Abstract
Background
Because growing bone possesses a greater capacity to adapt to mechanical loading than does mature bone, it is important for girls to engage in weight-bearing activities, especially since the prevalence of osteoporosis among older women is considerably higher than that of older men. In recent years, the osteogenic potential of weight-bearing activities performed by children and adolescents has received increasing attention and accumulating evidence suggests that this type of activity may improve bone health prior to adulthood and help prevent osteoporosis later in life.
Objective
Because previous interventions have varied with respect to the exercise parameters studied and sometimes produced conflicting findings, this meta-analysis was undertaken to evaluate the impact of weight-bearing exercise on the bone health of female children and adolescents and quantify the influence of key moderating variables (e.g. pubertal stage, exercise mode, intervention strategy, exercise duration, frequency of exercise, programme length and study design) on skeletal development in this cohort.
Methods
A comprehensive literature search was conducted using databases such as PubMed, MEDLINE, CINAHL, Web of Science, Physical Education Index, Science Direct and ProQuest. Search terms included ‘bone mass’, ‘bone mineral’, ‘bone health’, ‘exercise’ and ‘physical activity’. Randomized- and non-randomized controlled trials featuring healthy prepubertal, early-pubertal and pubertal girls and measurement of areal bone mineral density (aBMD) or bone mineral content (BMC) using dual energy x-ray absorptiometry were examined. Comprehensive Meta-Analysis software was used to determine weighted mean effect sizes (ES) and conduct moderator analyses for three different regions of interest [i.e. total body, lumbar spine (LS), and femoral neck].
Results
From 17 included studies, 72 ES values were retrieved. Our findings revealed a small, but significant influence of weight-bearing exercise on BMC and aBMD of the LS (overall ES 0.19; 95 % confidence interval (CI) 0.05, 0.33 and overall ES 0.26, 95 % CI 0.09, 0.43, respectively) and BMC of the femoral neck (ES 0.23; 95 % CI 0.10, 0.36). For both aBMD and BMC, overall ES was not affected by any moderator variables except frequency of exercise, such that weight-bearing activity performed for more than 3 days per week resulted in a significantly greater ES value for LS aBMD compared with programmes lasting 3 or fewer days per week [Cochran’s Q statistic (Qbetween) = 4.09; p < 0.05].
Conclusion
The impact of weight-bearing activities seems to be site specific, and a greater frequency of weight-bearing activities is related to greater aBMD of LS in growing girls. Future investigations are warranted to better understand the dose-response relationship between weight-bearing activity and bone health in girls and explore the mediating role of pubertal status in promoting skeletal development among female youth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The prevalence of osteoporosis in the US among women aged 50 years and older has increased by 60 % from 1967 to 1999 [1]. More than 1.5 million vertebral, hip and wrist fractures occur annually and one in two women and one in four men aged 50 years and older will experience an osteoporosis-related fracture in their lifetime [2]. In addition, vertebral and hip fractures are associated with a greater mortality rate during the first 5 years after the occurrence of a fracture [3] and the impact of these types of bone injuries on economic costs and quality of life are added burdens of osteoporosis-related fractures [4, 5].
As peak bone mass is a major determinant of bone mass later in life [6], it is critical to maximize the potential of reaching the most optimal value through management of potential factors contributing to bone loss and osteoporosis-related fractures. Research has shown that environmental factors (e.g. sex hormones, dietary intake, calcium and vitamin D intake, medication use, sedentary lifestyle, smoking and alcohol use) account for approximately 25 % of the variance in peak bone mass [2], and from 50 % to 85–90 % of this variance can be explained by genetic factors (e.g. sex, age, body size, ethnicity and family history) [7, 8]. While peak bone mass is typically attained by 30 years of age, about 90 % of peak bone mass is reached by the age of 18 years in females and 20 years in males [2, 9]. Similar to dietary interventions that have been shown to improve bone health [10], physical activity and exercise during childhood have been identified as primary methods of preventing osteoporosis and enhancing skeletal development in children and adolescents [11–13].
Published findings indicate that high-impact physical activities (e.g. gymnastics) increase skeletal growth in pre-pubertal girls [14, 15] and that bone mineral content (BMC) and bone mineral density (BMD) of young gymnasts are higher compared with age-matched non-exercising controls [15] and swimmers [14]. It is well known that regional bone adaptations occur when intensive weight-bearing activity is initiated in childhood [16]. Additionally, retrospective studies documenting areal BMD (aBMD) values of former female gymnasts and age-matched controls have reported the persistence of effects on bone associated with previous participation in gymnastics [17, 18]. While findings from several exercise intervention studies have highlighted a positive influence on aBMD in pre-pubertal and early-pubertal females [19–21], some investigations have reported no effect of exercise on aBMD in girls [22–24] or yielded negative findings [25–27]. Hence, questions remain concerning the potential impact of activity and exercise programmes on bone development in female children and adolescents.
At present, relatively little is known regarding the impact of exercise intervention-related factors that may influence bone growth in girls who are still undergoing physical growth and maturation [13, 28–30]. Consequently, a series of meta-analyses were conducted to quantify the role of weight-bearing exercise (WBE) on skeletal development in young females and examine the moderating influence of selected variables (e.g., pubertal stage, mode of exercise, intervention strategy, exercise duration, exercise frequency and programme length) on the relationship between WBE and bone mass in female youth.
2 Methods
2.1 Search Procedure and Inclusion Criteria
The following databases were identified and searched from 1992 to December 2010: PubMed, MEDLINE, CINAHL, Web of Science, Physical Education Index, Science Direct and ProQuest. The initial reporting of hours of weight-bearing activity as a significant predictor of radius and hip BMD among children began in 1991 [31]; thus, the literature search start date was set at 1992 for the present analyses. Search terms included ‘bone mass’, ‘bone mineral’, ‘bone health’, ‘exercise’ and ‘physical activity’. Reference lists of the retrieved studies and review articles [13, 28–30] were scrutinized to identify additional eligible studies and the literature search was extended to available abstracts, theses and dissertations in the English language.
While acknowledging the contemporary use of magnetic resonance imaging and peripheral quantitative tomography (pQCT) to quantify changes in bone geometry, measures of aBMD and BMC obtained from dual-energy x-ray absorptiometry (DXA) or dual photon absorptiometry (DPA) were chosen as outcome measures because of their widespread use in the research literature [32] and their clinical relevance to fracture risk at specific body sites [33–35]. Potential studies for inclusion in the meta-analysis were identified based on the following criteria: (1) description of a weight-bearing exercise trial comparing intervention and controls groups in a randomized or non-randomized setting; (2) enrollment of healthy girls with no previous experience in organized physical training programmes; (3) indication of pubertal status; and (4) measurement of outcome variables such as BMC and/or aBMD of the total body (TB), lumbar spine (LS), or femoral neck (FN). Studies were excluded from the analysis when (1) the study population was previously exposed to organized physical activity programmes; (2) only radial aBMD and BMC were reported; (3) no indication of pubertal status was provided; and (4) boys and girls were combined in the analysis. In addition, when absolute values of changes in aBMD or BMC from pre- to post-intervention could not be calculated from available studies, they were excluded from the meta-analysis. Two independent reviewers screened studies that were potentially appropriate based on the aforementioned inclusion criteria, and agreement on eligibility was achieved for all studies that were subsequently included in the meta-analysis. Thus, reliability coefficients for agreement between two reviewers were unnecessary in the study selection process.
2.2 Assessment of Methodological Quality
Full texts of included studies were reviewed independently for methodological quality assessment using the Downs and Black checklist [36]. This checklist was developed for both randomized and non-randomized comparative studies and consists of 27 criteria organized under four domains (reporting, external validity, internal validity and power). The total maximum score on the checklist was 30. All discrepancies for quality rating between reviewers were resolved by consensus.
2.3 Data Extraction and Coding
The primary outcome variables were defined as changes in aBMD (g/cm2) and BMC (g) across three different regions of interest, including TB, LS, and FN. These variables were assessed using DXA or DPA, two imaging techniques featuring a high degree of precision and accuracy and low radiation exposure [32]. Because an increase in BMC may not necessarily reflect a rise in aBMD during periods of bone growth, and since discrepancies in levels of bone acquisition have been reported between aBMD and BMC [33], both variables were analysed separately. Moreover, because differences between aBMD and BMC can vary by location [34, 35], six meta-analyses (i.e. aBMD and BMC measures taken at the TB, LS and FN) were performed and necessary statistics computed to derive effect sizes (ES).
In four studies, pre- and early-pubertal stages were combined and reported. One of the four studies in which pre- and early-pubertal girls were combined in the statistical analysis was classified as pre-pubertal (80 % of the participants were identified as being prepubertal), while the remaining three studies were classified as early pubertal. To determine the influence of moderator variables on the overall ES values of aBMD and BMC for each region of interest, we extracted six variables (pubertal status, exercise mode, intervention strategy, exercise duration, frequency of exercise and programme length) from each included study. ‘Pubertal status’, classified as either prepubertal (Tanner stage I), early pubertal (Tanner stages II and III), or pubertal (Tanner stages IV and V), has been linked to skeletal mineralization [37] and reflects the widespread use of Tanner staging found in the studies under consideration [13]. We acknowledge that changes in pubertal status occurring over the course of a given study could potentially influence the amount of change observed in aBMD and BMC. However, of the 11 studies that included pre- or early-pubertal girls at baseline, two studies did not report changes in pubertal status, four studies reported that participants remained in the same pubertal stage from baseline to post-intervention assessment and five studies indicated that some participants changed from pre- to early-pubertal or early pubertal to pubertal stages. Among these latter five studies, less than 40 % of participants in each study advanced from one maturation stage to the next, except for one investigation [38], wherein 62 % of the girls in the control group and 41 % of the girls in the intervention group advanced from a pre- to an early-pubertal stage. Consequently, we defined pubertal classification as the pubertal status reported at the start of each study. ‘Exercise mode’ was classified as either plyometric (e.g. jumping, hopping or skipping) or non-plyometric training, based on evidence indicating that the type of skeletal loading can affect the magnitude of change in bone parameters [39]. ‘Intervention strategy’ was categorized as school based or non-school based and ‘exercise duration’ was classified based on a position stand from the American College of Sports Medicine (ACSM) [39] stating that 10–20 min of impact activities two or more times per day for at least 3 days per week should be performed to increase bone mineral growth in children and adolescents. Hence, exercise duration was categorized as either less than 60 min per week or 60 min or more per week. ‘Frequency’ of exercise was categorized as either more than 3 days per week of weight-bearing exercise, which aligns with current physical activity and bone health guidelines [39], or less than or equal to 3 days per week of weight-bearing exercise. ‘Programme length’ was classified as less than 12 months or 12 months or longer to ascertain whether variation in the length of the activity intervention produced differential effects on bone mass. Given the lack of consistency among studies in clearly delineating exercise intensity, this parameter was not included as a moderator variable.
2.4 Study Characteristics
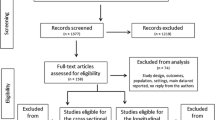
A total of 95 studies, including 61 publications, 22 abstracts, and 12 dissertations and theses, were identified for further review. Fifty-three studies were eliminated due to duplication or an inability to meet the inclusion criteria mentioned earlier. Contacts were made with corresponding authors of each remaining study to obtain relevant statistics. Among the 42 remaining studies, 25 with insufficient statistics to compute ES values were excluded from further analysis (please refer to Fig. 1 for a schematic flow diagram illustrating inclusion of potential studies). Overall, 17 studies met all inclusion criteria (see Table 1), with ten classified as randomized controlled trials (RCTs) and seven classified as non-RCTs.
Schematic flow diagram describing exclusions of potential studies and final number of included studies [71]
A total of 72 ES values were retrieved for inclusion into six different meta-analyses [TB: 16 ES from 15 studies for BMC and 10 ES from 9 studies for aBMD (Fig. 2); FN: 12 ES from 11 studies for BMC and 9 ES from 8 studies for aBMD (Fig. 3); LS: 15 ES from 14 studies for BMC and 10 ES from 9 studies for aBMD (Fig. 4)]. The methodological quality of included studies ranged from 16 to 24 [mean ± standard deviation (SD) 20.95 ± 2.68], based on the 30-point Downs and Black checklist [34]. Average scores for each measurement domain were fairly robust (reporting: 8.73 of 12; external validity: 2.53 of 3; internal validity: 9.68 of 13; power: 0.78 out of 2). However, there was a significant difference in the total methodological quality score between RCT (22.83 ± 2.66; n = 12) and non-RCT (19.86 ± 1.77; n = 7), t (16.54) = −3.06, p = 0.008, d = 1.32. Additional moderator analyses were conducted to quantify the effect of study designs (i.e. RCT and non-RCT) on overall ES values across regions of interest.
2.5 Statistical Analysis
To compute ES measures, mean group differences in aBMD and BMC between pre- and post-intervention time periods were divided by a pooled SD using SDs of pre-intervention values for each group. In situations in which only the SD of changes in aBMD and BMC between pre- and post-intervention time periods was available, an alternative estimation for pooled SD was applied based on the assumption of a large-sized correlation (r = 0.5) between pre- and post-intervention variables for each group. Standardized mean differences adjusted for sampling error were subsequently estimated as a measure of individual ES by assigning more weight to studies with larger sample sizes (see Lipsey and Wilson [40] for detailed information regarding ES calculations).
Weighted mean ES values, along with 95 % CIs, were estimated using a random-effects model for all outcomes. Heterogeneity of weighted mean ES was examined through moderator analyses using Cochran’s Q statistics (Q) [41], under the null hypothesis of homogenous weighted mean ES among sublevels of each moderator. ES values were described as small (0.2), moderate (0.5) or large (0.8), based on Cohen’s criteria [42]. A significance test using Fisher’s Z-transformation was performed for each weighted mean ES under the null hypothesis of no difference between weighted mean ES and zero. Given the number and extensive nature of variables examined in the moderator analyses, we elected to use a more liberal alpha level (i.e. 0.05) in this set of exploratory analyses. All statistical analyses were conducted using Comprehensive Meta-Analysis Software version 2.2.040 (BioStat, Inc., Englewood, NJ, USA), a user-friendly program that provides a complete set of analytical features to perform meta-analyses [43].
Funnel plots to assess potential publication bias were not constructed, as all but one included study was published in peer-reviewed journals. It is also possible that funnel plots would have been asymmetric, which would increase the potential for bias in effect sizes due to moderator variables compared with bias attributable solely to publication status. Moreover, the existence of asymmetric funnel plots could have led to greater heterogeneity of overall ES values [44].
3 Results
3.1 Overall Effect Sizes (ES)
Table 2 provides weighted mean ES values for weight-bearing exercise for aBMD and BMC for each region of interest. In general, results indicated that all weighted mean ES values were small in magnitude. Weighted mean ES for aBMD of LS (ES 0.26; 95 % CI 0.09, 0.43; p = 0.004), BMC of LS (ES 0.19; 95 % CI 0.05, 0.33, p = 0.007) and BMC of FN (ES 0.23; 95 % CI 0.10, 0.36; p = 0.001) were statistically significant, while other mean ES values contained the absolute zero value within the 95 % CI, indicating no effect of weight-bearing exercise on bone mineral acquisition. Forest plots are presented in Figs. 2, 3 and 4 to illustrate the individual effect size value for each study for aBMD and BMC at FN, LS, and TB.
3.2 Moderator Analyses
3.2.1 Total Body Bone Mineral Content (BMC) and Areal Bone Mineral Density (aBMD)
Table 3 depicts results of moderator analyses for BMC and aBMD measures of TB. No significant heterogeneity was detected in any moderator variable, meaning that pubertal stage, mode of exercise, intervention strategy, exercise duration, frequency of exercise and programme length did not exert a significant impact on the overall weighted mean ES values of TB BMC and aBMD. Although heterogeneity in ES values across levels of pubertal status was not observed, a significant difference in TB BMC was noted between the control and intervention groups (ES 0.88; p < 0.05), such that early-pubertal girls in the intervention group displayed a greater TB BMC compared with the control group.
3.2.2 Femoral Neck BMC and aBMD
Table 4 depicts results of moderator analyses for BMC and aBMD measures of the FN. Similar to findings for moderator analyses of TB BMC and aBMD, significant heterogeneity in ES values was not observed across levels of any of the moderator variables that could have affected the overall ES values of FN BMC and aBMD. To extend the interpretation of our results, individual ES values per level of each moderator variable were examined. While no significant difference was detected in FN aBMD between intervention and control groups per level of each moderator variable, significant differences in FN BMC between control and intervention groups were noted for specific levels of all moderator variables. In terms of pubertal stage, studies that featured early-pubertal and pubertal girls reported significant differences in FN BMC between control and the intervention groups (ES 0.10 and ES 0.21, respectively). In considering mode of exercise, while no significant difference was present in FN BMC between intervention and control groups relative to non-plyometric exercise programmes, studies that incorporated plyometric exercise displayed a greater FN BMC in the intervention group compared with the control group (ES 0.23). Moreover, significant differences in FN BMC values between intervention and control groups were observed among interventions featuring (a) school-based programmes (ES 0.23); (b) an exercise duration of less than 60 min per week (ES 0.36); (c) a frequency of exercise of less than or equal to 3 days per week (ES 0.32); and (d) programme lengths of less than 12 months and more than or equal to 12 months (ES 0.30 and ES 0.18, respectively).
3.2.3 Lumbar Spine BMC and aBMD
As shown in Table 5, while no significant moderator effect was present for LS BMC, a significant moderator effect of exercise frequency was noted for LS aBMD (Qbetween 4.09, p < 0.05). Specifically, studies that included weight-bearing exercises on more than 3 days per week (ES 0.52) resulted in a significantly greater LS aBMD compared with studies incorporating weight-bearing exercises for 3 or fewer days per week (ES 0.18).
When ES values were examined per level of each moderator variable relative to BMC and aBMD at LS, significant differences in LS BMC and aBMD values were observed between intervention and control groups for all moderator variables. More specifically, a significant difference was detected in LS BMC between control and intervention groups for early-pubertal status, insofar as studies featuring weight-bearing physical activity performed by early-pubertal girls showed greater LS BMC in the intervention group compared with the control group (ES 0.27), while no significant difference in LS BMC between groups was present for prepubertal and pubertal stages (ES 0.17 and ES 0.09, respectively). In contrast, a significant between-group difference was noted for LS aBMD for prepubertal stage, wherein studies including prepubertal girls reported greater LS aBMD in the intervention group compared with the control group (ES 0.28), while no statistical significance was observed for early-pubertal and pubertal stages. Regarding exercise mode, a significant difference was noted in LS BMC and aBMD between intervention and control groups, such that studies that implemented plyometric exercises displayed greater LS BMC and aBMD values in the intervention group relative to the control group (ES 0.19 and ES 0.30, respectively). With respect to intervention strategy, there was a significant between-group difference in LS BMC and aBMD, meaning that school-based programmes resulted in greater LS BMC and aBMD values in the intervention group compared with the control group (ES 0.27 and ES 0.34, respectively). While a significant between-group difference in LS BMC was observed for exercise programmes of less than 60 min per week (ES 0.32), exercise programmes of greater than or equal to 60 min per week led to higher LS aBMD values in the intervention group than in the control group (ES 0.30). Though significant heterogeneity was not present for exercise frequency in LS BMC, weight-bearing exercise occurring more than 3 days per week resulted in greater LS BMC in the intervention group compared with the control group (ES 0.29). Lastly, a programme length of longer than or equal to 12 months resulted in a significant difference in LS BMC and aBMD values between intervention and control groups (ES 0.25 and ES 0.31, respectively).
3.2.4 Effect of Study Designs on Overall ES
For all three regions of interest (i.e., TB, FN, and LS), there were no statistically significant differences in overall weighted mean ES values for BMC and aBMD between randomized control trials and non-randomized control trials (See Table 6).
4 Discussion
4.1 Overall ES
The primary goal of this meta-analysis was to document the impact of weight-bearing exercise on aBMD and BMC at the TB, LS, and FN in female children and adolescents. Among the three overall weighted ES values that were statistically significant, the largest ES was reported for aBMD of LS, followed by BMC of LS and BMC of FN. Our findings support previous research highlighting the advantage of performing high-impact, weight-bearing activity on bone mineral accrual during prepubescence [14, 15] and imply that even non-competitive levels of weight-bearing exercise can exert a positive influence on the bone health of young girls.
A clear majority of studies (13 of 17) included in our analysis featured plyometric training as the intervention of choice, and results from these investigations demonstrated that physical activities such as jumping, hopping and skipping produced a more favourable effect on bone mass at the LS compared with the FN. In general, resistance exercise is thought to be more effective in augmenting bone mass at the LS, while weight-bearing activity has been linked to increased bone mass at the FN [45]. Because plyometric training yielded gains in both BMC and aBMD at the LS and BMC at the FN, this exercise mode appears to combine aspects of both resistance exercise and weight-bearing activity. Given that bone responds to two types of mechanical loading (i.e. ground-reaction forces and muscle-joint forces) [45], plyometric-type training may have increased trunk muscle strength, exceeded the minimal strain threshold for enhanced bone remodelling [46] and generated greater muscle-joint forces, resulting in improved BMC and aBMD at the LS. In addition, changes in TB measures of BMC and aBMD due to high-impact, weight-bearing activity were less noticeable compared with LS and FN measures of BMC and aBMD. It is possible that this difference in response may be related to the regional nature of bone response [34, 35], meaning that bone regions that experience focused mechanical stress tend to show greater bone development compared with areas that do not directly receive such stress. Because exercises described in this analysis involved the lower extremities, this may explain the observation of greater increases in BMC and aBMD of LS and BMC of FN compared with TB. Viewed collectively, the aforementioned findings support the notion that mechanical loading of bone through plyometric exercise, particularly at the lumbar region, heightens the sensitivity of bone cells and enhances osteogenesis in maturing female youth [16, 47].
4.2 Moderator Analyses
4.2.1 Total Body BMC and aBMD
For TB, heterogeneity was not detected among moderator variables that could have impacted the overall weighted mean ES values of BMC and aBMD. More specifically, overall ES values of TB BMC and aBMD were not affected by any differences in ES values across levels of pubertal status. Similarly, overall ES values of TB BMC and aBMD were not influenced by differences in ES values across levels of exercise mode (plyometric vs. non-plyometric), intervention strategy (school-based vs. non-school based), exercise duration (<60 vs. ≥60 min per week), frequency of exercise (≤3 vs. >3 days per week), and programme length (<12 vs. ≥12 months). While no significant heterogeneity in pubertal status was observed relative to the overall ES of TB aBMD, a significant difference in TB aBMD was noted between control and intervention groups when only the early-pubertal stage was considered. While speculative, this finding implies that early puberty is a key maturational stage during which the TB bone density of females may be enhanced by performing weight-bearing activities.
4.2.2 Femoral Neck BMC and aBMD
Results from the moderator analysis for FN BMC demonstrated no significant heterogeneity in overall ES relative to pubertal status. However, the presence of a significant difference in FN BMC between intervention and control groups during early puberty and puberty suggests that these stages of sexual maturation may be ideal time periods for girls to increase BMC at the femoral neck, a common site of osteoporosis that is sensitive to weight-bearing activity [2, 48]. This finding is partly consistent with data showing an increased responsiveness of bone to mechanical loading during pre- and early-pubertal periods [12, 29, 47] and implies that BMC at the FN may continue to rise throughout puberty. According to Gunter and colleagues [11], childhood and adolescence may be particularly opportune periods to engage in weight-bearing exercise to maximize bone mass, particularly with respect to BMC at the FN. The benefits of weight-bearing exercise during growth in optimizing peak bone mass in younger and older girls have also been noted in reviews by Ondrak and Morgan [29] and McDevitt and Ahmed [12].
Similar to findings for pubertal status, homogeneity was observed for mode of exercise, implying that variation in the manner in which impact loading is applied does not strongly affect overall ES values in each region of interest. When combined with a small, but significant effect of weight-bearing exercise on the BMC of FN, these results add credence to the idea that a variety of weight-bearing physical activities such as plyometrics (i.e. jumping, hopping and skipping), resistance exercise (i.e. weight lifting and strength training) or aerobic exercise (i.e. walking and running) may promote skeletal development in female youth. However, when examining specific ES values for each level of pubertal status, a significant group difference in BMC of FN was found in studies employing plyometric training. Caution is warranted in interpreting this finding, however, as only 4 of 17 studies included non-plyometric exercises. Nonetheless, because most studies featured plyometric training and the significant overall ES value for BMC at FN may have potentially reflected this training mode, high-intensity weight-bearing activity (i.e. plyometric exercise) may be a more effective means of augmenting bone growth in younger and older girls compared with general resistance training. Interestingly, the notion that plyometric exercise may foster bone mineral accrual is consistent with ACSM bone health recommendations for children and adolescents [39].
Despite the presence of homogeneity in intervention strategy, a group difference was present in BMC at FN between control and intervention groups relative to school-based studies, such that the intervention group exhibited greater BMC at the FN compared with the control group. This finding suggests that school-based programmes may be an effective approach in improving bone health in young girls. While it is not possible to identify specific school-based factors that may promote gains in BMC among female youth, we speculate that the availability of a developmentally appropriate physical education curriculum featuring plyometric-type activities and high-impact sports (e.g. running, jumping rope, basketball, volleyball) may facilitate ongoing skeletal growth and development in female youth. It is also possible that structured and unstructured physical activity occurring during recess, before and after school, during breaks from classroom instruction or as part of intramural programming, may lead to better bone health in female children and adolescents. Based on data suggesting that subtle gains in bone mass made during the first decade of life may be an important factor in reducing the risk of adult fractures [49–51], future research is needed to clarify the mediating role of regular participation in weight-bearing and impact-loading activities in school settings among younger and older girls.
In considering exercise duration, frequency of exercise, and programme length, moderator analyses revealed no significant heterogeneity in ES values between levels of these moderator variables. Because the studies we examined lacked sufficient data regarding intensity of exercise, additional efforts should be directed towards quantifying the unique and interactive effects of this quartet of factors [52–54] on the overall influence of weight-bearing exercise on bone health in pre-pubertal and pubertal girls.
4.2.3 Lumbar Spine BMC and aBMD
The absence of heterogeneity for pubertal status suggests that girls exhibiting varying levels of sexual maturation may benefit from weight-bearing exercise to enhance skeletal health. However, statistical analyses revealed a significant difference in LS BMC between intervention and control groups in studies featuring early-pubertal girls. When combined with results of moderator analyses for FN BMC, which indicated a significant difference between intervention and control groups for early-pubertal girls, this collection of findings suggests that the potential to increase BMC at the FN and LS may be greater during early puberty and reinforces the benefits of engaging in weight-bearing activity during this maturational period [11]. Although there was no statistically significant heterogeneity relative to exercise mode, studies that incorporated plyometric exercises displayed higher ES values for LS BMC and aBMD compared with the control group. This finding agrees with current ACSM physical activity guidelines for promoting bone health [39], which state that performing high-impact activities (e.g. jumping, gymnastics, soccer, and basketball) for 10–20 min at least twice a day on at least 2 days per week can enhance the accrual of bone mineral in children and adolescents [39].
No significant difference between school- and non-school-based interventions was detected, which could have influenced overall weighted ES values of BMC and aBMD at the LS. However, a significant difference was present between intervention and control groups for school-based interventions, which highlight the potential of this intervention strategy to influence skeletal development at the LS region. The absence of significant heterogeneity for weekly exercise duration and overall programme length also implies that activity programmes that vary with respect to these two moderator variables may lead to gains in bone strength. However, data from the present study revealing significant group differences in LS and FN BMC during weight-bearing exercise programmes lasting less than 60 min per week raises the intriguing possibility that shorter programmes comprising more frequent exercise bouts may also serve as a positive stimulus to bone growth [55] in female children and adolescents.
With respect to exercise frequency, overall ES values for LS aBMD among girls who engaged in weight-bearing exercise more than 3 days per week was significantly greater compared with ES values for girls who participated in this type of exercise for 3 or fewer days per week. This finding is consistent with ACSM recommendations indicating better bone health in children and adolescents who perform 10–20 min of high-impact activities 2 or more times per day for at least 3 days per week [39]. In light of evidence showing that programme interventions occurring more than 3 days per week and lasting for at least a year resulted in higher ES values for LS BMC and aBMD, a potentially effective approach to improving bone development in female youth might be to emphasize regular participation in longer-term, school- or community-based programmes incorporating weight-bearing exercise.
4.3 Strengths and Limitations
A primary strength of this meta-analysis was the consideration of regional bone responses to exercise [34]. Given the importance of biological maturation when assessing the impact of exercise interventions in female youth [56], another unique feature of our analysis was the use of pubertal status as a moderating variable to evaluate mean group differences in BMC and aBMD at specific regions of interest. With respect to limitations, it should be noted that assessment of pubertal staging varied across studies and may have contributed to a lack of precision in clearly differentiating the moderating effects of biological maturation on bone growth occurring from early to late puberty. In addition, it is acknowledged that constraints exist in the use of DXA to detect small changes in areal measures of BMD and BMC, and the availability of measurement tools such as pQCT, high-resolution pQCT, and magnetic resonance imaging may enhance the ability to detect subtle modifications in bone structure [11]. While the selection of non-randomized controlled exercise trials increased the number of studies included in our meta-analysis, these types of studies cannot fully control for confounding factors that may influence bone development [36, 57]. However, moderator analyses demonstrated that variation in study design (RCT vs. non-RCT) did not noticeably affect the overall weighted ES values of BMC and aBMD at the LS, FN, or TB. The exclusion of foreign-language journals and potentially relevant articles lacking sufficient data to compute ES values were other limitations of our analysis [58]. Because adequate nutrition accompanied by exercise may promote the attainment of peak bone mass [27–30], subsequent analyses documenting the combined impact of exercise and diet on bone health may aid in explaining differences in the magnitude of effectiveness between physical activity alone and combined activity and nutritional interventions [29, 30]. Sustainability of skeletal benefits due to weight-bearing activity during childhood is another topic for further investigation [59, 60].
5 Conclusions
Results from our meta-analyses indicate that the effect of weight-bearing exercise in prepubertal, early-pubertal and pubertal girls is small, but statistically significant, for BMC and aBMD at the LS and BMC at the FN. Examination of selected moderator variables on BMC and aBMD also revealed that participation in weight-supporting physical activity for more than 3 days per week exerted a significantly greater impact on lumbar aBMD compared with exercise performed for 3 or fewer days per week. Although many questions remain to be addressed relative to the impact of physical activity and exercise on promoting bone health in female children and adolescents, greater attention should be devoted towards developing and implementing site-specific activity and exercise programmes. Additional research is also needed to more fully describe the interplay among pubertal status and various parameters of physical activity (i.e. intensity, frequency, duration and mode) and overall programme length on selected descriptors of bone growth and development in female children and adolescents.
References
Ahlborg HG, Rosengren BE, Jarvinen TL, et al. Prevalence of osteoporosis and incidence of hip fracture in women: secular trends over 30 years. BMC Musculoskelet Disord. 2010;11:48.
National Institutes of Health. Osteoporosis and Related Bone Diseases National Resource Center [online]. http://www.niams.nih.gov/bone (Accessed 31 July 2011).
Ioannidis G, Papaioannou A, Hopman WM, et al. Relation between fractures and mortality: results from the Canadian Multicentre Osteoporosis Study. CMAJ. 2009;181:265–71.
Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–75.
Salaffi F, Cimmino MA, Malavolta N, et al. The burden of prevalent fractures on health-related quality of life in postmenopausal women with osteoporosis: the IMOF study. J Rheumatol. 2007;34:1551–60.
Hui SL, Slemenda CW, Johnston CC Jr. The contribution of bone loss to postmenopausal osteoporosis. Ostoporos Int. 1990;1:30–4.
Ralson SH, de Crombrugghe B. Genetic regulation of bone mass and susceptibility to osteoporosis. Genes Dev. 2006;20:2492–506.
Recker RR, Deng HW. Role of genetics in osteoporosis. Endocrine. 2002;17:55–66.
Haapasalo HH. Physical activity and growing bone: development of peak bone mass with special reference to the effects of unilateral physical activity. Ann Chir Gynaecol. 1998;87:250–2.
Johnston CC Jr, Miller JZ, Slemenda CW, et al. Calcium supplementation and increases in bone mineral density in children. N Engl J Med. 1992;327:82–7.
Gunter KB, Almstedt HC, Janz KF. Physical activity in childhood may be the key to optimizing lifespan skeletal health. Exerc Sport Sci Rev. 2012;40:13–21.
McDevitt H, Ahmed SF. Establishing good bone health in children. Paediatr Child Health. 2010;20:83–7.
Hind K, Burrows M. Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. Bone. 2007;40:14–27.
Courteix D, Lespessailles E, Jaffre C, et al. Bone mineral acquisition and somatic development in highly trained girl gymnasts. Acta Paediatr. 1999;88:803–8.
Ward KA, Roberts SA, Adams JE, et al. Bone geometry and density in the skeleton of pre-pubertal gymnasts and school children. Bone. 2005;36:1012–8.
Bachrach LK. Acquisition of optimal bone mass in childhood and adolescence. Trends Endocrinol Metab. 2001;12:22–8.
Bass S, Pearce G, Bradney M, et al. Exercise before puberty may confer residual benefits in bone density in adulthood: studies in active pre-pubertal and retired female gymnasts. J Bone Miner Res. 1998;13:500–7.
Kirchner EM, Lewis RD, O’Connor PJ. Effect of past gymnastics participation on adult bone mass. J Appl Phyisol. 1996;80:226–32.
Linden C, Ahlborg HG, Besjakov J, et al. A school curriculum-based exercise program increases bone mineral accrual and bone size in prepubertal girls: two-year data from the pediatric osteoporosis prevention (POP) study. J Bone Miner Res. 2006;21:829–35.
MacKelvie KJ, Khan KM, Petit MA, et al. A school-based exercise intervention elicits substantial bone health benefits: a 2-year randomized controlled trail in girls. Pediatrics. 2003;112:e447–52.
Valdimarsson O, Linden C, Johnell O, et al. Daily physical education in the school curriculum in prepubertal girls during 1 year is followed by an increase in bone mineral accrual and bone width: data from the prospective controlled Malmo pediatric osteoporosis prevention study. Calcif Tissue Int. 2006;78:65–71.
Hasselstrom HA, Karlsson MK, Hansen SE, et al. A 3-year physical activity intervention program increases the gain in bone mineral and bone width in prepubertal girls but not boys: the prospective Copenhagen school child interventions study (CoSCIS). Calcif Tissue Int. 2008;83:243–50.
McKay HA, Petit MA, Schutz RW, et al. Augmented trochanteric bone mineral density after modified physical education classes: a randomized school-based exercise intervention study in prepubescent and early pubescent children. J Pediatr. 2000;136:156–62.
Van Langendonck L, Claessens AL, Vlietinck R, et al. Influence of weight-bearing exercises on bone acquisition in prepubertal monozygotic female twins: a randomized controlled prospective study. Calcif Tissue Int. 2003;72:666–74.
Alwis G, Linden C, Stenevi-Lundgren S, et al. A one-year exercise intervention program in pre-pubertal girls does not influence hip structure. BMC Musculoskelet Disord. 2008;9:9.
Burr DB, Yoshikawa T, Teegarden K, et al. Exercise and oral contraceptive use suppress the normal age-related increase in bone mass and strength of the femoral neck in women 18–31 years of age. Bone. 2000;27:855–63.
French SA, Story M, Fulkerson JA, et al. Increasing weight-bearing physical activity and calcium-rich foods to promote bone mass gains among 9–11 year old girls: outcomes of the Cal-Girls study. Int J Behav Nutr Phys Act. 2005;2:8.
Karlsson MK, Nordqvist A, Karlsson C. Physical activity increases bone mass during growth. Food Nutr Res. 2008;52.
Ondrak KS, Morgan DW. Physical activity, calcium intake and bone health in children and adolescents. Sports Med. 2007;37:587–600.
Rizzoli R, Bianchi ML, Garabedian M, et al. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46:294–305.
Slemenda CW, Miller JZ, Hui SL, et al. Role of physical activity in the development of skeletal mass in children. J Bone Miner Res. 1991;6:1227–33.
Faulkner KG, Gluer CC, Majumdar S, et al. Noninvasive measurements of bone mass, structure, and strength: current methods and experimental techniques. AJR AM J Roentgenol. 1991;157:1229–37.
NIH Consensus Development Panel on Osteoporosis Prevention. Diagnosis, and therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–95.
Haapasalo H, Kannus P, Sievanen H, et al. Long-term unilateral loading and bone mineral density and content in female squash players. Calcif Tissue Int. 1994;54:249–55.
Kannus P, Haapasalo H, Sievanen H, et al. The site-specific effects of long-term unilateral activity on bone mineral density and content. Bone. 1994;15:279–84.
Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomized and non-randomized studies of health care interventions. J Epidemiol Commun Health. 1998;52:377–84.
Slemenda CW, Reister TK, Hui SL, et al. Influences of skeletal mineralization in children and adolescents: evidence for varying effects of sexual maturation and physical activity. J Pediatr. 1994;125:201–7.
MacKelvie KJ, McKay HA, Khan KM, et al. A school-based exercise intervention augments bone mineral accrual in early pubertal girls. J Pediatr. 2001;139:501–8.
Kohrt WM, Bloomfield SA, Little KD, et al. American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc. 2004;36:1985–96.
Lipsey MW, Wilson DB. Practical meta-analysis. Thousand Oaks: SAGE Publications, Inc.; 2001. p. 247.
Hedges LV, Olkin I. Statistical methods for meta-analysis. Orlando: Academic Press Inc.; 1985. p. 361.
Cohen J. Statistical power for the behavioral sciences. 2nd rev. ed. Hillsdale: Erlbaum; 1999. p. 569.
Bax L, Yu LM, Ikeda N, et al. A systematic comparison of software dedicated to meta-analysis of causal studies. BMC Med Res Methodol. 2007;7:40.
Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Morseth B, Emaus N, Jorgensen L. Physical activity and bone: the importance of the various mechanical stimuli for bone mineral density. A review. Nor Epidemiol. 2011;20:173–9.
Ferretti JL, Schiessl H, Frost HM. On new opportunities for absorptiometry. J Clin Densitom. 1998;1:41–53.
Greene DA, Naughton GA. Adaptive skeletal responses to mechanical loading during adolescence. Sports Med. 2006;36:723–32.
Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures: the Study of Osteoporotic Fractures Research Group. Lancet. 1993;34:72–5.
Gunter K, Baxter-Jones AD, Mirwald RL, et al. Impact exercise increases BMC during growth: an 8-year longitudinal study. J Bone Miner Res. 2008;23:986–93.
Gunter K, Baxter-Jones AD, Mirwarld RL, et al. Jump starting skeletal health: a 4-year longitudinal study assessing the effects of jumping on skeletal development in pre and circum pubertal children. Bone. 2008;42:710–8.
Janz KF, Letuchy EM, Eichenberger Gilmore JM, et al. Early physical activity provides sustained bone health benefits later in childhood. Med Sci Sports Exerc. 2010;42:1072–8.
Mackelvie KJ, Khan KM, McKay HA. Is there a critical period for bone response to weight-bearing exercise in children and adolescents? A systematic review. Br J Sports Med. 2002;36:250–7.
Baxter-Jones AD, Kontulainen SA, Faulkner RA, et al. A longitudinal study of the relationship of physical activity to bone mineral accrual from adolescence to young adulthood. Bone. 2008;43:1101–7.
Cullen DM, Smith RT, Akhter MP. Bone loading response varies with strain magnitude and cycle number. J Appl Physiol. 2001;91:1971–6.
Robling AG, Hinant FM, Burr DB, et al. Shorter, more frequent mechanical loading sessions enhance bone mass. Med Sci Sports Exerc. 2002;34:196–202.
Baxter-Jones AD, Eisenmann JC, Sherar LB. Controlling for maturation in pediatric exercise science. Pediatr Exerc Sci. 2005;17:18–30.
Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomized controlled trials: the QUOROM statement. Lancet. 1999;354:1896–900.
Flather MD, Farkouh ME, Pogue JM, et al. Strengths and limitations of meta-analysis: larger studies may be more reliable. Control Clin Trials. 1997;18:568–79.
Scerpella TA, Dowthwaite JN, Rosenbaum PF. Sustained skeletal benefit from childhood mechanical loading. Osteoporos Int. 2011;22:2205–10.
Karlsson MK, Nordqvist A, Karlsson C. Sustainability of exercise-induced increases in bone density and skeletal structure. Food Nutr Res. 2008 (Epub 2008 Oct 1).
Blimkie CJR, Rice S, Webber CE, et al. Effects of resistance training on bone mineral content and density in adolescent females. Can J Physiol Pharmacol. 1996;74:1025–33.
Iuliano-Burns S, Saxon L, Naughton G, et al. Regional specificity of exercise and calcium during skeletal growth in girls: a randomized controlled trial. J Bone Miner Res. 2003;18:156–62.
Laing EM, Wilson AR, Modlesky CM, et al. Initial years of recreational artistic gymnastics training improves lumbar spine bone mineral accrual in 4- to 8-year-old females. J Bone Miner Res. 2005;20:509–19.
Macdonald HM, Kontulainen SA, Petit MA, et al. Does a novel school-based physical activity model benefit femoral neck bone strength in pre- and early pubertal children? Osteoporos Int. 2008;19:1445–56.
Morris FL, Naughton GA, Gibbs JL, et al. Prospective ten-month exercise intervention in premenarcheal girls: positive effects on bone and lean mass. J Bone Miner Res. 1997;12:1453–62.
Nichols DL, Sanborn CF, Love AM. Resistance training and bone mineral density in adolescent females. J Pediatr. 2001;139:494–500.
Stear SJ, Prentice A, Jone SC, et al. Effect of a calcium and exercise intervention on the bone mineral status of 16–18-y-old adolescent girls. Am J Clin Nutr. 2003;77:985–92.
Stewart SR. The effects of an 18-month weight-training and calcium-supplementation program on bone mineral of adolescent girls [dissertation]. Vancouver: University of British Columbia; 1997. p. 357.
Weeks BK, Young CM, Beck BR. Eight months of regular in-school jumping improves indices of bone strength in adolescent boys and girls: the POWER PE Study. J Bone Miner Res. 2008;23:1002–11.
Witzke KA, Snow CM. Effects of plyometric jump training on bone mass in adolescent girls. Med Sci Sports Exerc. 2000;32:1051–7.
Moher D, Liberati A, Tezlaff J, The PRISMA Group, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Acknowledgments
This work was not supported by any funding. All authors state that they have no conflicts of interest. We thank Dr. Heather Macdonald for providing us with information needed for this analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ishikawa, S., Kim, Y., Kang, M. et al. Effects of Weight-Bearing Exercise on Bone Health in Girls: A Meta-Analysis. Sports Med 43, 875–892 (2013). https://doi.org/10.1007/s40279-013-0060-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-013-0060-y