Abstract
Background
Advanced computing capabilities and novel visual analytics tools now allow us to move beyond the traditional cross-sectional summaries to analyze longitudinal prescription patterns and the impact of study design decisions. For example, design decisions regarding gaps and overlaps in prescription fill data are necessary for measuring adherence using prescription claims data. However, little is known regarding the impact of these decisions on measures of medication possession (e.g., medication possession ratio). The goal of the study was to demonstrate the use of visualization tools for pattern discovery, hypothesis generation, and study design.
Method
We utilized EventFlow, a novel discrete event sequence visualization software, to investigate patterns of prescription fills, including gaps and overlaps, utilizing large-scale healthcare claims data. The study analyzes data of individuals who had at least two prescriptions for one of five hypertension medication classes: ACE inhibitors, angiotensin II receptor blockers, beta blockers, calcium channel blockers, and diuretics. We focused on those members initiating therapy with diuretics (19.2 %) who may have concurrently or subsequently take drugs in other classes as well. We identified longitudinal patterns in prescription fills for antihypertensive medications, investigated the implications of decisions regarding gap length and overlaps, and examined the impact on the average cost and adherence of the initial treatment episode.
Results
A total of 790,609 individuals are included in the study sample, 19.2 % (N = 151,566) of whom started on diuretics first during the study period. The average age was 52.4 years and 53.1 % of the population was female. When the allowable gap was zero, 34 % of the population had continuous coverage and the average length of continuous coverage was 2 months. In contrast, when the allowable gap was 30 days, 69 % of the population showed a single continuous prescription period with an average length of 5 months. The average prescription cost of the period of continuous coverage ranged from US$3.44 (when the maximum gap was 0 day) to US$9.08 (when the maximum gap was 30 days). Results were less impactful when considering overlaps.
Conclusions
This proof-of-concept study illustrates the use of visual analytics tools in characterizing longitudinal medication possession. We find that prescription patterns and associated prescription costs are more influenced by allowable gap lengths than by definitions and treatment of overlap. Research using medication gaps and overlaps to define medication possession in prescription claims data should pay particular attention to the definition and use of gap lengths.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Big data provides an unprecedented level of detail regarding prescribing patterns among large cohorts. |
Visual analytics tools can harness the rich information in big data to provide unique insight into health services utilization among large cohorts as well as to generate hypotheses involving the use and cost of health services. |
Study parameters such as allowable gaps can significantly affect the medication coverage period and associated prescription costs; it is important for both researchers and decision makers to be aware of the impact of these parameters. |
1 Introduction
Since poor adherence to medication therapy is a major contributor to poor health outcomes [1, 2] and higher costs of care [3], it is essential that researchers are able to understand and measure adherence at the population level. The most common, extensively used measurements of drug adherence have historically been medication possession ratio (MPR), proportion of days covered (PDC), and fraction compliant [4–7]. However, big data, combined with new computing technology such as information visualization software, allow us to move beyond cross-sectional investigations of prescription drug utilization and the associated descriptive statistics in order to analyze and describe population adherence over time. This study evaluates the utility of EventFlow for the investigation of longitudinal fill patterns for antihypertensive medications. EventFlow is an interactive visualization software with a graphical user interface that enables users to analyze temporal patterns to visually inspect and search the prescription patterns of individual patients [8, 9]. The software also provides a visual overview of population-wide patterns by automatically grouping patients based on their prescriptions patterns (which drug they start on, their subsequent use of drugs of interest, and gaps in coverage) and presents the results in a visually comprehensible display that includes information about the timing between prescriptions and duration of coverage gaps.
The study of prescription fill patterns is not new. Studies have investigated trends in antihypertensive prescribing [10] and evaluated the concordance between observed prescription patterns and the guidelines of the seventh report of the joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure [11]. Medication adherence has been investigated in prior studies using direct and indirect measures, which include drug assays or markers, self-reporting, pill counts, electronic monitoring systems, and reviews of pharmacy records or administrative data [12]. Researchers have often used administrative (i.e., claims) data, which offer advantages over other data sources: they are inexpensive, convenient, and can be used for large patient populations [13]. Further, administrative data are non-invasive and objective [12], and estimates derived from pharmacy databases are also more likely than those obtained from clinical trial populations to reflect real-world settings [5]. However, one well known limitation of using administrative data to measure adherence is the inability to confirm that filled prescriptions correlate to medication usage. Yet, studies have found a high concordance between prescription claims data and pill counts, suggesting that the rate at which patients refill their medications is usually consistent with the rate at which they consume them [13, 14].
When processing longitudinal refill data, it is necessary to consider the fact that specific study design decisions can impact the measure of medication possession and, consequently, any summary statistic. For example, one study demonstrated that the choice of gap measurement affects the calculated rate of adherence [15]. Specifically, decisions regarding gaps and overlaps in prescription fill data can materially influence our measures of medication possession. Despite the fact that these decisions are commonly made with input from clinical and statistical experts, information about their impact is limited. It is important, then, to understand how extensively and in what ways these measurement decisions influence study findings on medication possession and associated costs. Visual information about fill patterns may be useful in helping researchers determine appropriate thresholds for gap length or overlap length, particularly when novel software facilitates investigation of variation over time and across individuals.
In this study, we focused on refill patterns, gaps and overlaps for antihypertensive medications. We worked within the context of hypertension because of its prevalence [16, 17], long-term medication use, and poor adherence [18]. An estimated 50–70 % of patients are non-adherent to their antihypertensive medications, with variations in this range due to differences in study groups, duration of follow-up, methods of adherence assessment, and different drug regimens [3]. Using a combination of summary statistics and results from EventFlow, we characterized longitudinal patterns in prescription fills for antihypertensive medications and investigated ways in which study design decisions regarding gap length and overlaps can affect a study’s findings about prescription fill patterns and costs.
2 Methods
2.1 Data and Study Parameters
In this study, we used a cohort of commercially insured members who each fill at least two prescriptions for hypertensive medications. Each prescription in the data consisted of a de-identified member ID, drug code, service date, and days-supply, as well as other descriptive columns. We included five drug classes: ACE inhibitors (ACE-I), angiotensin II receptor blockers (ARB), beta blockers (Beta), calcium channel blockers (CCB), and diuretics. Each class has been shown to be effective in randomized clinical trials, and during the study period all but ARB were available as generics and in single daily dose formulations. The study period extended from October 1, 2008 to September 30, 2010. The study excluded members taking combined dosage forms (i.e., fixed dose drug/drug combinations like Beta plus diuretic), as well as members with any negative costs or claims reflecting data entry errors.
Since patients rarely collect a follow-up prescription on the same day that they consume the last dose of their previous prescription, drug treatment patterns constructed from prescribing or dispensing events in administrative databases often show an overlap or a gap between two prescriptions [19]. These gaps and overlaps are typically processed by defining an allowable length of time or minimum period of time for grouping or separating prescription fills. The ‘allowable gap’ (i.e., the length of time a patient can be without medication before being considered non-adherent) has been defined in multiple ways in the literature. One study considered gaps as small as 7 days to be an indicator of non-adherence [20], while another study used a gap of 30 days [21]. In a study of patients using liraglutide, discontinuation was defined as a gap of at least 90 days, which researchers interpreted as non-persistence [22]. Overlaps are defined as instances in which a patient is in possession of two or more filled prescriptions at once. In a single-drug case, the second prescription is often shifted forward [20]. In the case of multi-drug overlap, researchers must be able to distinguish between instances of concurrent use and instances where a patient is switching medication. However, there is limited guidance available for handling overlaps in the case of multi-drug regimens.
2.2 Graphical Interface and Data Modeling in EventFlow
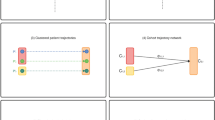
In this section, we introduce EventFlow [9] and discuss the interpretation of the program’s data output. We start with a small data sample for clarity of presentation (see Fig. 1, representing 10 patients prescribed two different drugs: a and b).
In Fig. 1, the timeline on the right shows details of prescription fills data for individual patients, including the sequencing and timing of prescriptions. Each horizontal bar represents a prescription interval, color-coded by the drug (with small vertical lines at both ends of prescription intervals, to make overlaps more apparent). In the center, we see an overview of all the records. It aggregates groups of records with the same sequence of prescriptions into horizontal stripes of colored bars representing each interval. The height of each bar is proportional to the number of records in the group, and the width of the bar is the average duration of the interval (i.e., prescription[s]). When prescriptions overlap, the colors are merged. Reading from the left, we can see that all records start with a prescription for Drug A (red). We then identify three different patterns (i.e., groups of records). The largest group is at the top (7 out of 10 patients). Patients in this group completed the Drug A prescription and did not start another prescription either before or at the time that the prescription for Drug A ended. Six of these patients continue to a prescription for Drug B (blue), while one patient discontinues treatment. The next group (2 patients) is starting a prescription for Drug B before the end of Drug A. The overlap is identified by the purple bar. Lastly, one patient refills a prescription for Drug A before the first prescription is complete, and the duration of the prescription overlap is shown by the darker red bar. In addition, EventFlow’s interactive features provide summary statistics and distributions of durations of different events.
EventFlow has an interactive search panel that makes it possible for users to graphically specify complex queries involving temporal constraints and the absence of events (e.g., which patients used both Drug A and Drug B for at least 30 days?) or search and replace operations (see Fig. 2). The combination of these techniques allows users to sharpen the focus of an analysis to records that exhibit particular event sequences. (For detailed explanations of EventFlow’s capabilities and how the overview is constructed please see [23]).
EventFlow provides a simple interface that allows multiple interval events (prescriptions) in the same category to be merged into a single interval event (detail view in Fig. 2). This can be done in two ways: allowing gaps of certain duration or eliminating overlaps of certain duration. In Fig. 2b, we eliminate a gap that is smaller than the allowable gap parameter. In Fig. 2c, the overlap is smaller than the single-drug overlap parameter and, as a result, EventFlow will take the length of the overlap and extend the interval by that amount. For example, if a patient refills his or her prescription 4 days early, the second prescription will be shifted 4 days and the two prescriptions will be merged into a single, longer interval. The interactive user interface allows users to investigate the impact of different merging parameters on the grouping of the prescription patterns.
In this study, we use the search and replace features to analyze the effects of different parameter values for allowable gaps and for single- and multi-drug overlaps. In Fig. 3 we demonstrate how we applied the search and replace feature twice to distinguish between two cases of overlap: when the multi-drug overlap period is less than our parameter and when the multi-drug overlap period is greater than our parameter.
Illustration of how we distinguished between switching pattern and concurrent use pattern. In this example, the multi-drug overlap parameter is set to 30 days. If the overlap is greater than 30 days, the visual display represents concurrent use. Otherwise, it is considered a switch. The EventFlow search and replace feature was used to specify the data transformation
In defining our base parameters, we first set the allowable gap to 15 days, the single-drug overlap parameter to 15 days and the multi-drug overlap parameter to 0 day. EventFlow’s interactive features make it very easy to change the value of those parameters and visually inspect the results. This allowed us to rapidly explore the parameter space, which in turn guided the sensitivity analysis.
Finally we set the observation window to 2 years following the first prescription in the data set for an antihypertensive agent. This is also easily done interactively within EventFlow by aligning all records by their first event, then setting a temporal window parameter.
While EventFlow’s overviews can be reproduced as figures in the paper, many other interactive features allow the researchers to gain a more detailed understanding of the data. For example, the overview shows only the average durations between events, but the temporal distributions, exact values of counts and percentages are revealed by holding the cursor on elements of the display.
3 Results
3.1 Population Statistics
Our overall population comprised 790,609 members. The majority, 61.8 %, only took medications from one drug class, while 25.4 % had at least one prescription for two drug classes, 9.8 % had a prescription for three drug classes and 2.7 and 0.3 % had prescriptions for four and all five drug classes, respectively. The average age was 52.4 years (calculated at the time of individual’s first prescription) and 53.1 % of the sample was female. Overall, females are more likely to use fewer drug classes, and members using fewer drug classes are on average younger than members with claims for multiple classes. Table 1 summarizes the study population and breaks it down in more detail based on drug class. For example, we note that 37,612 members filled a prescription for both ACE-I and diuretics, and that those two drug classes are the second most common combination of classes (the more common combination is ACE-I and Beta).
3.2 Members Starting on Diuretics
In clinical practice, newly diagnosed hypertensive patients are typically prescribed a diuretic, either alone or in combination with other antihypertensive agents. The JNC 7 (The seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure) [24] recommended thiazide diuretics as initial therapy for most patients diagnosed with stage one hypertension without compelling indications. The guideline also recommends ACE-I, ARBs, Beta, and CCB, alone or in combination. The recent JNC 8 (The eight report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure) [25] recommends thiazide diuretics, ACE-I, ARBs, and CCBs, alone or in combination, as first-line agents for patients who are non-Black without chronic kidney disease.
In order to investigate prescription patterns among patients on multi-drug regimens, in this paper we focus on individuals who begin treatment with diuretics [Fig. A-1 in the Appendix gives an overview of population analysis in EventFlow and Fig. A-2 analyzes the population that have their first prescription for diuretics, alone or in combination with other drug classes; see electronic supplementary material (ESM)]. The analysis of all 151,566 members that started on thiazide diuretics, possibly together with additional drug classes, reveals that 54 % of the population initiates therapy with diuretics only (average duration of initial period is 4 months, 27 days), followed by at least one gap. There are also large groups that either start on diuretics and then initiate a second drug class without a gap, or who immediately begin dual medication therapy. Table 2 summarizes the number of members on dual therapy; the most common second class is ACE-I, followed by Beta, CCBs, and finally ARBs.
In certain patient subgroups (e.g., diabetes mellitus, chronic kidney disease), ACE-I and ARB are the drugs recommended for use with diuretics. In our data, 26,747 members that started on diuretics also had at least one prescription for ACE-I or ARB (but no prescriptions for Beta or CCB) during the study period. When the patterns of these members are analyzed, the majority (56.6 %) started on both diuretics and ACE-I/ARB, and their usage patterns varied greatly; very few (1.7 %) had continuous use of both classes for the duration of the 2-year study period. Of all other members who started on both, about 13 % only had a single 30-day prescription, close to 20 % had an initial usage duration between 31 and 90 days, and close to 67 % had an initial duration longer than 90 days. However, a significant proportion of the population started out on diuretics alone (for about 2 months on average), then the members either added the second medication class without a gap, had a gap, or switched medications: they initiated ACE-I and/or ARB therapy immediately after diuretic use. Overall, we observed that switching (with or without a gap in between drugs) is not a common pattern among these members. The Appendix discusses these patterns in more detail, and the visualization is included in Fig. A-3 (see ESM).
3.3 Sensitivity Analysis
In this section, we consider the implications of decisions regarding gaps and overlaps for analyzing fill patterns among patients who initiate on diuretics. We investigated the effects of three parameters—the allowable gap, the single-drug overlap, and the multi-drug overlap—on the duration of the initial treatment and the first treatment gap, and on prescription costs and adherence.
An increase in the allowable gap parameter will merge more prescriptions together, therefore increasing the length of a continuous coverage period; we demonstrate this fact using data from members who took diuretics only (please refer to Appendix B.1 for visual analytics of the effects of the allowable gap parameter; see ESM). The allowable gap parameter affects any measure of the number of gaps, average duration, and distributions of gap length. Table 3 summarizes some population statistics as a function of the allowable gap. For example, when the allowable gap is zero, only 34.4 % of the population has continuous coverage, and the average length of continuous coverage is 2 months, 8 days (median 1 month). In contrast, when the allowable gap is 30 days, 68.7 % of the population shows a single continuous prescription period with an average length of 5 months and 25 days (median 3 months). We also note, as we increase the allowable gap, that the average length of the observable gaps increases (as shorter gaps have been eliminated). Table 3 reports the average cost (the paid amount by the insurer) for antihypertensive therapy for the initial period across various gap lengths. We find that the average prescription costFootnote 1 of the initial on-study treatment episode ranges from US$3.44 (when the maximum gap is 0 day) to US$9.08 (when the maximum gap is 30 days). This is due to the fact that, as the allowable gap increases, more prescriptions become a part of the initial treatment episode, its duration is extended, and as a result the cost increases. Further, if we calculate the average capped MPR during the initial on-study treatment episode, we note that the average MPR is 1 when the allowable gap is zero. This is due to the fact that when the allowable gap is zero, no gaps are a part of the initial episode. As the allowable gap parameter increases, the average MPR slowly decreases, as more and more small gaps are considered a part of the initial episode.
Next we assessed the effects of changing the overlap parameter for a single drug class. Our base parameter is 15 days—that is, if a prescription overlap was ≤15 days, the overlap was appended to the duration of the drug, otherwise the second drug was considered a replacement and the overlap was merged without appending. The short overlaps affect very few members and as a result the effects are minimal as is summarized in Table 4, which reports the average prescription cost for the initial period across various lengths for single-drug overlap. The average cost of the initial treatment episode ranges from US$7.29 (when the maximum overlap is 0 day) to US$7.61 (when the maximum overlap is 30 days). The increase in costs is small, reflecting the small changes to the initial coverage period. Similarly, we only observe a minimal change in the average capped MPR.
The last parameter is multi-drug overlap. Adjusting this parameter affects how we distinguish between those switching medications and those with concurrent use. When the parameter is set at zero, any overlap in medication is considered concurrent use; in contrast, when the parameter is set at 30 days, only overlap of more than 30 days is considered concurrent use.
We studied this parameter using the data on the 26,747 members that started on diuretics and also had at least one prescription for ACE-I or ARB (but no prescriptions for Beta or CCB) during the study period. Overall, when the parameter changes from zero to 30 days, it only affects the patterns of 17 % of the population; there is a reduction in concurrent use and an increase in the number of switchers and single class use (for details please refer to Appendix B.3 in the ESM). Smaller changes to this parameter have marginal effects. For example, changing the parameter from 0 to 15 only affects 122 members (0.5 %), and changing it from 0 to 29 affects 157 members (0.6 %). In each of these cases, the majority of the patterns change from diuretic only use to concurrent use followed by ACE/ARB use, to diuretic only use followed immediately by use of ACE-I/ARB only. Therefore, the critical setting for this parameter is whether or not investigators require more than 30 days of concurrent use to establish simultaneous use. From a payer’s cost perspective there is no change in cost. Whether a member’s claim is considered a switch or concurrent use does not change the fact that a prescription was filled and paid for.
4 Discussion
Medication non-adherence has significant effects on healthcare expenditures, as it increases physician visits, emergency incidents, re-hospitalizations, and nursing home re-admissions [26]. Therefore, adherence studies examining outcomes and associated costs can inform important pharmacoeconomic decisions. However, as documented in a systematic review of randomized controlled trials, there is no consensus on how medication adherence is defined, analyzed or reported, and in fact, the literature shows substantial heterogeneity [27].
Traditionally, descriptive summary statistics have been used to report prescription drug usage and adherence. However, these statistics do not provide an intuitive, interactive way to investigate the implications of changes in gap lengths and overlaps across patient groups or on the associated cost effects, and research in this area has been limited. In this paper, we have illustrated the use of EventFlow for visualizing large-scale prescription claims data. EventFlow revealed the diversity of those patterns and informed the description of the longitudinal patterns in prescription fills for antihypertensive medications. The software also helped us investigate the implications of decisions regarding the merging of gaps and overlaps, and examine the impact on the average cost of the initial episode. We have found that EventFlow is a useful tool for analyzing high-level patterns, but it also allows for a drill down to the patient-level data; for example, to investigate adverse events in the context of drug patterns.
The literature does not currently offer a ‘best standard’ for assessing medication adherence, or guidance in identifying the important parameters for measuring medication possession. We show how key study parameters affect the observed prescription patterns, which has direct implications for adherence studies. Studies have shown that changing measures will change the rate of adherence [15], and this in turn has a direct effect on cost evaluation. A study by Jonsson et al. [15] highlights the importance of choosing an adherence measure most appropriate for specific study factors and drug properties. This is vital since, depending on the pharmacokinetic property of the medications under consideration, one method of measuring adherence may be of greater clinical value than another. For example, occasional missed doses may be less important than long gaps in treatment for drugs that have a long half-life. It is reasonable to consider that, in addition to pharmacokinetic properties, other factors (disease-related and patient group characteristics, for instance) may also affect the suitability of an adherence measure. Our sensitivity analysis highlights that it is not only the method of adherence evaluation that is important, but the study design parameters as well. EventFlow’s interactive features made it easy to vary those parameters and develop hypotheses about their effect on the overall patterns, which could be later quantified.
EventFlow can be used to explore the implications of alternative design decisions; for example, lengths for a washout (i.e., ‘prescription-free’) period. For each member, one can define the start date of his/her eligibility as an event. It is then easy to apply a filter, to only show those members with at least 6 months (or any time period) between their first (the start of their eligibility) and second event (their first hypertension prescription). EventFlow can also be used to inform the statistical analysis: (1) to visually inspect the patterns of prescription drug use in clinically important strata (e.g., patients grouped according to the Charlson Comorbidity Index, disease severity, performance status, mental health status, etc.); (2) to understand how the timing and length of medication possession varies over time for patients; (3) to investigate the use of medications and the periods of medication possession in their relationship to a health outcome of interest (e.g., a hospitalization or length of stay of the hospitalization). Findings from EventFlow could help determine how to measure medication possession, whether it is the medication use or interval length of medication possession that is more relevant for predicting a hospitalization, and whether specific subgroups are important to examine separately. These insights can help determine how a covariate enters a regression model built to test the relationship between medication use/adherence and health outcomes. Current analytical tools do not provide this nuanced information, which can be important for study design and statistical model specification.
Since pharmacoeconomic evaluations assess the cost and effect tradeoff, the impact of poor compliance and persistence on medication effectiveness is as important as the impact on costs [28]. Although, here again, no single measure can be deemed the ‘best’ [5], it would be useful in future studies to understand the extent to which design decisions regarding prescription patterns will affect the cost outcomes of a study.
5 Conclusions
This proof-of-concept study illustrates the use of visual analytics tools in characterizing longitudinal medication possession. Given the limited information regarding their impact on measures of medication possession, we investigated the role of decisions regarding gaps and overlaps in prescription fill data. We found that decisions regarding gap length have a stronger effect on the average prescription cost of the initial period compared with decisions regarding single-drug overlaps. Information regarding the cost implications of decisions about gap length and overlaps is lacking, and this will be an important area for future research. There is also a need for consensus guidance regarding evaluation of complex fill patterns among individuals on multiple drugs. We have shown that complex patterns can be investigated using a visual analytics tool like EventFlow, and future research can build on these findings to investigate the implications of these patterns for adherence and associated cost studies. In particular, future research using medication gaps and overlaps to define medication possession in prescription claims data should pay particular attention to the definition and use of gap lengths.
Notes
The start-date of the first treatment episode is different for each patient, as a result the year of the cost differs from one patient to the next.
References
National Institutes of Health (1997) The sixth report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. National Institutes of Health; National Heart Lung and Blood Institute, Bethesda, Maryland.
Psaty B, Koepsell TW, LoGerfo J, Inui T. The relative risk of incident coronary heart disease associated with recently stopping the use of beta-blockers. J Am Med Assoc. 1990;263(12):1653–7.
World Health Organization. Adherence to long term therapies: evidence for action. Geneva: Switzerland; 2003.
Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication compliance and persistence: terminology and definitions. Value Health. 2008;2(1):44–7.
Andrade SE, Kahler KH, Frech F, Chan K. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565–74.
Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. Prospective validation of eight different adherence measures for use with administrative claims data among patients with schizophrenia. Value Health. 2009;12(6):989–95.
Patel BV, Remigio-Baker RA, Mehta D, Thiebaud P, Frech-Tamas F, Preblick R. Effects of initial antihypertensive drug class on patient persistence and compliance in a usual-care setting in the United States. J Clin Hypertens. 2007;9(9):692–700.
Plaisant C, Monroe M, Meyer T, Shneiderman B. Interactive visualization. In: Marconi K, Harold L, editors. Big data and health analytics. Taylor and Francis: CRC Press; 2014.
Monroe M, Lan R, Plaisant C, Shneiderman B. Temporal event sequence simplification. IEEE Trans Vis Comput Graph. 2013;19(12):2227–36.
Stafford R, Monti V, Furberg C, Ma J. Long-term and short-term changes in antihypertensive prescribing by office-based physicians in the United States. Hypertension. 2006;48(2):213–8.
Ranchana P, Anuradha H, Shivamurthy M. Anti hypertensive prescribing patterns and cost analyis for primary hypertension: a retrospective study. J Clin Diagn Res. 2014;8(9):HC19–22.
Hess LM, Raebel MA, Conner DA. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40:1280–8.
Grymonpre R, Cheang M, Math M, Fraser M, Metge C, Sitar DS. Validity of a prescription claims database to estimate medication adherence in older persons. Med Care. 2006;44(5):471–7.
Lau HS, de Boer A, Beuning KS, Porsius A. Validation of pharmacy records in drug exposure assessment. J Clin Epidemiol. 1997;50(5):619–25.
Jonsson A, Schioler L, Lesen E, Sundell KA, Mardby A. Influence of refill adherence method when comparing level of adherence for different dosing regimens. Eur J Clin Pharmacol. 2014;70:589–97.
Hannings A, Firm AL, Bertrand CA, Galdo JA. Hypertension 101—a review of JNC 8. In: Cohen HE, editor. Pharmacist. New York: Jobson Medical Information; 2015. pp. 64-69.
Centers for Disease Control. High blood pressure. 2015. http://www.cdc.gov/bloodpressure/. Retrieved 28 Apr 2015.
Mazzaglia G, Ambrosioni E, Alacqua M, Filippi A, Sessa E, Immordino V, Borghi C, Brignoli O, Caputi AP, Cricelli C, Mantovani LG. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120(16):1598–605.
Gardarsdottir H, Egberts TC, Heerdink ER. The association between patient-reported drug taking and gaps and overlaps in antidepressant drug dispensing. Ann Pharmacother. 2010;44:1755–61.
Vink N, Klungel O, Stolk R, Denig P. Comparison of various measures for assessing medication refill adherence using prescription data. Pharmcoepidemiol Drug Saf. 2009;18:159–65.
Hansen R, Dusetzina SB, Dominik RC, Gaynes BN. Prescription refil records as a screening tool to identify antidepressant non-adherence. Pharmacoepidemiol Drug Saf. 2010;19:33–7.
Buysman EK, Fang L, Hammer M, Langer J. Impact of medication adherence and persistence on clinical and economic outcomes in patients with Type 2 diabetes treated with liraglutide: a retrospective cohort study. Adv Ther. 2015;32(4):341–55.
Human-Computer Interaction Lab, University of Maryland. Eventflow user manual. http://www.cs.umd.edu/hcil/eventflow/manual. Accessed 10 Aug 2015.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Greene LA, Izzo JL Jr., Jones DW, Materson BJ, Oparil S, Wright JT Jr., Rocella EJ, National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72.
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Narva AS, Oritz E. 2014 evidence-based guideline for the management of high blood pressure in adults report from the panel members appointed to the eighth Joint National Committee (JNC 8). J Am Med Assoc. 2014;311(5):507–20.
Giannetti VJ, Kamal KM. Adherence with therapeutic regimens: behavioral and pharmacoeconomic perspectives. J Pharm Pract. 2014 [Epub ahead of print].
Zhang Z, Peluso MJ, Gross CP, Viscoli CM, Kernan WN. Adherence reporting in randomized controlled trials. Clin Trials. 2014;11:195–204.
Dyfrig H, Cowell W, Koncz T, Cramer J. Methods for integrating medication compliance and persistence in pharmacoeconomic evaluations. Value Health. 2007;10(6):498–509.
Author contributions
The interpretation and reporting of the results are the sole responsibility of the authors. Dr. Bjarnadottir will act as the guarantor of the work presented in this paper. Dr. Bjarnadottir contributed to the study design, data collection, conduct and interpretation of the analysis, and drafted and revised the manuscript with input from all co-authors. Sana Malik created all figures in the paper, and contributed to the study design, the application of EventFlow to the data, interpretation of the analysis, and commented on/edited all drafts of the manuscript. Dr. Onukwugha contributed to the study design, interpretation of the analysis, drafted and revised the manuscript. Dr. Plaisant contributed to the study design, the application of EventFlow and the interpretation of the analysis, reviewed and commented on/edited all drafts of the manuscript. Tanisha Gooden contributed to the interpretation of the analysis, drafted and/or reviewed and commented on/edited all drafts of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was partially funded by The University of Maryland/Mpowering the State through the Center for Health-Related Informatics and Bioimaging.
Conflicts of interest
Dr. Onukwugha reports consulting income from AstraZeneca and Janssen Analytics. Dr. Bjarnadottir has no conflicts of interest to declare. Sana Malik has no conflicts of interest to declare. Tanisha Gooden has no conflicts of interest to declare. Dr. Plaisant reports being a stake-holder in EventFlow through indirect benefit from commercial use of the software. The EventFlow software has been disclosed as an invention with the University of Maryland Office of Technology Commercialization (OTC) so that the OTC can negotiate commercial licenses with companies interested in licensing the software. The IP is owned by the campus and the income is distributed among different entities on campus (OTC, Colleges and departments of the inventors) with a small percentage of the income from those licenses returned to the inventors and used to support further research. The OTC website specifies the availability of the software and the policy of distributing a portion of the income to the inventors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bjarnadóttir, M.V., Malik, S., Onukwugha, E. et al. Understanding Adherence and Prescription Patterns Using Large-Scale Claims Data. PharmacoEconomics 34, 169–179 (2016). https://doi.org/10.1007/s40273-015-0333-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-015-0333-4