}\) was not considered for analysis. Cochrane and Clark [28] did not provide standard deviations for histamine challenge data before training or after training and we were unable to obtain data from them. Therefore, inclusion into the meta-analysis was not possible. Results of all meta-analyses are presented in Fig. 2.
Effect of exercise training (EXT) on a asthma symptoms (ASymp); b bronchial hyperresponsiveness (BHR); c exercise-induced bronchoconstriction (EIB); d forced expiratory volume in one second (FEV 1 ); e peak expiratory flow (PEF); f maximal oxygen consumption \({(\dot{V}{\text{O}}_{\it{2},\it{max}} )}\); g maximal working capacity (W max) and h exercise endurance. Only those studies providing the necessary information are included in this forest plot. CI confidence interval, Con control, IV inverse variance, Std. standardized
Since FEV1 was the only variable assessed in more than ten studies, a funnel plot is only presented for this parameter (Fig. 3). Results of the analysis of risk of bias in individual studies are given in the Electronic Supplementary Material, Table S3.
3.3.1 Asthma Symptoms
A meta-analysis of AQoL and ASymp was deemed inappropriate due to the use of different questionnaires and questions with different directions of improvements. Therefore, an analysis was performed for the most commonly used item which was the number of symptom-free days per month. The EXT group was clearly favoured compared with controls (MD 8.90 symptom-free days, 95 % CI 8.18–9.61, p < 0.001).
3.3.2 Bronchial Hyperreactivity
The meta-analysis for BHR in response to an inhaled substance revealed a tendency in favour of EXT compared with controls (MD 0.21 mg mL−1, 95 % CI −0.03 to 0.45, p = 0.09). The meta-analysis for EIB (% FEV1-reduction post-exercise) showed no significant difference between EXT and controls (MD −2.81 %, 95 % CI −7.05 to 1.43, p = 0.19).
3.3.3 Lung Function
The meta-analysis for FEV1 approached significance in favour of the EXT group (MD 0.09 L, 95 % CI −0.00 to 0.17, p = 0.05). Bias analysis across studies (Fig. 3) showed no risk of publication bias in FEV1.
The meta-analysis for PEF showed no significant difference between EXT and control groups after a period of training (MD 0.45 L s−1, 95 % CI −0.16 to 1.07, p = 0.15). Also, heterogeneity was considerable (I 2 = 81 %, p < 0.001).
3.3.4 Exercise Capacity
The meta-analysis for all performance variables showed a significant difference in favour of the EXT group compared with controls; i.e., \({\dot{V}{\text{O}}_{2,\rm{max}}}\) (MD 4.06 mL min−1 kg−1, 95 % CI 3.02–5.10, p < 0.001), W max (MD 24.03 W, 95 % CI 20.15–27.91, p < 0.001) and exercise endurance (SMD 0.81, 95 % CI 0.13–1.48, p < 0.02). For W max there was considerable heterogeneity (I 2 = 87 %, p < 0.0001), despite selecting only cycling tests in an attempt to minimize this. However, subgroup analysis of adults [21, 29] and children [52, 74] demonstrated a substantial reduction of heterogeneity (I 2 = 21 %, p = 0.26 and I 2 = 63 %, p = 0.10, respectively) with significance still present (p < 0.001 and p = 0.002, respectively). For endurance exercise, heterogeneity was also considerable, but could be reduced by separating constant-load tests from time trials (data not shown).
3.4 Analysis of Relative Changes
The analysis of percent changes in the EXT group when corrected for control group effects showed improvements in AQoL (17 ± 14 %, p = 0.011), BHR (53 ± 19 %, p = 0.043), EIB (9 ± 7 %, p = 0.036), FEV1 (3 ± 7 %, p = 0.019), \({\dot{V}{\text{O}}_{2,\rm{max}}}\) (17 ± 8 %, p = 0.001), W max (11 ± 8 %, p = 0.003) and endurance (32 ± 42 %, p = 0.005). Results are shown in Fig. 4.
Relative changes comparing after versus before training (where possible controlled against the control group) for a asthma-related quality of life (AQoL), bronchial hyperresponsiveness (BHR), exercise-induced bronchoconstriction (EIB), b forced expiratory volume in one second (FEV 1 ), peak expiratory flow rate (PEF) and c maximal oxygen consumption \({(\dot{V}{\text{O}}_{2,\rm{max}} )}\), maximal working capacity (W max) and endurance. Open circles non-controlled trials, light grey circles controlled trials, closed circles randomized controlled trials. The size of the circles represents the number of subjects included in the study. % Changes >0 (positive) are improvements; % changes <0 (negative) are deteriorations; dashed lines represent the weighted relative mean improvement of the controlled and randomized controlled trials. In several instances [7–9, 22, 25, 26, 36, 37, 55, 60, 63, 65, 69], the study design for the selected variables differs from the original study design. Therefore, if a control group was present but not relevant or not appropriate for this review, a non-controlled design was assumed, although this might not be true for the original study design
Subgroup analyses for training with/without education were not possible because of the small number of studies using the same type of intervention. Similarly, data pooling for subgroup analysis on the use of asthma medication was deemed inappropriate because too many different measures of use of asthma medication were reported: medication scores [24, 37, 56, 76]; amount of oral corticosteroids per day [70]; amount of inhaled corticosteroids per day in one subgroup [56]; number of days taking oral corticosteroids [33]; number of subjects having changed, unchanged or increased daily dose of inhaled corticosteroids [34]; number of subjects using less frequent oral theophylline tablets and rescue inhaler or with less frequent prescriptions of rescue inhaler or oral corticosteroids [50]; number of exacerbations requiring systemic corticosteroids [39]; and use of asthma medication per week [45].
3.5 Multiple Linear Regression Analysis
The multiple linear regression models (Table 1) revealed that (i) reduction in airway hyperreactivity (BHR + EIB; p < 0.001) and number of training hours (p < 0.001 and p < 0.01, respectively) significantly contributed to both improvements in QoL and exercise capacity; and (ii) improvements in lung function (p < 0.001) contributed to increases in QoL while changes in lung function (p = 0.054) only tended to contribute to improvements in exercise capacity. The effect of training hours was significant but its contribution was minimal.
4 Discussion
The present analysis shows that the QoL of asthmatics considerably improves with physical training and that changes in airway hyperreactivity and lung function significantly contribute to this improvement. Also, these two factors, in particular the change in airway hyperreactivity (BHR and EIB), contribute to improved physical fitness. Unfortunately, the influence of asthma severity or age on the different outcomes could not be analysed due to the heterogeneity of information assessed and/or provided in the different publications.
4.1 Quality of Life
Since studies assessing AQoL used different types of questionnaires with different scales or did not provide SDs, no meta-analysis could be performed on these data. However, when considering percent changes of single studies independent of scale used, AQoL improved by 17 % more on average in the EXT group compared with the control group (6 RCTs, 1 CT). Several factors can contribute to this improvement; some of them are directly related to asthma symptoms. In fact, meta-analysis of the number of days per month free of asthma symptoms showed a clear improvement. Also, the majority of studies analysing the use of asthma medication reported a reduction in medication intake [24, 26, 34, 39, 50, 56, 70], two studies [31, 32] reported no change, and 58 studies did not analyse changes on the use of medication. Three [24, 34, 39] of the studies that reported a reduction also assessed QoL, and although using different types of questionnaires, they all showed an improvement. This is not surprising since it is known that QoL is influenced by patients’ dependence on medication [79]. Taken together, these facts demonstrate that physical training improves asthma-associated symptoms and QoL as also suggested by Chandratilleke and colleagues [10].
4.2 Airway Hyperreactivity and its Influence on QoL
The meta-analysis of three studies comparing BHR after EXT and control phase demonstrated a trend for lower and thus improved BHR in the EXT compared with the control group. This result is supported by the analysis of percent changes in all five controlled EXT groups, showing a significant average improvement of 53 %. With respect to EIB, the meta-analysis of two studies did not show any difference between EXT and control groups at the end of the study. In contrast, the weighted, average percent change of all eight controlled EXT groups was significant and came up to 9 %. This apparent contradiction regarding improvements in EIB may result from the larger number of studies in the latter analysis, or from the fact that the latter analysis assessed changes from baseline while in the meta-analysis, only post-training values were included (as suggested by Higgins and Green [16]); i.e., non-significant differences in baseline values were not accounted for and may have impacted the result. For example, in the study of Matsumoto et al. [52], the mean post-training difference between the training and control group was 5 %, while the difference of the percent change of the EXT groups (corrected for control changes) was 21 %.
Interestingly, BHR improved much more (53 %) than EIB (9 %). One possible explanation could lie in the standardization of BHR and EIB tests, as performance of BHR challenges seem much better standardized; i.e., in particular, investigators can adhere to these protocols more easily since fewer variables need to be observed [80]. In fact, although recommendations for standardization of EIB challenges have existed for a long time [81], studies included in this review applied many different tests to assess EIB. Also, with the test used in the current recommendations, EIB has a coefficient of variation (CV) of about 21 % [82], a variability that might mask ‘true’ changes. Another possible explanation for the discrepancy between changes in BHR and EIB may result from the different mechanisms by which bronchoconstriction is provoked. Bronchoconstriction in BHR tests of the present analysis was triggered directly, meaning that inhaled substances such as methacholine and histamine directly act on airway smooth muscles. EIB challenges, however, trigger bronchoconstriction indirectly by respiratory water loss and an increase in osmolarity of the airway surface, which in turn leads to the release of mediators causing airway smooth muscle contractions [83]. Due to this mechanism, an EIB challenge depends more on environmental conditions, such as air temperature and humidity [84], than a BHR challenge, a fact that may also attenuate the test result.
It is expected that changes in BHR and EIB would be accompanied by a change in inflammation. Due to the many different approaches to assess inflammation, we were unable to perform a meta-analysis. However, several studies found an improvement in inflammatory variables after the physical training phase [13, 25, 40, 51, 54]. Some studies assessed systemic inflammation [13, 51], which is known to improve with exercise [2, 3], while local airway inflammation is of special interest in asthmatics, particularly with respect to BHR. Four studies measured biomarkers of local airway inflammation, namely induced sputum cell count [54], exhaled breath condensate [25] and exhaled nitric oxide [13, 25, 40, 54]. While three studies found no improvement after physical exercise [13, 25, 40], one study reported reduced airway inflammation in the EXT group, namely a significant decrease in induced sputum cell count and exhaled nitric oxide concentration [54]. From a clinical point of view, reduced airway inflammation should lead to a reduction in medication intake, e.g. inhaled corticosteroids [85]. One study indeed reported that 52 % of all patients in the EXT group reported a reduction in inhaled corticosteroids use after the training intervention [34] and three studies reported a reduction in systemic corticosteroid intake [39, 50, 71], but none of them assessed parameters related to inflammation.
However, the present results are consistent with data from animal studies that show a reduction in airway reactivity and inflammatory parameters in a murine asthma model after an aerobic exercise intervention. Interestingly, animal studies even showed a reversal of inflammation-associated airway remodelling [86–89]. Therefore, in humans, physical training might also be advantageous over anti-inflammatory medication, which appears to reverse only some of the processes involved in airway remodelling by suppressing inflammation, but does not seem able to inhibit structural airway changes [90] unless high doses of inhaled corticosteroids are used [91].
The multiple linear regression models revealed that the observed changes in airway hyperreactivity in the present study may partly explain the improvement in QoL.
4.3 Lung Function and its Influence on QoL
In contrast to the meta-analysis performed by Chandratilleke et al. [10], we found a significant improvement in FEV1 in the present meta-analysis. The difference likely results from the three additional studies that we found with our search strategy [22, 63, 77]. Further support is given by the analysis of all 24 studies assessing training compared with controls (16 RCTs, 8 CTs). The weighted average percent improvement in the EXT groups was significantly greater (3 %) than that of the controls. However, despite significant FEV1 improvements, it can be questioned whether these are relevant. On the one hand, the biological variability (without any intervention) ranges around 0.2 L (or 5 %), on the other hand it is assumed that only differences around 10 % are perceived [17]. Nevertheless, the linear regression model showed, although only including four studies, that the changes in FEV1 could explain part of the improvement in QoL.
The funnel plot analysis of FEV1 (Fig. 3) gives the impression that no risk of bias across studies was present. However, it seems conspicuous that small studies showing either positive or negative results are completely missing. A closer look at the data, however, shows that two of the 11 included studies have a relatively small study population (Weisgerber et al. [76] with five EXT and three controls, and Varray et al. [14] with seven subjects in each group). Since the SDs in these studies were rather small, study populations were very homogenous, which may indicate a small publication bias.
Given the significant change in FEV1, it may be surprising that neither the meta-analysis nor the analysis of relative percent changes (data not shown) detected a significant change in PEF. However, PEF variability is reported to be around 30 % or even more in severe asthmatics [1] (three out of the four studies included in the meta-analysis), which may explain this difference. In addition, PEF is reported to be inferior to FEV1 as a clinically measured parameter due to the possibility of underestimating airway obstruction in individuals with airway remodelling [17].
4.4 Exercise Capacity
Not surprisingly, the meta-analysis showed that exercise capacity after intervention period differed significantly in favour of EXT compared with controls with a difference of 4.06 mL min−1 kg−1. This is consistent with the findings of Chandratilleke et al. [10]. In addition, we analysed W max during cycling where a difference of 24 W was observed in favour of EXT compared with controls. For W max, however, heterogeneity was considerable but this can partly be explained by the different age groups included in the analysis: the mean difference was 30 W for adults and 11 W for children. Analysis of the percent changes of all maximal exercise tests (8 RCTs and 5 CTs for \({\dot{V}{\text{O}}_{2,\rm{max}}}\), 4 RCTs and 5 CTs for W max) revealed significant improvements of 17 % for \({\dot{V}{\text{O}}_{2,\rm{max}}}\) and 11 % for W max, compared with controls. This is in accordance with the findings of the meta-analysis. In addition to maximal performance, we also analysed changes in endurance performance (e.g., constant-load tests and time trials). Three studies could be included in the meta-analysis and, again, a significant effect in favour of the EXT group was found compared with controls, although heterogeneity was considerable. Separating the constant-load test [74] from time trials [66, 72] greatly reduced heterogeneity. This may be explained by the fact that constant-load tests are known to have a higher CV (over 10 %) compared with time trials (5 %) [92]. Analysis of changes of all endurance tests (5 RCTs and 1 CT), showed an average improvement of 32 % in the EXT group compared with controls.
The multiple linear regression model showed that improvements in exercise capacity can partly be explained by the improvement in airway hyperreactivity. Interestingly, two studies [34, 39] also showed a significant improvement in dyspnoea or breathlessness, while three others [20, 31, 72] found no changes. However, in the latter three studies, performance was higher in the test after the training phase which suggests that breathlessness would have decreased at similar load levels. Together with the improvement in airway hyperreactivity, these subjective measures suggest a reduced impairment of exercise performance by the respiratory system after a phase of physical training.
The number of training hours correlates positively with the improvement in exercise capacity, although the estimate was quite small. However, considering that this improvement is partially at the expense of QoL (very small decrease in QoL with increasing training load), an increase in frequency or duration of training might not always be good advice. This may also be due to a loss of enjoyment or a reduced amount of remaining leisure time. The optimal amount of training for the greatest benefit still remains to be determined.
5 Conclusion
In conclusion, this is the first systematic review showing a positive effect of regular physical activity on QoL in asthmatics, with improvements in BHR and FEV1 shown to positively influence this change. Improvements in exercise capacity were also shown to be affected by changes in airway hyperreactivity. Thus, physical activity should be recommended as a supplementary therapy to medication. The optimum training volume, however, needs further investigation, since it should be noted that a higher training volume or frequency also has a negative impact on QoL despite improving exercise capacity. More well controlled studies should be performed assessing the relationships between physical activity, QoL, airway hyperreactivity, lung function and especially airway inflammation as well as medication intake.
References
The global strategy for asthma management and prevention, Global Initiative for Asthma (GINA) 2011. http://www.ginasthma.org/. Accessed 2013 Jan 30.
Ahmed HM, Blaha MJ, Nasir K, et al. Effects of physical activity on cardiovascular disease. Am J Cardiol. 2012;109(2):288–95.
Warnberg J, Cunningham K, Romeo J, et al. Physical activity, exercise and low-grade systemic inflammation. Proc Nutr Soc. 2010;69(3):400–6.
Weiler JM, Anderson SD, Randolph C, et al. Pathogenesis, prevalence, diagnosis, and management of exercise-induced bronchoconstriction: a practice parameter. Ann Allergy Asthma Immunol. 2010;105(6 Suppl.):S1–47.
Welsh L, Roberts RG, Kemp JG. Fitness and physical activity in children with asthma. Sports Med. 2004;34(13):861–70.
Godfrey S, Konig P. Inhibition of exercise-induced asthma by different pharmacological pathways. Thorax. 1976;31(2):137–43.
Robinson DM, Egglestone DM, Hill PM, et al. Effects of a physical conditioning programme on asthmatic patients. N Z Med J. 1992;105(937):253–6.
Freeman W, Nute MG, Williams C. The effect of endurance running training on asthmatic adults. Br J Sports Med. 1989;23(2):115–22.
Hallstrand TS, Bates PW, Schoene RB. Aerobic conditioning in mild asthma decreases the hyperpnea of exercise and improves exercise and ventilatory capacity. Chest. 2000;118(5):1460–9.
Chandratilleke MG, Carson KV, Picot J, et al. Physical training for asthma. Cochrane Database Syst Rev 2012; 5: CD001116.
Pacheco DR, Silva MJ, Alexandrino AM, et al. Exercise-related quality of life in subjects with asthma: a systematic review. J Asthma 2012;49(5):487–95
Atlantis E, Chow CM, Kirby A, et al. An effective exercise-based intervention for improving mental health and quality of life measures: a randomized controlled trial. Prev Med. 2004;39(2):424–34.
Moreira A, Delgado L, Haahtela T, et al. Physical training does not increase allergic inflammation in asthmatic children. Eur Respir J. 2008;32(6):1570–5.
Varray AL, Mercier JG, Terral CM, et al. Individualized aerobic and high intensity training for asthmatic children in an exercise readaptation program: is training always helpful for better adaptation to exercise? Chest. 1991;99(3):579–86.
Wang JS, Hung WP. The effects of a swimming intervention for children with asthma. Respirology. 2009;14(6):838–42.
Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]: The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org. Accessed 2013 Jan 30.
Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99.
Lawani MM, Hounkpatin S, Akplogan B. Modification of expiratory peak flow (EPF) in 14 asthmatic subjects from Benin by short duration exercise training [in French]. Dakar Med. 2006;51(3):165–71.
Mitsui J, Akimaru T, Yamazaki Y. Asthmatic children and swimming: the distinctive feature on the time of living. Jap J Phys Fitness Sports Med. 1985;34(3):158–66.
Afzelius-Frisk I, Grimby G, Lindholm N. Physical training in patients with asthma. Poumon Coeur. 1977;33(1):33–7.
Ahmaidi SB, Varray AL, Savy-Pacaux AM, et al. Cardiorespiratory fitness evaluation by the shuttle test in asthmatic subjects during aerobic training. Chest. 1993;103(4):1135–41.
Araki H, Kano S, Nishima S, et al. Effects of physical training on children with bronchial asthma [in Japanese]. Arerugi. 1991;40(3 Pt 1):205–14.
Arandelovic M, Stankovic I, Nikolic M. Swimming and persons with mild persistant asthma. Scientific World Journal 2007;7:1182–8.
Basaran S, Guler-Uysal F, Ergen N, et al. Effects of physical exercise on quality of life, exercise capacity and pulmonary function in children with asthma. J Rehabil Med. 2006;38(2):130–5.
Bonsignore MR, La Grutta S, Cibella F, et al. Effects of exercise training and montelukast in children with mild asthma. Med Sci Sports Exerc. 2008;40(3):405–12.
Bundgaard A, Ingemann-Hansen T, Schmidt A, et al. Effect of physical training on peak oxygen consumption rate and exercise-induced asthma in adult asthmatics. Scand J Clin Lab Invest. 1982;42(1):9–13.
Cambach W, Chadwick-Straver RV, Wagenaar RC, et al. The effects of a community-based pulmonary rehabilitation programme on exercise tolerance and quality of life: a randomized controlled trial. Eur Respir J. 1997;10(1):104–13.
Cochrane LM, Clark CJ. Benefits and problems of a physical training programme for asthmatic patients. Thorax. 1990;45(5):345–51.
Counil FP, Varray A, Matecki S, et al. Training of aerobic and anaerobic fitness in children with asthma. J Pediatr. 2003;142(2):179–84.
Dogra S, Jamnik V, Baker J. Self-directed exercise improves perceived measures of health in adults with partly controlled asthma. J Asthma. 2010;47(9):972–7.
Emtner M, Herala M, Stalenheim G. High-intensity physical training in adults with asthma. A 10-week rehabilitation program. Chest. 1996;109(2):323–30.
Emtner M, Finne M, Stalenheim G. High-intensity physical training in adults with asthma. A comparison between training on land and water. Scand J Clin Lab Invest. 1998;30(4):201–9.
Engstrom I, Fallstrom K, Karlberg E, et al. Psychological and respiratory physiological effects of a physical exercise programme on boys with severe asthma. Acta Paediatr Scand. 1991;80(11):1058–65.
Fanelli A, Cabral AL, Neder JA, et al. Exercise training on disease control and quality of life in asthmatic children. Med Sci Sports Exerc. 2007;39(9):1474–80.
Farid R, Azad FJ, Atri AE, et al. Effect of aerobic exercise training on pulmonary function and tolerance of activity in asthmatic patients. Iran J Allergy Asthma Immunol. 2005;4(3):133–8.
Fesharaki M, Paknejad SMJO, Kordi R. The effects of aerobic and strength exercises on pulmonary function tests and quality of life in asthmatic patients [in Persian]. Teheran Univ Med J. 2010;68(6):348–54.
Fitch KD, Morton AR, Blanksby BA. Effects of swimming training on children with asthma. Arch Dis Child. 1976;51(3):190–4.
Fitch KD, Blitvich JD, Morton AR. The effect of running training on exercise-induced asthma. Ann Allergy. 1986;57(2):90–4.
Foglio K, Bianchi L, Bruletti G, et al. Long-term effectiveness of pulmonary rehabilitation in patients with chronic airway obstruction. Eur Respir J. 1999;13(1):125–32.
Goncalves RC, Nunes MPT, Cukier A, et al. Effects of an aerobic physical training program on psychosocial characteristics, quality-of-life, symptoms and exhaled nitric oxide in individuals with moderate or severe persistent asthma. Rev Bras Fisioter. 2008;12(2):127–35.
Graff-Lonnevig V, Bevegard S, Eriksson BO, et al. Two years’ follow-up of asthmatic boys participating in a physical activity programme. Acta Paediatr Scand. 1980;69(3):347–52.
Haas F, Pasierski S, Levine N, et al. Effect of aerobic training on forced expiratory airflow in exercising asthmatic humans. J Appl Physiol. 1987;63(3):1230–5.
Henriksen JM, Nielsen TT, Dahl R. Effects of physical training on plasma citrate and exercise-induced asthma. Scand J Clin Lab Invest. 1981;41(3):225–9.
Henriksen JM, Nielsen TT. Effect of physical training on exercise-induced bronchoconstriction. Acta Paediatr Scand. 1983;72(1):31–6.
Hildenbrand K, Nordio S, Freson T, et al. Development of an aquatic exercise training protocol for the asthmatic population. Int J Aquatic Res Educ. 2010;4:278–99.
Hirt M. Physical conditioning in asthma. I. Preliminary results. Ann Allergy. 1964;22(5):229–37.
Hirt M. Physical conditioning in asthma. II. Changes in VEBTPS, VO2, and external work. Int Arch Allergy Appl Immunol. 1965;26(4):191–203.
Holzer FJ, Schnall R, Landau LI. The effect of a home exercise programme in children with cystic fibrosis and asthma. Aust Paediatr J. 1984;20(4):297–301.
Huang SW, Veiga R, Sila U, et al. The effect of swimming in asthmatic children—participants in a swimming program in the city of Baltimore. J Asthma. 1989;26(2):117–21.
King MJ, Noakes DT, Weinberg EG. Physiological effects of a physical training program in children with exercise-induced asthma. Pediatr Exerc Sci. 1989;1(2):137–44.
Leisti S, Finnila MJ, Kiuru E. Effects of physical training on hormonal responses to exercise in asthmatic children. Arch Dis Child. 1979;54(7):524–8.
Matsumoto I, Araki H, Tsuda K, et al. Effects of swimming training on aerobic capacity and exercise induced bronchoconstriction in children with bronchial asthma. Thorax. 1999;54(3):196–201.
Mendes FA, Goncalves RC, Nunes MP, et al. Effects of aerobic training on psychosocial morbidity and symptoms in patients with asthma: a randomized clinical trial. Chest. 2010;138(2):331–7.
Mendes FA, Almeida FM, Cukier A, et al. Effects of aerobic training on airway inflammation in asthmatic patients. Med Sci Sports Exerc. 2011;43(2):197–203.
Millman M, Grundon WG, Kasch F, et al. Controlled exercise in asthmatic children. Ann Allergy. 1965;23(5):220–5.
Neder JA, Nery LE, Silva AC, et al. Short term effects of aerobic training in the clinical management of moderate to severe asthma in children. Thorax. 1999;54(3):202–6.
Nickerson BG, Bautista DB, Namey MA, et al. Distance running improves fitness in asthmatic children without pulmonary complications or changes in exercise-induced bronchospasm. Pediatrics. 1983;71(2):147–52.
Orenstein DM, Reed ME, Grogan FT Jr, et al. Exercise conditioning in children with asthma. J Pediatr. 1985;106(4):556–60.
Petersen KH, McElhenney TR. Effects of a physical fitness program upon asthmatic boys. Pediatrics. 1965;35(2):295–9.
Rothe T, Kohl C, Mansfeld HJ. Controlled study of the effect of sports training on cardiopulmonary functions in asthmatic children and adolescents [in German]. Pneumol. 1990;44(9):1110–4.
Schmidt SM, Ballke EH, Nuske F, et al. Effect of ambulatory sports therapy on bronchial asthma in children [in German]. Pneumol. 1997;51(8):835–41.
Schnall R, Ford P, Gillam I, et al. Swimming and dry land exercises in children with asthma. Aust Paediatr J. 1982;18(1):23–7.
Scichilone N, Morici G, Zangla D, et al. Effects of exercise training on airway closure in asthmatics. J Appl Physiol. 2012;113(5):714–8.
Shaw BS, Shaw I. Pulmonary function and abdominal and thoracic kinematic changes following aerobic and inspiratory resistive diaphragmatic breathing training in asthmatics. Lung. 2011;189(2):131–9.
Sidiropoulou MP, Fotiadou EG, Tsimaras VK, et al. The effect of interval training in children with exercise-induced asthma competing in soccer. J Strength Cond Res. 2007;21(2):446–50.
Silva CS, Torres LA, Rahal A, et al. Comparison of morning and afternoon exercise training for asthmatic children. Braz J Med Biol Res. 2006;39(1):71–8.
Sly RM, Harper RT, Rosselot I. The effect of physical conditioning upon asthmatic children. Ann Allergy. 1972;30(2):86–94.
Svenonius E, Kautto R, Arborelius M Jr. Improvement after training of children with exercise-induced asthma. Acta Paediatr Scand. 1983;72(1):23–30.
Swann IL, Hanson CA. Double-blind prospective study of the effect of physical training on childhood asthma. In: Oseid A, Edwards A, editors. The asthmatic child—in play and sport. London: Pitman Books Limited; 1983. p. 318–25.
Tanizaki Y, Komagoe H, Sudo M, et al. Swimming training in a hot spring pool as therapy for steroid-dependent asthma [in Japanese]. Arerugi. 1984;33(7):389–95.
Tanizaki Y. Improvement of ventilatory function by spa therapy in patients with intractable asthma. Acta Med Okayama. 1986;40(1):55–9.
Turner S, Eastwood P, Cook A, et al. Improvements in symptoms and quality of life following exercise training in older adults with moderate/severe persistent asthma. Respiration. 2011;81(4):302–10.
van Veldhoven NHM, Wijnroks L, Bogaard JM, et al. Effects of an exercise program (PEP) for children with asthma: results of a pilot study. Pediatr Exerc Sci. 2000;12(3):244–57.
van Veldhoven NH, Vermeer A, Bogaard JM, et al. Children with asthma and physical exercise: effects of an exercise programme. Clin Rehabil. 2001;15(4):360–70.
Varray AL, Mercier JG, Prefaut CG. Individualized training reduces excessive exercise hyperventilation in asthmatics. Int J Rehabil Res. 1995;18(4):297–312.
Weisgerber MC, Guill M, Weisgerber JM, et al. Benefits of swimming in asthma: effect of a session of swimming lessons on symptoms and PFTs with review of the literature. J Asthma. 2003;40(5):453–64.
Weisgerber M, Webber K, Meurer J, et al. Moderate and vigorous exercise programs in children with asthma: safety, parental satisfaction, and asthma outcomes. Pediatr Pulmonol. 2008;43(12):1175–82.
Wicher IB, Ribeiro MA, Marmo DB, et al. Effects of swimming on spirometric parameters and bronchial hyperresponsiveness in children and adolescents with moderate persistent atopic asthma. J Pediatr (Rio J). 2010;86(5):384–90.
van den Bemt L, Kooijman S, Linssen V, et al. How does asthma influence the daily life of children? Results of focus group interviews. Health Qual Life Out. 2010; 8:5.
Crapo RO, Casaburi R, Coates AL, et al. Guidelines for methacholine and exercise challenge testing–1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161(1):309–29.
Anderson SD, Silverman M, Konig P, et al. Exercise-induced asthma. Br J Dis Chest. 1975;69(1):1–39.
Anderson SD, Rodwell LT, Du Toit J, et al. Duration of protection by inhaled salmeterol in exercise-induced asthma. Chest. 1991;100(5):1254–60.
Anderson SD, Daviskas E. The mechanism of exercise-induced asthma is …. J Allergy Clin Immunol. 2000;106(3):453–9.
Joos GF, O’Connor B, Anderson SD, et al. Indirect airway challenges. Eur Respir J. 2003;21(6):1050–68.
Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–15.
Hewitt M, Estell K, Davis IC, et al. Repeated bouts of moderate-intensity aerobic exercise reduce airway reactivity in a murine asthma model. Am J Respir Cell Mol Biol. 2010;42(2):243–9.
Silva RA, Vieira RP, Duarte AC, et al. Aerobic training reverses airway inflammation and remodelling in an asthma murine model. Eur Respir J. 2010;35(5):994–1002.
Vieira RP, Claudino RC, Duarte AC, et al. Aerobic exercise decreases chronic allergic lung inflammation and airway remodeling in mice. Am J Respir Crit Care Med. 2007;176(9):871–7.
Vieira RP, de Andrade VF, Duarte AC, et al. Aerobic conditioning and allergic pulmonary inflammation in mice. II. Effects on lung vascular and parenchymal inflammation and remodeling. Am J Physiol Lung Cell Mol Physiol. 2008;295(4):L670–9.
Murray CS. Can inhaled corticosteroids influence the natural history of asthma? Curr Opin Allergy Clin Immunol. 2008;8(1):77–81.
Durrani SR, Viswanathan RK, Busse WW. What effect does asthma treatment have on airway remodeling? Current perspectives. J Allergy Clin Immunol. 2011;128(3):439–48; quiz 449–50.
Currell K, Jeukendrup AE. Validity, reliability and sensitivity of measures of sporting performance. Sports Med. 2008;38(4):297–316.
Acknowledgments
We would like to thank Dr. Nicolette van Veldhoven and Augusto Mendes for providing us with raw or unpublished data. We are also grateful to Yuya Sugano, Veronique Werkle and Christoph Brändle for helping with publications in Japanese, Tomas Hejhal with Czech, Dr. Peter Rasmussen with Danish, Swedish and Norwegian, Laura Tsialkagaras with Greek, Lajos Pécsi with Hungarian, Dr. Arezoo Daryadel with Persian, Dr. Michal Rajski with Polish, Dr. Alexander Akhmedov and Ekaterina Kurakevich with Russian, and Dr. Branko Simic with Serbian publications. We gratefully thank Dr. Ulrike Held for the support with statistical analysis as well as the ETH Zurich and the Swiss Federal Office of Sport (grant no. 12-07) for providing financial support.
The authors have no conflicts of interest to declare that are directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Eichenberger, P.A., Diener, S.N., Kofmehl, R. et al. Effects of Exercise Training on Airway Hyperreactivity in Asthma: A Systematic Review and Meta-Analysis. Sports Med 43, 1157–1170 (2013). https://doi.org/10.1007/s40279-013-0077-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-013-0077-2