Abstract
Background
Chemotherapy prolongs survival for stage III colon cancer patients but community-level evidence on the effectiveness and cost effectiveness of treatment for elderly patients is limited. Comparisons were between patients receiving no chemotherapy, 5-fluorouracil (5-FU), and FOLFOX (5-FU + oxaliplatin).
Methods
A retrospective cohort study was conducted using the Surveillance Epidemiology, and End Results (SEER)–Medicare linked database. Patients (≥65 years) with American Joint Committee on Cancer stage III colon cancer at diagnosis in 2004–2009 were identified. The 3-way propensity score matched sample included 3,534 patients. Effectiveness was measured in life-years and quality-adjusted life-years (QALYs). Medicare costs (2010 US dollars) were estimated from diagnosis until death or end of study.
Results
FOLFOX patients experienced 6.06 median life-years and 4.73 QALYs. Patients on 5-FU had 5.75 median life-years and 4.50 median QALYs, compared to 3.42 and 2.51, respectively, for the no chemotherapy patients. Average total healthcare costs ranged from US$85,422 for no chemotherapy to US$168,628 for FOLFOX. Incremental cost-effectiveness ratios (ICER) for 5-FU versus no chemotherapy were US$17,131 per life-year gained and US$20,058 per QALY gained. ICERs for FOLFOX versus 5-FU were US$139,646 per life-year gained and US$188,218 per QALY gained. Results appear to be sensitive to age, suggesting that FOLFOX performs better for patients 65–69 and 80+ years old while 5-FU appears most effective and cost effective for the age groups 70–74 and 75–79 years.
Conclusion
FOLFOX appears more effective and cost effective than other strategies for colon cancer treatment of older patients. Results were sensitive to age, with ICERs exhibiting a U-shaped pattern.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Overall, 5-fluorouracil was cost effective relative to FOLFOX (5-fluorouracil + oxaliplatin) and no chemotherapy and total healthcare costs increase concomitantly as treatment strategies become more effective. As cancer treatments become more costly, public and private payers and agencies that recommend treatment guidelines may consider efficiency along with effectiveness, safety, and equity in establishing reimbursement policy and treatment guidelines. |
Effectiveness varies with age and cost effectiveness of treatments changes accordingly. Effectiveness may decline with age due to biological constraints associated with development of resistant tumors and increased toxicity. Clinicians and patients may be responding to these changes accordingly for older patients. |
1 Introduction
Chemotherapy has been shown to prolong survival in patients with various malignancies. As noted in breast cancer clinical trials and pooled analyses, the efficacy of chemotherapy decreases with age, from a 27 % proportional reduction in 10-year mortality for women less than 50 years of age to 14 % for women aged 50–59 years and 8 % for women aged 60–69 years, and no benefit for women 70 years or older [1–4]. This pattern has not been reported for men and women with colon cancer. The pooled analysis from seven phase III randomized trials (involving a total of 3,351 patients) showed that adjuvant chemotherapy [5-fluorouracil (5-FU) + leucovorin or 5-FU + levamisole] for stage II and III colon cancer was equally efficacious in prolonging survival for patients <70 and ≥70 years [5]. The combination regimen FOLFOX4 (oxaliplatin + 5-FU/leucovorin) was also efficacious in reducing mortality for patients <70 and ≥70 years [2]. Therefore, evidence-based clinical guidelines on chemotherapy from the US National Institutes of Health (NIH) and other health authorities only recommend chemotherapy for women with breast cancer less than 70 years old, whereas chemotherapy is strongly recommended for patients of all age groups with high-risk stage II or III colon cancer [5–7]. Although randomized controlled clinical trials are the gold standard for determining the efficacy of a therapy, participants often do not represent a cross-section of community patients, and older patients are often under-represented [8, 9]. When randomized trials are not possible due to ethical or logistical issues, well-conducted observational studies can potentially determine the effectiveness and cost effectiveness of therapies. There is a critical need for information on whether chemotherapy is effective and cost effective for patients of varying ages.
The objective of this study was to determine the effectiveness and cost effectiveness of chemotherapy for elderly patients diagnosed with stage III colon cancer from 2004 through 2009. Comparisons are between patients receiving no chemotherapy, 5-FU, and FOLFOX (5-FU + oxaliplatin). For men and women with stage III colon cancer, adjuvant chemotherapy consisting of 5-FU alone or with the addition of leucovorin was recommended as the standard of care by the NIH 1990 consensus conference until November 2004 when the US Food and Drug Administration (FDA) approved the addition of oxaliplatin to the 5-FU/leucovorin, so-called FOLFOX4, for the treatment of patients with stage III colon cancer [10, 11]. Because a substantial number of patients with stage III colon cancer did not receive chemotherapy and some continued to use 5-FU/leucovorin, it is important to compare the effectiveness and cost effectiveness of all treatment groups.
Studies have examined chemotherapy cost and the cost effectiveness of specific agents for the treatment of colorectal cancer [12–14]. Evaluations were based on hypothetical cohorts or modeling of disease progression, whereas health outcomes and costs were based on parameters derived from the literature [15]. While disease stage is included, age has not been examined as an independent factor in the assessment of cost effectiveness of chemotherapy for colon cancer. Although Surveillance Epidemiology, and End Results (SEER)–Medicare data have previously been used to estimate the cost of cancer treatment, these data have not been widely employed for cost-effectiveness analysis of chemotherapy stratified by age [16, 17]. The current study advances the field by applying an economic framework and population-based SEER–Medicare data to assess the cost effectiveness of chemotherapy by age and disease stage under ‘real-world’ conditions for patients with colon cancer.
2 Methods
2.1 Data Source, Population, and Chemotherapy Regimens
Data were obtained from the SEER–Medicare linked database. SEER is a population-based cancer registry with data on tumor site, stage at diagnosis, patient characteristics, and initial treatment after diagnosis. SEER–Medicare data linkage provides information on cancer patients 65 years and older from SEER and their healthcare administrative claims from Medicare. Accuracy and validity of these data have been established [18]. Patients diagnosed with colon cancer as the first primary tumor without other primary tumors at age 65 years or older from January 2004 to December 2009 were selected. Patients were excluded if the diagnosis was based on autopsy, death certificate as the outcome (i.e., death) had already occurred in the patients, and the treatment cannot be assessed. Also, patients were excluded if they died within 30 days of diagnosis as sufficient follow-up data would not be available for such patients. Patients were required to be enrolled in both Medicare Parts A and B without any Health Maintenance Organization (HMO) enrollment from the time of diagnosis to death or end of study. A total of 11,204 patients with AJCC (American Joint Committee on Cancer) stage III colon cancer treated with surgical resection were identified from 16 SEER areas. Chemotherapy receipt was ascertained if associated Medicare claims codes were identified within a year following diagnosis. Patients were characterized into three treatment groups: no chemotherapy, 5-FU, and FOLFOX (5-FU + oxaliplatin).
Multinomial logistic regression analysis was conducted to obtain propensity scores for each treatment group (5-FU as reference). Covariates included age, race, sex, marital status, tumor grade, co-morbidity score, socioeconomic status, year of diagnosis, and state of residence. A 1:1:1 propensity score matching was performed using the nearest neighbor approach [19]. Details about the matching are provided in the Electronic Supplementary Material. The matched sample consisted of 3,534 patients, with 1,178 patients in each group. Patient and tumor characteristics were compared using the chi-square test for categorical and dichotomous variables.
2.2 Effectiveness
Treatment effectiveness was measured in life-years gained and quality-adjusted life-years (QALYs) gained. QALYs were derived after adjusting overall survival time with health state utilities (obtained from literature) specific to disease phase, time since diagnosis, chemotherapy receipt (with or without adverse event), and occurrence of relapse [15, 20–22]. Best et al. [20] derived the health state utilities for adjuvant chemotherapy with/without neuropathy (minor or major) among a combined sample of colorectal cancer patients and community members using the time trade-off technique. Ramsey et al. [21] surveyed colorectal cancer patients and assessed the health state utility of each patient using the health utilities index and grouping them based on time since diagnosis. Patient survival was divided into initial, continuing, and terminal phases, where the initial phase was the first year after diagnosis, the terminal phase was the last year of life, and the continuing phase was the time between the initial and terminal phase. Patients surviving until the end of study (31 December 2010 for Medicare data) were assigned to the initial and continuing phase, while patients surviving for less than a year were assigned to the terminal phase. Patients with less than 2 years of survival had their last year of life assigned to the terminal phase and the remaining to the initial phase [23]. Literature-derived health state utilities are shown in Table 1 for base-, best-, and worst-case scenarios [15, 20–22]. Patients in the initial phase were assigned utilities based on receipt of any chemotherapy and the severity of chemotherapy-related adverse events. Adverse event severity was assessed as ‘moderate’ if reported in outpatient claims and as ‘severe’ if reported in inpatient claims [24]. Adverse events included paresthesia, peripheral neuropathy, neutropenia, thrombocytopenia, and anemia. Continuing phase utilities were based on the time since diagnosis. If there was a gap in chemotherapy for more than 4 months (±15 days) in the continuing phase, a ‘relapse’ was considered to have occurred and a relapse utility was assigned for the remaining continuing phase [25]. Terminal phase utilities were applied based on clinical input and existing literature [21, 22].
QALYs for each phase were computed by multiplying the time spent by each patient in that phase with the associated utility, and total QALYs was obtained by summing all phase-specific QALYs. Since more than 50 % of the data were censored, median QALYs and life-years gained for each chemotherapy regimen were computed using parametric regression with Weibull distribution as it is known to fit survival data well and has been used in the cancer literature previously [26–28]. A 3 % annual discounting was applied to both life-years and QALYs gained.
2.3 Cost Analysis
The Medicare amount paid for each claim was used, and hence a US national payer perspective was adopted for healthcare costs. Total healthcare costs by disease phase were estimated from diagnosis until death or the end of study using claims for inpatient services, outpatient visits and procedures, physician services, skilled nursing facility, hospice, and durable medical equipment as cancer treatment may affect overall morbidity. Cost data from 2004 to 2010 were adjusted for geographical location and inflation using price adjusters developed by Brown et al. [29] (additional information can be found in the Electronic Supplementary Material). Price adjusters were matched with patient’s county at diagnosis using the registry code variable and Federal Information Processing Standard (FIPS) county code from the SEER data. Price adjusters allowed the conversion of costs to 2009 US dollars, and adjustment to 2010 US dollars was conducted using the medical care consumer price index [30]. Similar to clinical effectiveness, a 3 % annual discounting was applied to costs.
2.4 Cost-Effectiveness Analysis
Incremental cost-effectiveness ratios (ICERs) were computed as the ratio of the average incremental total healthcare costs of a chemotherapy regimen divided by the incremental median life-years gained or QALYs gained, i.e., ICER = Δ Cost/Δ Effectiveness. ICERs were calculated for 5-FU versus no chemotherapy and FOLFOX versus 5-FU using life-years gained and QALYs gained as effectiveness measures. Statistical uncertainty was assessed using the bootstrapping methodology to derive 95 % confidence intervals for the ICERs. Using the ICERs from bootstrap samples, proportions of ICERs below a range of willingness-to-pay thresholds were calculated and used to compute cost-effectiveness acceptability curves (CEACs). ICERs were separately calculated for best-case and worst-case scenarios and for different age groups. A fourth treatment group of ‘other-chemo’ was also identified and a secondary analysis was conducted using all four chemotherapy treatment groups. Given the limitations of propensity score matching for more than three groups, an inverse probability treatment weight (IPTW) approach was used to adjust for selection bias [31, 32].
3 Results
Of the 11,204 patients, 10,252 received either no chemotherapy (n = 5,322), 5-FU (n = 1,860), or FOLFOX (n = 3,070). The remaining 952 patients were categorized in the other chemotherapy group. The proportion of patients receiving chemotherapy was consistent with previous studies [33, 34]. For example, the study by Schrag et al. [33] found 55 % of stage III colon cancer patients received adjuvant chemotherapy. The matched sample consisted of 3,534 patients, with 1,178 patients in each group. Patient and tumor characteristics for the matched sample are presented in Table 2 (characteristics for unmatched sample and multinomial regression results are shown in tables 1 and 2 of the Electronic Supplementary Material, respectively). Matched groups were similar across all the characteristics, with no statistically significant differences between the groups. Electronic Supplementary Material table 3 shows the total healthcare costs per month for each chemotherapy regimen and disease phase. Higher costs were observed in the initial and terminal phases of care relative to the continuing phase. During the initial phase, FOLFOX costs were highest at US$9,771/month followed by 5-FU (US$6,553/month). Continuing phase costs for the no-chemotherapy (US$1,029/month) and FOLFOX (US$1,874/month) groups were relatively lower than for the 5-FU group (US$2,012/month). In contrast, the no-chemotherapy group (US$13,222/month) was most expensive in the terminal phase of disease followed by FOLFOX (US$10,115/month) and 5-FU (US$8,586/month).
Table 3 shows FOLFOX had the longest median life-years (6.06) and median QALYs (4.73). A larger relative difference was seen between 5-FU and no chemotherapy regimen for median life-years (5.75 vs. 3.42) and median QALYs (4.50 vs. 2.51) than between FOLFOX and 5-FU for median life-years (6.06 vs. 5.75) and median QALYs (4.73 vs. 4.50). Average total healthcare costs for chemotherapy regimens ranged from US$85,422 for no chemotherapy to US$168,628 for FOLFOX. The ICER for 5-FU versus no chemotherapy was US$17,131 (95 % CI 6,262–25,378) per life-year gained and US$20,058 (95 % CI 6,298–34,720) per QALY gained. Alternatively, ICERs for FOLFOX versus 5-FU were US$139,646 (95 % CI −261,578–313,229) per life-year gained and US$188,218 (95 % CI −177,778–279,719) per QALY gained. See Electronic Supplementary Material table 4 for scenario results.
The cost-effectiveness plane for 5,000 bootstrap samples and the CEAC computed from bootstrap samples are shown in Electronic Supplementary Material figures 1 and 2, respectively. Almost all of the replicates were in the North-East quadrant of the cost-effectiveness planes, indicating that the effectiveness increases with a corresponding increase in costs. The CEAC curves (Electronic Supplementary Material Figure 1) demonstrated that at a willingness to pay of US$25,000 per QALY, 5-FU had a 90 % chance of being cost effective as compared with no chemotherapy. FOLFOX was observed to be 90 % cost effective relative to 5-FU at a willingness to pay of US$75,000 per QALY. However, a higher degree of uncertainty was observed.
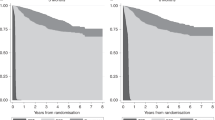
Overall, life-years gained and QALYs gained decreased as age increased (Fig. 1). QALYs for FOLFOX decreased steadily and notably for age categories 70–74 and 75–79 years, falling below the corresponding QALYs for 5-FU. As a result, FOLFOX was dominated (less effective and more costly) by 5-FU in patients 70–74 and 75–79 years old. As compared with no chemotherapy the ICER/QALY for 5-FU was US$14,132 for 70–74 years and US$10,582 for 75–79 years (Table 4). In contrast, FOLFOX showed extended dominance (lower ICER) over 5-FU for the age group 65–69 years (ICER/QALY: US$26,327) and the age group 80+ years (ICER/QALY: $141,727) compared with no chemotherapy. Cost-effectiveness analyses that included the ‘other’ chemotherapy group and all of the 11,204 patients using the IPTW approach showed results similar to the propensity score approach (see Electronic Supplementary Material table 5).
4 Discussion
Overall, 5-FU was cost effective relative to FOLFOX and no chemotherapy using a willingness-to-pay threshold of US$100,000 per QALY. Chemotherapy effectiveness declined with age and the cost effectiveness of alternative treatments varied by age. FOLFOX median life-years steadily declined from 8.38 for patients 65–69 years old to 4.43 for those 80+ years old. For 5-FU median life-years were relatively stable for patients 65–79 years old, ranging from 6.21 to 6.66, and then falling to 3.85 for patients 80+ years old. FOLFOX was most cost effective for patients 65–69 and 80+ years of age, while 5-FU was most cost effective (dominating FOLFOX) in the 70–74 and 75–79 age groups. Effectiveness may be expected to decline with age due to biological constraints such as the development of more resistant tumors, and increased sensitivity to chemotherapy toxicities leading to the administration of lower doses for older patients. These decisions may well be in accord with patient preferences. Dose reduction for older patients was observed in this study as the percentage of patients receiving four cycles of 5-FU decreased as age increased, from 69 % in 65–69 years to 62 % in those aged 80 years or older. Similarly, the percentage of patients receiving eight cycles of FOLFOX decreased from 68 % in patients 65–69 years to 54 % in those aged 80 years or older. However, the finding that FOLFOX was more effective than 5-FU for the youngest and oldest age groups requires additional investigation. Another explanation for the differential effectiveness observed may be related to smaller sample sizes in the older age subgroups.
The MOSAIC (Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer) trial was used to estimate the cost effectiveness of oxaliplatin compared with 5-FU/leucovorin in adjuvant treatment of stage III colon cancer from the US Medicare program perspective [35]. Initial treatment utilization and cost data were obtained from the trial and the cost of toxicities and recurrence of disease were estimated based on standard treatment pathways. MOSAIC included patients with a median age of 61 years (range 19–75) compared with a median age of 75 years (range 65–97) in the current study. Thus, life-years and QALYs using the FOLFOX regimen were higher in the MOSAIC study at 12.34 and 9.94, compared with the current study gain with FOLFOX of 6.06 life-years and 4.73 QALYs. Comparing FOLFOX to 5-FU/leucovorin, estimated gains in life-years of 0.83 and QALYs of 0.75 were also greater in the MOSAIC study than in the current study (gain in life-years = 0.31, gain in QALYS = 0.23). MOSAIC costs in 2003 US dollars were much lower for FOLFOX and 5-FU/leucovorin at US$56,320 and US$39,285 than in the current study: US$168,628 and US$125,338 (2010 US dollars). The latter costs were based on total healthcare costs, whereas the former were cancer-related costs. Consequently, the ICER for FOLFOX compared with 5-FU/leucovorin is much higher in the current study (life-years = US$139,646, QALYs = US$188,218) than in MOSAIC (life-years = US$20,603, QALYS = US$22,804). The cost-effectiveness results were similar to MOSAIC for life-years gained for the age group 65–69 years in the current study: ICER = US$20,030 compared with US$20,603 for all participants in MOSAIC. In contrast, the current study found that FOLFOX was dominated (higher cost and less effective) by 5-FU/leucovorin in the age groups 70–74 and 75–79 years. FOLFOX compared with 5-FU/leucovorin had an ICER for life-years and QALYs of US$90,188 and US$108,977, respectively, for the 80+ years group. Howard et al. utilized 1995–2005 SEER–Medicare data to estimate the cost effectiveness of chemotherapeutic agents applied to five groups of patients with stage IV metastatic colorectal cancer defined by treatment regimens [36]. Agents included irinotecan hydrochloride, capecitabine, oxaliplatin, bevacizumab, and cetuximab. Costs were based on Medicare reimbursements inflated to 2006 US dollars. Effects were estimated from survival curves and adjusted for quality of life with an assumed health utility after diagnosis of 0.8. The cost per QALY gained was US$99,100 comparing patients treated with chemotherapeutic agents with those not treated with chemotherapeutic agents. Results were sensitive to the utility weight assumptions. This ICER is much greater than the estimated ICER in the current study (US$20,058) for QALYS comparing the no-chemotherapy group to the 5-FU/leucovorin group; this is due partially to the inclusion of patients with more advanced metastatic disease in the Howard et al. study [36].
In order to reduce selection bias, 1:1:1 propensity score matching was employed to balance treatment groups across patient and tumor characteristics in the current study. Patients were dropped from the analytical sample and the approach was limited to comparisons among three groups. The three group limitation was addressed by conducting an IPTW analysis that used the entire cohort of 11,204 patients and included the ‘other’ chemotherapy group. Propensity score matching does not control for unobservable factors that may render the no-chemotherapy patients with a worse effectiveness profile. Given that some stage III colon cancer patients have a long survival and more than 50 % of the study patients were alive at the study end date, a Weibull distribution parametric regression model was applied to estimate the median overall survival [26, 28, 37, 38]. Health state utilities from the literature were assigned based on time since diagnosis, presence/absence of toxicity, and occurrence of relapse. Alternate scenarios (best case and worst case) accounted for methodological uncertainty for health state utilities. The study only includes stage III colon cancer patients 65 years and older from participating SEER regions with continuous Medicare Parts A and B without HMO enrollment from date of diagnosis to death or end of study. Thus, the findings may not be generalizable to younger populations, HMO members, and non-SEER participating regions of the USA. Only direct healthcare costs were assessed, and thus it lacks societal valuation. However, the payer perspective is important for healthcare program decision makers.
5 Conclusion
Patients diagnosed with stage III colon cancer who are over 65 years old gain life-years and QALYs when treated with FOLFOX rather than 5-FU or no chemotherapy. Total healthcare costs increase concomitantly as treatment strategies become more effective. Given the cost differential, the ICER for 5-FU compared with no chemotherapy is well within the commonly referenced US$100,000 per QALY gained threshold, and FOLFOX compared with 5-FU is well beyond the threshold [39]. Of note is that the results of this analysis vary according to age. Specifically, FOLFOX appears to be more effective and cost effective than other treatments for the 65–69 years age group and the 80+ years age group. In contrast, 5-FU appears to be the most effective and cost effective for age groups 70–74 and 75–79 years, and is well within the US$100,000 threshold. These findings raise questions about why results shift by age group and need to be replicated in additional studies and larger populations. While Medicare is currently prohibited from using cost-effectiveness analysis results in decision making, other influential national agencies such as the Veterans Administration Health System and the NIH expert panel and guideline committees may consider cost-effectiveness data in making recommendations [40]. Physician groups support the need to consider cost relative to the effectiveness of treatment alternatives given limited resources and the increasing cost of drugs and other medical services [41, 42].
References
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–717.
Cole BF, Gelber RD, Gelber S, Coates AS, Goldhirsch A. Polychemotherapy for early breast cancer: an overview of the randomised clinical trials with quality-adjusted survival analysis. Lancet. 2001;358(9278):277–86.
Polychemotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;352(9132):930–42.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Clarke M, Coates AS, Darby SC, Davies C, Gelber RD, et al. Adjuvant chemotherapy in oestrogen-receptor-poor breast cancer: patient-level meta-analysis of randomised trials. Lancet. 2008;371(9606):29–40.
Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller DG, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345(15):1091–7.
Goldberg RM, Tabah-Fisch I, Bleiberg H, de Gramont A, Tournigand C, Andre T, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24(25):4085–91.
NIH Consensus Conference. Adjuvant therapy for breast cancer. J Natl Cancer Inst. 2001;93:979–89.
Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061–7.
Lewis JH, Kilgore ML, Goldman DP, Trimble EL, Kaplan R, Montello MJ, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21(7):1383–9.
Grothey A, Sargent DJ. FOLFOX for stage II colon cancer? A commentary on the recent FDA approval of oxaliplatin for adjuvant therapy of stage III colon cancer. J Clin Oncol. 2005;23(15):3311–3.
Food and Drug Administration. Eloxatin: new or modified indication. Washington, DC: US Food and Drug Administration; 2004.
Hillner BE, Schrag D, Sargent DJ, Fuchs CS, Goldberg RM. Cost-effectiveness projections of oxaliplatin and infusional fluorouracil versus irinotecan and bolus fluorouracil in first-line therapy for metastatic colorectal carcinoma. Cancer. 2005;104(9):1871–84.
Mullins CD, Hsiao F, Onukwugha E, Pandya NB, Hanna N. Comparative and cost-effectiveness of oxaliplatin-based or irinotecan-based regimens compared with 5-fluorouracil/leucovorin alone among US elderly stage IV colon cancer patients. Cancer. 2012;118(12):3173–81.
Pandor A, Eggington S, Paisley S, Tappenden P, Sutcliffe PA. The clinical and cost-effectiveness of oxaliplatin and capecitabine for the adjuvant treatment of colon cancer: systematic review and economic evaluation. Health Technol Assess. 2006;10(41):1–204.
Attard C, Maroun J, Alloul K, Grima D, Bernard L. Cost-effectiveness of oxaliplatin in the adjuvant treatment of colon cancer in Canada. Curr Oncol. 2010;17(1):17.
Etzioni R, Ramsey SD, Berry K, Brown M. The impact of including future medical care costs when estimating the costs attributable to a disease: a colorectal cancer case study. Health Econ. 2001;10(3):245–56.
Brown ML, Riley GF, Potosky AL, Etzioni RD. Obtaining long-term disease specific costs of care: application to Medicare enrollees diagnosed with colorectal cancer. Med Care. 1999;37(12):1249–59.
Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8):IV-3–-18.
Rassen JA, Doherty M, Huang W, Schneeweiss S. Pharmacoepidemiology toolbox; 2013. http://www.drugepi.org/wp-content/uploads/2013/10/Using_the_Pharmacoepi_Toolbox_in_SAS_2.4.15.pdf. Accessed 20 Feb 2014.
Best JH, Garrison LP, Hollingworth W, Ramsey SD, Veenstra DL. Preference values associated with stage III colon cancer and adjuvant chemotherapy. Qual Life Res. 2010;19(3):391–400.
Ramsey SD, Andersen MR, Etzioni R, Moinpour C, Peacock S, Potosky A, et al. Quality of life in survivors of colorectal carcinoma. Cancer. 2000;88(6):1294–303.
Allen E, Nicolaidis C, Helfand M. The evaluation of rectal bleeding in adults. J Gen Intern Med. 2005;20(1):81–90.
Sail KR, Franzini L, Lairson DR, Du XL. Clinical and economic outcomes associated with adjuvant chemotherapy in elderly patients with early stage operable breast cancer. Value Health. 2012;15(1):72–80.
Havrilesky LJ, Broadwater G, Davis DM, Nolte KC, Barnett JC, Myers ER, et al. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol. 2009;113(2):216–20.
Earle CC, Nattinger AB, Potosky AL, Lang K, Mallick R, Berger M, et al. Identifying cancer relapse using SEER-Medicare data. Med Care. 2002;40(8):IV-75–81.
Gray AM, Clarke PM, Wolstenholme JL, Wordsworth S. Applied methods of cost-effectiveness analysis in healthcare. Oxford: Oxford University Press; 2010.
Wu B, Dong B, Xu Y, Zhang Q, Shen J, Chen H, et al. Economic evaluation of first-line treatments for metastatic renal cell carcinoma: a cost-effectiveness analysis in a health resource-limited setting. PLoS One. 2012;7(3):e32530.
Zhu H, Xia X, Yu C, Adnan A, Liu S, Du Y. Application of Weibull model for survival of patients with gastric cancer. BMC Gastroenterol. 2011;11(1):1.
Brown ML, Riley GF, Schussler N, Etzioni R. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002;40(8):IV-104–17.
Medical care—consumer price index; 2013. http://data.bls.gov/timeseries/CUUR0000SAM?output_view=pct_12mths. Accessed 20 Feb 2014.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424.
Chitnis AS, Aparasu RR, Chen H, Johnson ML. Effect of certain angiotensin-converting enzyme inhibitors on mortality in heart failure: a multiple-propensity analysis. Res Soc Admin Pharm. 2012;8(2):145–56.
Schrag D, Cramer LD, Bach PB, Begg CB. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 2001;93(11):850–7.
Panchal JM, Lairson DR, Chan W, Du XL. Geographic variation and sociodemographic disparity in the use of oxaliplatin-containing chemotherapy in patients with stage III colon cancer. Clin Colorectal Cancer. 2013;12(2):113–21.
Aballéa S, Chancellor JV, Raikou M, Drummond MF, Weinstein MC, Jourdan S, et al. Cost-effectiveness analysis of oxaliplatin compared with 5-fluorouracil/leucovorin in adjuvant treatment of stage III colon cancer in the US. Cancer. 2007;109(6):1082–9.
Howard DH, Kauh J, Lipscomb J. The value of new chemotherapeutic agents for metastatic colorectal cancer. Arch Intern Med. 2010;170(6):537.
Bradburn M, Clark T, Love S, Altman D. Survival analysis part II: multivariate data analysis–an introduction to concepts and methods. Br J Cancer. 2003;89(3):431.
Bradburn M, Clark T, Love S, Altman D. Survival analysis part III: multivariate data analysis—choosing a model and assessing its adequacy and fit. Br J Cancer. 2003;89(4):605.
Malin JL. Wrestling with the high price of cancer care: should we control costs by individuals’ ability to pay or society’s willingness to pay? J Clin Oncol. 2010;28(20):3212–4.
Mason A, Drummond M, Ramsey S, Campbell J, Raisch D. Comparison of anticancer drug coverage decisions in the United States and United Kingdom: does the evidence support the rhetoric? J Clin Oncol. 2010;28(20):3234–8.
American College of Physicians. Information on cost-effectiveness: an essential product of a national comparative effectiveness program. Ann Intern Med. 2008;148:956–61.
Murden RA, Seiber EE. How can cost-effectiveness information help control unsustainable growth in U.S. health care spending? [letter]. Ann Intern Med. 2009;150(1):58.
Acknowledgments
This study was supported by a grant from the Agency for Healthcare Research and Quality (R01-HS018956) and in part by a grant from Cancer Prevention Research Institute of Texas (RP130051). The authors have no conflicts of interest to declare. Authors D.L., R.P., and X.D. were primarily responsible for study design, data analysis, and manuscript writing. Authors J.C. and W.C. were responsible for study design, interpretation of data, and critical review of the manuscript. D.L. is the overall guarantor for the content.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lairson, D.R., Parikh, R.C., Cormier, J.N. et al. Cost–Utility Analysis of Chemotherapy Regimens in Elderly Patients with Stage III Colon Cancer. PharmacoEconomics 32, 1005–1013 (2014). https://doi.org/10.1007/s40273-014-0180-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-014-0180-8