Abstract
Objective
Our objective was to estimate the cost effectiveness of ofatumumab plus chlorambucil (OChl) versus chlorambucil in patients with chronic lymphocytic leukaemia for whom fludarabine-based therapies are considered inappropriate from the perspective of the publicly funded healthcare system in Canada.
Methods
A semi-Markov model (3-month cycle length) used survival curves to govern progression-free survival (PFS) and overall survival (OS). Efficacy and safety data and health-state utility values were estimated from the COMPLEMENT-1 trial. Post-progression treatment patterns were based on clinical guidelines, Canadian treatment practices and published literature. Total and incremental expected lifetime costs (in Canadian dollars [$Can], year 2013 values), life-years and quality-adjusted life-years (QALYs) were computed. Uncertainty was assessed via deterministic and probabilistic sensitivity analyses.
Results
The discounted lifetime health and economic outcomes estimated by the model showed that, compared with chlorambucil, first-line treatment with OChl led to an increase in QALYs (0.41) and total costs ($Can27,866) and to an incremental cost-effectiveness ratio (ICER) of $Can68,647 per QALY gained. In deterministic sensitivity analyses, the ICER was most sensitive to the modelling time horizon and to the extrapolation of OS treatment effects beyond the trial duration. In probabilistic sensitivity analysis, the probability of cost effectiveness at a willingness-to-pay threshold of $Can100,000 per QALY gained was 59 %.
Conclusions
Base-case results indicated that improved overall response and PFS for OChl compared with chlorambucil translated to improved quality-adjusted life expectancy. Sensitivity analysis suggested that OChl is likely to be cost effective subject to uncertainty associated with the presence of any long-term OS benefit and the model time horizon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Improved progression-free survival and overall response rates translated to higher quality-adjusted life-years (QALYs) for patients with chronic lymphocytic leukaemia treated with ofatumumab plus chlorambucil (OChl) than for patients treated with chlorambucil. |
These health improvements were accompanied by an increase in first-line treatment costs associated with OChl; however, these costs were partially offset by reductions in the costs associated with treatment in subsequent lines of therapy. |
Cost-effectiveness estimates suggested that OChl is likely to be cost effective at a threshold of $Can100,000 per QALY gained; the cost effectiveness of OChl was most sensitive to the time horizon and to the presence of a long-term overall survival benefit. |
1 Introduction
Chronic lymphocytic leukaemia (CLL) is a haematological cancer characterized by the clonal expansion of malignant B cells over several years, resulting in impaired immune function and eventually progressive bone marrow failure. The majority of CLL patients are aged ≥65 years at the time of diagnosis [1]. The estimated prevalence of CLL in western countries is approximately 30 per 100,000 people [2], and the annual incidence in Canada is between 5.0 and 8.0 per 100,000 people [3, 4]. CLL is usually incurable; however, patients often can be managed successfully for many years with chemotherapeutic and immunotherapeutic agents [5, 6].
The recommended management approach for patients with early CLL is a watch-and-wait strategy [5, 6]. For patients with advanced or symptomatic disease requiring treatment, the current first-line standard of care comprises a combination of fludarabine, cyclophosphamide and rituximab, which has shown significant clinical benefit (improvement in both progression-free survival [PFS] and overall survival [OS]) in previously untreated CLL patients [7]. However, for many elderly or less fit patients with CLL, first-line standard-of-care treatment is inappropriate due to toxicities associated primarily with fludarabine [8]. The optimal first-line treatment choice for fludarabine-ineligible patients is unclear [9], and while alemtuzumab may be considered for patients with specific genetic markers [8], chlorambucil monotherapy remains a recommended choice [10, 11]. Recently, the pan-Canadian Oncology Drug Review (pCODR) recommended bendamustine as another option for fludarabine-ineligible patients [12]; currently, the treatment is being funded in some Canadian provinces [13].
Ofatumumab is a fully human anti-CD20 monoclonal antibody approved in Canada in combination with chlorambucil for the first-line treatment of CLL [14]. Approval of ofatumumab plus chlorambucil (OChl) in Canada was based on the results of a randomized, open-label, multi-centre clinical trial (COMPLEMENT-1) comparing OChl with chlorambucil in this population [15, 16]. The results of the COMPLEMENT-1 trial indicated clear clinical benefits for OChl in comparison with chlorambucil: a longer median PFS (22.4 vs. 13.1 months, P < 0.001) with a hazard ratio of 0.57 (95 % confidence interval [CI] 0.45–0.72; P < 0.001) and an improved overall response rate (ORR) (82 vs. 69 %; P = 0.001) [15]. Ofatumumab also has received a ‘Notice of compliance with conditions’ in Canada for CLL patients who are refractory to fludarabine and alemtuzumab [14].
The objective of this economic evaluation was to examine whether OChl is cost effective in comparison with chlorambucil [10] as a first-line treatment for CLL in patients for whom fludarabine-based treatment is inappropriate. The patient population and treatment comparison considered in the evaluation mirrors the COMPLEMENT-1 trial. The analysis adopted the perspective of the publicly funded healthcare system in Canada.
2 Methods
The economic evaluation used a semi-Markov decision model comprising pre-progression health states based on efficacy and safety data from the COMPLEMENT-1 trial and post-progression health states based on Canadian treatment practices, treatment patterns identified from clinical guidelines and published literature. Clinical and economic input data for the model were identified by systematic reviews and supplemented by a survey of Canadian haematologists and oncologists who actively treat patients with CLL. The model structure and input data were reviewed and validated by clinical experts and health economists during advisory boards convened in Canada and the UK. The model was built and analyses were conducted in accordance with guidelines for decision-analytic modelling [17–20]. Model validation was performed according to best practices guidelines [21]. Please refer to the Electronic Supplementary Material (ESM) for additional details on the systematic reviews, physician survey, advisory boards and model validation.
2.1 Patient Population and Treatment Interventions
The patient characteristics for the model population were based on the population enrolled in the COMPLEMENT-1 trial [16]. OChl and chlorambucil were administered for a minimum of three 28-day cycles and for a maximum of 12 cycles or until best response, unacceptable toxicity, disease progression or death. Patients in both groups received oral chlorambucil 10 mg/m2 on days 1 through 7. Patients in the OChl group also received intravenously infused ofatumumab 300 mg on day 1 of the first cycle, 1000 mg on day 8 of the first cycle, and 1000 mg on day 1 of all subsequent cycles.
2.2 Model Structure
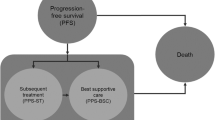
A diagram of the model structure is presented in Fig. 1. The health states prior to disease progression included complete response, partial response and stable disease and were consistent with a recently published model for first-line CLL treatment [22] appraised by the UK National Institute for Health and Care Excellence (NICE) and by the Scottish Medicines Consortium [23, 24]. After patients begin first-line treatment for CLL, they may respond, their disease may progress or their disease may remain stable (i.e. no response and no disease progression). This approach allows the model to capture differences in drug utilization, PFS, utility weights and disease-management costs between levels of response. The structure after disease progression included separate states for re-treatment with the study drug, up to three subsequent lines of therapy, best supportive care (BSC), and death. Patients in any of the health states can move to the BSC health state or the dead health state.
Diagrammatic representation of the model structure. Patients transitioned from the starting state to their best-response state after 3 months (at the end of the first model cycle). BSC best supportive care, Chl chlorambucil, CLL chronic lymphocytic leukaemia, OChl ofatumumab plus chlorambucil, PML progressive multifocal leukoencephalopathy, tx treatment
The proportions of patients who are in the post-progression health states or who have died at the end of each cycle were governed by Kaplan–Meier (K–M) curves for PFS and parametric survival functions for OS, respectively, as derived from patient-level data from the COMPLEMENT-1 trial. If patients initially respond to first-line treatment and do not progress within 12 months, they may receive re-treatment with their original therapy. Patients in the model may receive up to three further lines of active therapy. The specific therapies used in subsequent lines and the percentages of patients receiving them were based on recommendations from Canadian clinical experts and from the results of a survey of Canadian physicians who actively treat patients with CLL (see the ESM for additional details).
Adverse events (AEs) associated with first- and subsequent-line treatments were modelled as acute events that may occur within any health state while receiving treatment. The exception was progressive multifocal leukoencephalopathy (PML), a rare event that can result in long-term neurological disability. PML was not observed in the COMPLEMENT-1 trial but was included as a possible event for patients receiving OChl because there is a small risk of PML for the class of therapies to which ofatumumab belongs [14]. The risk of PML was assumed to be zero for chlorambucil and all subsequent-line therapies, as no events were reported in the key studies identified for subsequent-line therapies [25–29].
The model used a lifetime time horizon, defined as 25 years based on a long-term survival analysis of the North American Intergroup Study C9011, which investigated chlorambucil in previously untreated CLL patients and found that fewer than 10 % of patients survived more than 18 years [30]. The longer horizon was selected to allow for improved outcomes for cancer patients since enrolment for Study C9011 (1990–1994). The cycle length of the model was 3 months to align with the time at which response was assessed and could result in treatment discontinuation. A half-cycle correction was applied using the life-table method [31]. Costs and health outcomes were discounted at 5.0 % per annum [19]. The cost year for the study was 2013.
2.3 Efficacy and Safety Data
The primary measures of effectiveness for the first-line treatments were drawn from the COMPLEMENT-1 trial data and included best overall response, PFS, and OS. Definitions and methods for assessing response, including investigator assessment and independent review committee assessment, and PFS have been described elsewhere [15]. The median follow-up was 29 months. Best overall response data are summarized in Table 1.
PFS was modelled in the base case through transition probabilities, calculated from K–M data (independent review committee-assessed PFS) by best overall response (Fig. 2), and in variability analyses through parametric survival functions fitted with best overall response as a covariate. Separate analyses for independent review committee-assessed and investigator-assessed PFS were conducted. A range of alternative functions were explored (exponential, Weibull, log-logistic, log-normal, Gompertz and gamma). Functions were fitted with treatment as a covariate and to separate datasets for each treatment arm. Functions were selected for inclusion in the economic model based on Akaike Information Criterion and Bayesian Information Criterion results, diagnostic plots (e.g. log-cumulative hazard plots), visual inspection of the fit to the K–M curves and plausibility of the extrapolation. The parameters for selected functions are presented in Table 2.
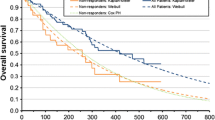
Follow-up for OS in the COMPLEMENT-1 trial was limited; the survival proportion was more than 70 % in both arms at 4 years. Therefore, there was substantial uncertainty in the extrapolation for OS. Conventional survival functions fitted to COMPLEMENT-1 OS data produced long-term predictions that were unlikely to be clinically plausible. For example, a Weibull function predicted a 20-year survival of 24 % for the chlorambucil arm; this estimate was much higher than clinical experience with chlorambucil treatment or long-term follow-up studies suggest (e.g. Rai et al. [30]; see Fig. 3). In a novel approach [32], long-term observations of OS for patients receiving chlorambucil in an external trial (C9011 [30]; see Fig. 3) were used to guide the extrapolation of OS beyond the end of the COMPLEMENT-1 trial period. The published K–M curve for the chlorambucil arm of the C9011 trial was digitized to estimate a patient-level data set by the method of Guyot et al. [33]. These data could not be used directly to predict OS for patients receiving chlorambucil because outcomes have improved substantially since patients were enrolled in Study C9011 (1990–1994), the chlorambucil dosing regimen differed and the observed OS differed from that observed in COMPLEMENT-1 (see Fig. 3). However, the OS curve from Study C9011 was used to guide the extrapolation of the COMPLEMENT-1 data using a method described by Hawe et al. [32] (see Table 2, footnote g). Functions were selected for inclusion in the model as previously described for PFS (Table 2). The base-case analysis assumed that the OS benefit observed during the COMPLEMENT-1 trial (hazard ratio 0.91 [95 % CI 0.57–1.43; P = 0.666]) [16] persisted beyond the end of the trial. Alternative assumptions about the OS treatment effect after the trial were explored, including scenarios with tapering treatment effect and with no treatment effect after the trial period.
Comparison of model predictions for overall survival with published long-term overall survival data for CLL patients with chronic lymphocytic leukaemia treated with chlorambucil. Notes: The model predictions used the Weibull function, guided by external data. The K–M data for OChl and Chl are from COMPLEMENT-1 [16] and for C9011 [30] were reported by Rai et al. [30]. Study C9011 investigated Chl at a dose of 40 mg/m2 once every 28 days for a maximum of 12 cycles. Chl chlorambucil, K–M Kaplan–Meier, OChl ofatumumab plus chlorambucil, OS overall survival
Transitions between post-progression health states were modelled using the weighted average time to progression (TTP) for the basket of subsequent therapies received. The mean TTP was taken from published trials (median PFS was used as a proxy if mean TTP was not reported) (see Table S1 in the ESM). The percentages of patients receiving each therapy in each line of treatment were estimated from a survey of Canadian physicians (see Table S2 in the ESM). According to Canadian clinical experts, the efficacy of treatments used in subsequent lines may not equal their efficacy in the first line; an estimated reduction in efficacy of 50 % was considered reasonable. This reduction was applied to the weighted average TTP for all subsequent lines, excluding re-treatment. The physician survey did not collect data on fourth-line therapy; therefore, the distribution of treatments in the fourth line was assumed to equal that in third-line therapy. In the base case, re-treatment with first-line therapies was not considered, and the distribution of treatments in subsequent treatment lines was assumed to be identical for patients receiving first-line OChl or chlorambucil. The weighted average TTP was used to calculate transition probabilities from the second-, third- and fourth-line treatment health states, assuming a constant hazard, but was not used to govern OS. OS in the post-progression states remained based on the survival analysis of the COMPLEMENT-1 trial as previously described.
AEs associated with first-line treatments were considered for inclusion in the model based on the AEs observed in COMPLEMENT-1 [16] and the recommendations of expert clinical advisors in Canada and the UK. AEs were included in the model if there was a risk difference of >2 % between the two treatment arms; if there was a risk of ≥5 % in any treatment arm; if the AEs were expected to have an impact on costs, patient quality of life and/or mortality; or if the AEs were included in previous CLL models [22]. For subsequent lines of therapy, the AEs were identical to those considered for first-line treatment, with the exception of PML.
2.4 Resource Use and Costs
The costs estimated by the model included the drug acquisition, administration and monitoring costs for each regimen in first-line treatment (Table 3) and subsequent-line treatments (Table 4); the costs of treating AEs (Table 5); and the costs of general disease management during the periods with response, stable disease, progression and BSC (Table 6). The variability analysis also considered the costs associated with re-treatment, which were calculated based on re-treatment response rates. Because no data were identified describing response to therapy or duration of therapy during re-treatment, the same data used for first-line treatment were assumed. Costs were inflated to 2013 where necessary using the Canadian Consumer Price Index for healthcare [35].
Drug consumption was based on the COMPLEMENT-1 study; OChl and chlorambucil patients received a mean of 6.4 and 6.0 cycles of therapy, respectively. Drug costs were modelled separately for each response category to reflect differences in the cost of therapy resulting from differences in response rates. Unit costs for the drugs used in the model were taken primarily from the Ontario Drug Benefit [36] and the IMS Brogan [42] databases. The unit cost of ofatumumab was provided by the manufacturer, and the unit cost of bendamustine was taken from the pCODR recommendation for bendamustine [12]. Additional sources included resource utilization observed in the COMPLEMENT-1 trial, the published literature, a survey of Canadian physicians, expert opinion solicited from clinical and economic experts during advisory boards and the Woods et al. [22] economic model (see the ESM for additional details on the physician survey and the advisory board). Canadian unit costs were applied to calculate the cost per AE.
2.5 Health-State Utility Values
The primary source of utility data for the model was EuroQol 5 Dimensions (EQ-5D) (3-level version) data collected in the COMPLEMENT-1 trial. A regression analysis was performed using a fixed-effects model to describe the change from baseline in EQ-5D index scores, with complete response, partial response, stable disease, disease progression and other predictive factors as covariates. The regression parameters (see Table S3 in the ESM) were used in the model to estimate the change from baseline utility for each modelled health state, incorporating correlations among health states within the patient-level data. The resulting mean utility values for each health state are presented in Table 6. Two alternative sources of utility weights for CLL identified by the systematic review were used in the variability analysis: data from Beusterien et al. [41] for the baseline and pre-progression health states and data from Ferguson et al. [43] for the post-progression health states.
Disutility data for AEs were not available from COMPLEMENT–1. For AEs included in the analysis by Beusterien et al. [41], the reported utility weights were used to calculate utility decrements. For AEs not included in that analysis, utility decrements were obtained from other published oncology utility studies [39, 40] or solicited from clinical experts. The default utility decrements for AEs included in the model are presented in Table 5.
2.6 Variability and Sensitivity Analysis
A range of variability analyses were conducted to assess the impact on the results of the analysis of alternative time horizons, discount rates, perspectives, post-progression treatment patterns, response assessment methods (independent review committee-assessed vs. investigator-assessed PFS), parametric survival functions, scenarios for the continuation of treatment effect after the COMPLEMENT-1 trial period and utility values.
One-way sensitivity analyses (OWSAs) and probabilistic sensitivity analyses (PSAs) were conducted to assess the impact of parameter uncertainty on the results. In the OWSAs, all input parameter values were varied ±20 % from the base-case values (except for survival function parameters, which were not suitable for univariate sensitivity analysis due to correlations between parameters). The PSA considered uncertainty in all model parameters (except drug prices, which were fixed). Estimates of uncertainty for the PSA were based on reported measures of uncertainty in the source data (e.g. standard error) where data were available. Where no estimates of uncertainty were reported, we assumed 95 % CIs of ±20 % around the mean value. Parameters were sampled from appropriate statistical distributions [44].
The PSA was performed by estimating the incremental costs and effects (life-years and quality-adjusted life-years [QALYs]) for each of 10,000 probabilistic simulations and the net monetary benefit for each iteration at a series of willingness-to-pay thresholds (incremental cost-effectiveness ratios [ICERs], defined as the incremental cost per QALY gained).
3 Results
3.1 Base-Case Analysis
The results of the base-case analysis are presented in Table 7. The reported estimates of costs, life-years and QALYs represent mean total expected lifetime per-patient values. Compared with treatment with chlorambucil, OChl led to improved long-term health outcomes and an increase in total direct costs. The incremental lifetime costs (in Canadian dollars [$Can]) and outcomes per patient for OChl versus chlorambucil were $Can27,866 and 0.41 QALYs, respectively. Treatment with OChl was more costly than chlorambucil in terms of first-line treatment costs ($Can29,325) and pre-progression general disease-management costs ($Can223). However, the costs associated with subsequent treatments and post-progression general disease management were lower for patients receiving OChl (−$Can1234 and −$Can448, respectively). The resulting ICERs were $Can68,647 per QALY gained and $Can54,428 per life-year gained.
3.2 Variability Analysis
Table 8 presents the scenarios tested in the variability analysis and the resulting impact on the base-case incremental cost per QALY gained. The scenarios with the greatest impact on the ICER included the consideration of shorter time horizons and alternate assumptions regarding the extrapolation of the OS treatment effect. Shorter time horizons ranging from 5 years (the duration of the COMPLEMENT-1 trial) to 15 years (the approximate mean life expectancy at age 70 years in Canada) led to higher ICERs ($Can217,243 to 81,638 per QALY, respectively) because additional QALYs and cost offsets associated with improved PFS and OS occurring in later years were not captured. Alternate assumptions for the OS treatment effect for OChl versus chlorambucil after the trial period led to ICERs ranging from $Can106,615 per QALY gained (hazard ratio 1.0 after trial period) to $Can90,457 per QALY gained (hazard ratio tapering to 1.0 over 5 years).
3.3 One-Way Sensitivity Analysis
The results of the OWSA for the base-case revealed that the variable with the largest impact on the results was the baseline utility value. In contrast to the variability analysis above, which varied the post-progression treatment patterns equally between OChl and chlorambucil, the OWSA suggests that differences in post-progression treatment patterns (the proportions of patients advancing to, and the costs of, subsequent lines of therapy) between the two arms may have a large effect on the ICER (see Fig. S2 in the ESM).
Due to the uncertainty around the extrapolation of OS treatment effect, OWSA was also conducted for the scenario where the OS hazard ratio is set to 1.0 after the trial period (see Fig. S3 in the ESM). The variables with the largest impact on the ICER in this supplementary analysis mirrored the results of the OWSA around the base case.
3.4 Probabilistic Sensitivity Analysis
Figure 4 presents the joint distribution of incremental costs and benefits for the base-case estimated in the PSA (10,000 simulations) on the cost-effectiveness plane. Approximately 33 % of the simulated points fell below a cost-effectiveness threshold of $Can50,000 per QALY gained, and approximately 59 % of the simulated points fell below a threshold of $Can100,000 per QALY gained (Fig. 5).
Joint distribution of incremental costs and effects for OChl versus Chl in the PSA (10,000 iterations). The figure presents the incremental mean discounted total expected lifetime costs and QALYs per patient (OChl minus Chl). Approximately 59 % of simulated points fell below the $Can100,000 per QALY gained threshold. Costs are presented in Canadian dollars. Chl chlorambucil, ICER incremental cost-effectiveness ratio, OChl ofatumumab plus chlorambucil, PSA probabilistic sensitivity analysis, QALY quality-adjusted life-year
PSA was also conducted for the scenario where the OS hazard ratio is set to 1.0 after the trial period (see Figs. S4 and S5 in the ESM). In this supplementary analysis, approximately 46 % of the simulated points fell below a threshold of $Can100,000 per QALY gained.
4 Discussion
The identification of effective CLL treatments with limited toxicity that can be tolerated by older or less fit patients (for whom fludarabine-based therapy is inappropriate) is a critical unmet need in CLL management. Trial results suggested that OChl was associated with improved PFS and better ORRs than standard-of-care chlorambucil. The comparison considered in this economic evaluation mirrored the head-to-head treatment comparison in COMPLEMENT-1 and its study population.
The base-case results (Table 7) indicated that improved PFS and ORR for OChl compared with chlorambucil were predicted to translate into improved long-term health outcomes in CLL patients for whom fludarabine-based therapies are considered inappropriate. The increase of mean total direct costs owing to an increase in first-line treatment costs associated with OChl and a small increase in the cost of pre-progression general disease management were partially offset by reductions in the costs associated with subsequent lines of therapy and in post-progression general disease management. The variability analysis scenarios indicated that the cost-effectiveness results were most sensitive to the time horizon and the continuation of the OS treatment effect beyond the COMPLEMENT-1 trial period. The results of the one-way sensitivity analysis indicated that patients’ baseline utility values and potential differences in post-progression treatment patterns between patients receiving OChl and chlorambucil also had an impact on the cost-effectiveness results.
While the model was developed and validated according to international and Canada-specific guidelines and treatment patterns [17–21], several limitations of this analysis should be discussed. These include the use of efficacy, safety and drug utilization data taken from a clinical trial (COMPLEMENT-1) for the model’s first-line therapies and for dosage regimens for subsequent-line therapies. Resource utilization in controlled settings typically differs from that experienced in real-world settings [46]. Where possible, real-world Canada-specific data were used in the analysis. However, further uncertainty remains in the assumptions around the percentage of patients retreated with OChl, the proportion of patients receiving later lines of therapy, and whether this proportion is equal for both arms. Moreover, COMPLEMENT-1 was relatively short in duration, with a median follow-up of 29 months, yet the model simulated costs and outcomes over the lifetime of the patient cohort. Another limitation was the use of data from Study C9011 to guide the shape of the long-term extrapolation of OS beyond the COMPLEMENT-1 trial follow-up period. Specifically, the assumption of proportional hazards (or accelerated failure time, depending on the function fitted) between the two studies cannot be verified without further long-term observation. The uncertainty around the continuation of OS treatment effect after the trial follow-up resulted in uncertainty in the ICER estimate. A review of published economic models in CLL similarly identified these limitations as key areas of uncertainty [47].
The COMPLEMENT-1 trial included patients from approximately 150 study locations in 17 countries (including four patients in Canada), including countries where CLL treatment patterns may differ from those in Canada. This could limit the generalizability of the results to the Canadian setting. However, the model included Canadian unit costs and, where possible, resource use data, all of which were confirmed by a panel of Canadian clinical experts. Finally, the health-related quality-of-life utility values estimated from COMPLEMENT-1 were valued by the UK general population. A targeted literature review did not find Canada-specific utility values, and published comparisons between UK and Canadian utility values are inconsistent [48, 49]. While the results showed some sensitivity to the choice of utility values in both the variability analysis and the OWSA, the magnitude of this sensitivity does not change the overall conclusions of the evaluation.
5 Conclusions
This analysis suggests that OChl is an important therapeutic option for previously untreated patients with CLL who are unfit for fludarabine-based therapies, with the improved overall response and PFS in comparison with chlorambucil observed in the COMPLEMENT-1 trial translating to long-term increases in quality-adjusted life expectancy. Sensitivity analyses indicated that the primary source of uncertainty in the cost effectiveness of OChl in comparison with chlorambucil was associated with the extrapolation of clinical benefits beyond the duration of the COMPLEMENT-1 trial. In an era of limited resources, pharmacy budget holders are faced with reimbursement decisions for multiple therapies across a range of conditions. The cost-effectiveness results presented in this study thus serve as important evidence to support reimbursement decisions for OChl in the first-line treatment of CLL in Canada.
6 Electronic Supplementary Material Contents
The ESM presents additional input parameter tables, sensitivity analysis results, details on the advisory boards convened during model development, an overview of the survey of Canadian haematologists and oncologists used to estimate Canada-specific resource utilization, and a summary of the economic and clinical systematic literature reviews conducted to inform model development.
References
Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER cancer statistics review, 1975–2010. In: National Cancer Institute. 2013. http://seer.cancer.gov/csr/1975_2010/. Accessed 27 Feb 2014.
Orphanet: prevalence of rare diseases: bibliographic data. Paris: Orphanet; 2011.
Seftel MD, Demers AA, Banerji V, Gibson SB, Morales C, Musto G, Pitz MW, Johnston JB. High incidence of chronic lymphocytic leukemia (CLL) diagnosed by immunophenotyping: a population-based Canadian cohort. Leuk Res. 2009;33:1463–8.
Public Health Agency of Canada. Chronic lymphocytic leukemia incidence [age-adjusted, 2009]. In: Chronic Disease Infobase. 2014. http://66.240.150.17:9600/PHAC/dimensionMembers.jsp?l=en-US,en;q=0.8&rep=iC3EE3A042BDA4B10ACDD99A9BB6ED430. Accessed 6 Mar 2014.
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, Hillmen P, Keating MJ, Montserrat E, Rai KR, Kipps TJ. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia (IWCLL) updating the National Cancer Institute-Working Group (NCI-WG) 1996 guidelines. Blood. 2008;111:5446–56.
Eichhorst B, Hallek M, Dreyling M, ESMO Guidelines Working Group. Chronic lymphocytic leukaemia: ESMO clinical practice guidelines for diagnostic, treatment, and follow-up. Ann Oncol. 2010;21(suppl 5):162–4.
Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, Hensel M, Hopfinger G, Hess G, von Grünhagen U, Bergmann M, Catalano J, Zinzani PL, Caligaris-Cappio F, Seymour JF, Berrebi A, Jäger U, Cazin B, Trneny M, Westermann A, Wendtner CM, Eichhorst BF, Staib P, Bühler A, Winkler D, Zenz T, Böttcher S, Ritgen M, Mendila M, Kneba M, Döhner H, Stilgenbauer S, International Group of Investigators, German Chronic Lymphocytic Leukaemia Study Group. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–74.
Del Giudice I, Mauro FR, Foa R. Chronic lymphocytic leukemia in less fit patients: “slow-go”. Leuk Lymphoma. 2011;52:2207–16.
Eichhorst BF, Busch R, Stilgenbauer S, Stauch M, Bergmann MA, Ritgen M, Kranzhöfer N, Rohrberg R, Söling U, Burkhard O, Westermann A, Goede V, Schweighofer CD, Fischer K, Fink AM, Wendtner CM, Brittinger G, Döhner H, Emmerich B, Hallek M, German CLL, Study Group (GCLLSG). First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114(16):3382–91.
Alberta Health Services. Chronic lymphocytic leukemia: clinical practice guideline LYHE-007 (version 3). 2015. http://www.albertahealthservices.ca/assets/info/hp/cancer/if-hp-cancer-guide-lyhe007-cll.pdf. Accessed 13 Aug 2015.
Woyach JA, Ruppert AS, Rai K, Lin TS, Geyer S, Kolitz J, Appelbaum FR, Tallman MS, Belch AR, Morrison VA, Larson RA, Byrd JC. Impact of age on outcomes after initial therapy with chemotherapy and different chemoimmunotherapy regimens in patients with chronic lymphocytic leukemia: results of sequential cancer and leukemia group B studies. J Clin Oncol. 2012;31:440–7.
Pan-Canadian Oncology Drug Review. Final recommendation for bendamustine (Treanda) for first line CLL. In: pCODR Expert Review Committee (pERC) final recommendation. 2013. http://www.pcodr.ca/idc/groups/pcodr/documents/pcodrdocument/pcodr-treandacll1st-fn-rec.pdf. Accessed 7 Aug 2015.
Pan-Canadian Oncology Drug Review. Bendamustine (Treanda) for CLL (first line). In: Provincial Funding Summary. 2013. http://www.pcodr.ca/idc/groups/pcodr/documents/webcontent/pcodr_provfund_treandacll1stln.pdf. Accessed 7 Aug 2015.
Arzerra (ofatumumab) [product monograph]. Mississauga (Ontario): GlaxoSmithKline Inc; 2014.
Hillmen P, Robak T, Janssens A, Babu KG, Kloczko J, Grosicki S, Doubek M, Panagiotidis P, Kimby E, Schuh A, Pettitt AR, Boyd T, Montillo M, Gupta IV, Wright O, Dixon I, Carey JL, Chang CN, Lisby S, McKeown A, Offner F, COMPLEMENT 1 Study Investigators. Chlorambucil plus ofatumumab versus chlorambucil alone in previously untreated patients with chronic lymphocytic leukemia (COMPLEMENT 1): a randomised, multicentre, open-label phase 3 trial. Lancet. 2015;385(9980):1873–83.
GlaxoSmithKline. A phase III, open label, randomized, multicenter trial of ofatumumab added to chlorambucil versus chlorambucil monotherapy in previously untreated patients with chronic lymphocytic leukemia. 2013. http://www.gsk-clinicalstudyregister.com/study/OMB110911#rs. Accessed 29 Jul 2015.
Caro JJ, Briggs AH, Siebert U, Kuntz KM, ISPOR-SMDM Modeling Good Research Practices Task Force. Modeling good research practices-overview: a report of the ISPOR-SMDM modeling good research practices task force-1. Value Health. 2012;15:796–803.
Siebert U, Alagoz O, Bayoumi AM, Jahn B, Owens DK, Cohen DJ, Kuntz KM. State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-3. Med Decis Making. 2012;32(5):690–700.
Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada. 3rd ed. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2006.
Mittmann N, Evans WK, Rocchi A, Longo CJ, Au H-J, Husereau D, Leighl N, Isogai P, Krahn M, Peacock S, Marshall D, Coyle D, Malfair Taylor SC, Jacobs P, Oh PI. Addendum to CADTH’s guidelines for the economic evaluation of health technologies: specific guidance for oncology products. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2009.
Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB, ISPOR-SMDM Modeling Good Research Practices Task Force. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Value Health. 2012;15(6):843–50.
Woods B, Hawkins N, Dunlop W, O’Toole A, Bramham-Jones S. Bendamustine versus chlorambucil for the first-line treatment of chronic lymphocytic leukemia in England and Wales: a cost-utility analysis. Value Health. 2012;15(5):759–70.
National Institute for Health and Care Excellence. Bendamustine for the first-line treatment of chronic lymphocytic leukaemia. In: NICE technology appraisal guidance 216. 2011. http://www.nice.org.uk/guidance/ta216/chapter/1-guidance. Accessed 7 Aug 2015.
Scottish Medicine Consortium. Bendamustine hydrochloride 25 mg, 100 mg powder for solution for infusion (Levact). In: SMC No. 694/11. 2011. http://www.scottishmedicines.org.uk/files/advice/bendamustine_Levact_CLL_FINAL_MARCH_2011_for_website.pdf. Accessed 7 Aug 2015.
Robak T, Dmoszynska A, Solal-Céligny P, Warzocha K, Loscertales J, Catalano J, Afanasiev BV, Larratt L, Geisler CH, Montillo M, Zyuzgin I, Ganly PS, Dartigeas C, Rosta A, Maurer J, Mendila M, Saville MW, Valente N, Wenger MK, Moiseev SI. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28(10):1756–65.
Fischer K, Cramer P, Busch R, Stilgenbauer S, Bahlo J, Schweighofer CD, Böttcher S, Staib P, Kiehl M, Eckart MJ, Kranz G, Goede V, Elter T, Bühler A, Winkler D, Kneba M, Döhner H, Eichhorst BF, Hallek M, Wendtner CM. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2011;29(26):3559–66.
Raphael B, Andersen JW, Silber R, Oken M, Moore D, Bennett J, Bonner H, Hahn R, Knospe WH, Mazza J, et al. Comparison of chlorambucil and prednisone versus cyclophosphamide, vincristine, and prednisone as initial treatment for chronic lymphocytic leukemia: long-term follow-up of an Eastern Cooperative Oncology Group randomized clinical trial. J Clin Oncol. 1991;9:770–6.
Knauf WU, Lissichkov T, Aldaoud A, Liberati A, Loscertales J, Herbrecht R, Juliusson G, Postner G, Gercheva L, Goranov S, Becker M, Fricke HJ, Huguet F, Del Giudice I, Klein P, Tremmel L, Merkle K, Montillo M. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27:4378–84.
Goede V, Fischer K, Humphrey K, Asikanius E, Busch R, Engelke A, Wendtner VM, Samoylova O, Chagorova T, Dilhuydy M-S, De La Serna Torroba J, Illmer T, Opat S, Owen C, Kreuzer KA, Langerak AW, Ritgen M, Stilgenbauer S, Wenger M, Hallek M, German CLL Study Group. Obinutuzumab (GA101) plus chlorambucil (Clb) or rituximab (R) plus Clb versus Clb alone in patients with chronic lymphocytic leukemia (CLL) and preexisting medical conditions (comorbidities): final stage 1 results of the CLL11 (BO21004) phase III trial. Abstract presented at the Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31–June 4 2013b. Abstract No. 7004.
Rai KR, Peterson BL, Appelbaum FR, Tallman MS, Belch A, Morrison VA, Larson RA. Long-term survival analysis of the North American Intergroup Study C9011 comparing fludarabine (F) and chlorambucil (C) in previously untreated patients with chronic lymphocytic leukemia (CLL). Abstract presented at the 51st Annual Meeting and Exposition of the American Society of Hematology, New Orleans, LA, 5–8 December 2009. https://ash.confex.com/ash/2009/webprogram/Paper20588.html. Accessed 7 Aug 2015.
Barendregt JJ. The half-cycle correction: banish it rather than explain it. Med Decic Mak. 2009;29(4):500–2.
Hawe E, Pearson I, Wolowacz S, Haiderali A. Use of external data to guide long-term survival extrapolations of trial data for chronic lymphocytic leukemia. 17th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes Research, Amsterdam, The Netherlands, 8–12 November 2014.
Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol. 2012;12:9.
Briggs A, Claxton K, Sculpher M, editors. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
Statistics Canada. Table 326-0021. Consumer Price Index (CPI), 2011 basket, annual (2002 = 100 unless otherwise noted), CANSIM (database). January 23, 2014. http://www5.statcan.gc.ca/cansim/a26?lang=eng&retrLang=eng&id=3260021&paSer=&pattern=&stByVal=1&p1=1&p2=37&tabMode=dataTable&csid. Accessed 18 Nov 2014.
Ontario Drug Benefit. 2014. https://www.healthinfo.moh.gov.on.ca/formulary/index.jsp. Accessed 17 Jan 2014.
Tam VC, Ko YJ, Mittmann N, Cheung MC, Kumar K, Hassan S, Chan KK. Cost-effectiveness of systemic therapies for metastatic pancreatic cancer. Curr Oncol. 2013;20:e90–106.
Ontario Ministry of Health and Long-term Care. 2014. http://www.health.gov.on.ca/en/public/programs/drugs/programs/odb/opdp_dispensing_fees.aspx. Accessed 30 Jan 2014.
Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84.
Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. Br J Can. 2006;95(6):683–90.
Beusterien KM, Davies J, Leach M, Meiklejohn D, Grinspan JL, O’Toole A, Bramham-Jones S. Population preference values for treatment outcomes in chronic lymphocytic leukaemia: a cross-sectional utility study. Health Qual Life Outcomes. 2010;8:50.
IMS Brogan. Delta PA online database. 2013. https://baeappsw.imshealth.com/IMAC/Dashboard.aspx?Module=DeltaPA. Accessed 27 Jan 2014.
Ferguson J, Tolley K, Gilmour L, Priaulx J. Health state preference study mapping the change over the course of the disease process in chronic lymphocytic leukemia (CLL). Abstract No. PCN79. Abstract presented at the 11th Annual International Meeting of the International Society for Pharmacoeconomics and Outcomes Research, Philadelphia, PA, 20–24 November 2008.
Briggs A. Probabilistic analysis of cost-effectiveness models: statistical representation of parameter uncertainty. Value Health. 2005;8(1):1–2.
Statistics Canada. Table 281-0027. Earnings, average weekly, by province and territory. CANSIM. 2013. http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/labr79-eng.htm. Accessed 11 Dec 2013.
Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programs. 3rd ed. Oxford: Oxford University Press; 2005.
Marsh K, Xu P, Orfanos P, Gordon J, Griebsch I. Model-based cost-effectiveness analyses for the treatment of chronic lymphocytic leukaemia: a review of methods to model disease outcomes and estimate utility. Pharmacoeconomics. 2014;32(10):981–93.
Bansback N, Tsuchiya A, Brazier J, Anis A. Canadian valuation of EQ-5D health states: preliminary value set and considerations for future valuation studies. PLoS One. 2012;7(2):e31115.
Szabo SM, Levy AR, Davis C, Holyoake TL, Cortes J. A multinational study of health state preference values associated with chronic myelogenous leukemia. Value Health. 2010;13(1):103–11.
Acknowledgments
The authors would like to acknowledge the contribution of RTI Health Solutions employees Santiago Zuluaga-Sanchez, James Brockbank and Christopher Graham for technical writing assistance; Emma Hawe and Adam Irving for statistical analyses of COMPLEMENT-1 (survival analyses, treatment consumption, and design of EQ-5D analyses) and overall survival extrapolation guided by external data; and Sarah Mitchell, Matthew Woods and James Brockbank for performing the clinical and economic systematic literature reviews.
The authors also would like to acknowledge the clinical input received from the following: Dr James Kaye of RTI Health Solutions; all of the UK and Canadian clinical and health economics experts who shared their expertise on CLL and economic modelling; Tingting Song of Pharmaceutical Product Development for the utility score regression analysis; and Thomas Delea and Alice Wang of Policy Analysis Inc. for the physician survey analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding for this study was provided to RTI Health Solutions under a research contract with GlaxoSmithKline. Additional funding for the manuscript preparation and revision was provided to RTI Health Solutions under a research contract with Novartis Pharmaceuticals Canada Inc. During the conduct of the study, Dr. Hamid Reza Nakhaipour and Dr. Amin Haiderali were employees of GlaxoSmithKline. Dr. Nakhaipour is currently an employee of Astellas Pharma Inc. Dr. Haiderali is currently an employee of Merck & Co. Inc. Kavisha Jayasundara is an employee of GlaxoSmithKline. Dr. William Herring, Dr. Molly Purser, Dr. Isobel Pearson and Dr. Sorrel Wolowacz are employees of RTI Health Solutions, an independent research organization, and contributed to the design and development of the model, including input parameter identification, data analysis and interpretation of results. All authors participated in the conceptualization of the model and in the preparation of the manuscript. Additionally, Dr. Nakhaipour and Ms Jayasundara contributed to the identification of Canadian-specific input parameters, including the development of the survey of Canadian physicians. Dr. Herring, Dr. Purser, Dr. Pearson and Dr. Wolowacz contributed to the identification of input parameters, the model programming, and the interpretation of analytical results. Dr. Herring will serve as the overall guarantor for the content of the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Herring, W., Pearson, I., Purser, M. et al. Cost Effectiveness of Ofatumumab Plus Chlorambucil in First-Line Chronic Lymphocytic Leukaemia in Canada. PharmacoEconomics 34, 77–90 (2016). https://doi.org/10.1007/s40273-015-0332-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-015-0332-5