Abstract
Background
Most existing models of smoking cessation treatments have considered a single quit attempt when modelling long-term outcomes.
Objective
To develop a model to simulate smokers over their lifetimes accounting for multiple quit attempts and relapses which will allow for prediction of the long-term health and economic impact of smoking cessation strategies.
Methods
A discrete event simulation (DES) that models individuals’ life course of smoking behaviours, attempts to quit, and the cumulative impact on health and economic outcomes was developed. Each individual is assigned one of the available strategies used to support each quit attempt; the outcome of each attempt, time to relapses if abstinence is achieved, and time between quit attempts is tracked. Based on each individual’s smoking or abstinence patterns, the risk of developing diseases associated with smoking (chronic obstructive pulmonary disease, lung cancer, myocardial infarction and stroke) is determined and the corresponding costs, changes to mortality, and quality of life assigned. Direct costs are assessed from the perspective of a comprehensive US healthcare payer ($US, 2012 values). Quit attempt strategies that can be evaluated in the current simulation include unassisted quit attempts, brief counselling, behavioural modification therapy, nicotine replacement therapy, bupropion, and varenicline, with the selection of strategies and time between quit attempts based on equations derived from survey data. Equations predicting the success of quit attempts as well as the short-term probability of relapse were derived from five varenicline clinical trials.
Results
Concordance between the five trials and predictions from the simulation on abstinence at 12 months was high, indicating that the equations predicting success and relapse in the first year following a quit attempt were reliable. Predictions allowing for only a single quit attempt versus unrestricted attempts demonstrate important differences, with the single quit attempt simulation predicting 19 % more smoking-related diseases and 10 % higher costs associated with smoking-related diseases. Differences are most prominent in predictions of the time that individuals abstain from smoking: 13.2 years on average over a lifetime allowing for multiple quit attempts, versus only 1.2 years with single quit attempts. Differences in abstinence time estimates become substantial only 5 years into the simulation. In the multiple quit attempt simulations, younger individuals survived longer, yet had lower lifetime smoking-related disease and total costs, while the opposite was true for those with high levels of nicotine dependence.
Conclusion
By allowing for multiple quit attempts over the course of individuals’ lives, the simulation can provide more reliable estimates on the health and economic impact of interventions designed to increase abstinence from smoking. Furthermore, the individual nature of the simulation allows for evaluation of outcomes in populations with different baseline profiles. DES provides a framework for comprehensive and appropriate predictions when applied to smoking cessation over smoker lifetimes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
• Future economic evaluations of smoking cessation will be enhanced by the application of discrete event simulation techniques.
• The model described in this paper makes predictions for treatment success and smoking outcomes based on individual smoker profiles, sequences of smoking cessation strategies, time interval between quit attempts, and relapse.
• Analyses conducted to confirm clinical trial replication validate the model’s prediction of treatment success.
• Allowing smokers to experience multiple quit attempts shows substantial differences in population health outcomes and costs, compared to a single quit attempt.
1 Background
Smoking is the leading cause of preventable death in the US and results in many serious smoking-related diseases, including lung cancer, coronary heart disease, stroke and chronic respiratory disease [1, 2]. While smoking prevalence in the US has recently declined, 19 % of US adults currently smoke [3]. While many smokers are motivated to attempt to quit, few find they are able to do so easily and many subsequently relapse [4–6]. Tobacco dependence can be viewed as a chronic disease [7] and often requires multiple attempts to quit. For example, in one study, approximately one in four individuals who made a quit attempt reported they had still stopped smoking 8–10 months later [5].
Unassisted quit attempts are common; however, the use of smoking cessation medications has been increasing and in several recent surveys, 40 % or more are using medications to support an attempt [8–10]. The US Public Health Service recommends the use of nicotine replacement therapy (NRT), bupropion, or varenicline (Chantix®) [7]. While smoking cessation medications have been shown to increase success rates, sustaining abstinence is difficult, since many relapse and return to smoking until they are motivated to make another attempt to stop [11, 12].
To date, many economic models evaluating the impact of smoking cessation interventions have evaluated the impact of a single quit attempt on long-term health and economic outcomes, and have often assumed that smokers who fail in their quit attempt or who succeed, but later relapse, remain smokers for the remainder of their life [13]. This simplifying assumption is clearly not reflective of the actual patterns of smoking cessation attempts, successes and failures, and could potentially lead to biased predictions of the long-term effectiveness and cost effectiveness of smoking cessation aids or strategies. While a few models have looked at multiple attempts, they have required major simplifying assumptions such as assuming a ‘natural’ quit rate [14] or a background quit attempt rate [15].
The discrete event simulation (DES) described here builds upon an earlier DES model which evaluated the impact of treatments over a 1-year time period. [16] The initial 1-year model used subject-level predictions for the success of quit attempts and relapse over a 52-week period. The current model also incorporates sequences of smoking cessation strategies, intervals between quit attempts, short- and long-term relapse risk and, depending on risk exposure, predicts incidence of smoking-related diseases, and consequent mortality and costs. Furthermore, whereas the initial model [16] predicted success and relapse based on two pivotal varenicline clinical trials, the current predictions are based on a broader pool of trial data. The objective of this study was to simulate the multiple quit attempts and relapses of individuals and to provide a realistic representation of smoking cessation behaviour over a lifetime. The model was developed from a comprehensive US healthcare payer perspective.
2 Methods
2.1 Model Overview
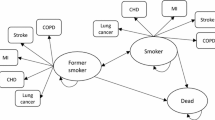
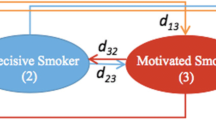
This study is based on a simulation of individuals’ lifetime smoking behaviours, attempts to quit, and the impact on health outcomes. Based on each individual’s smoking or abstinence patterns, the risks of developing smoking-related diseases (chronic obstructive pulmonary disease [COPD], lung cancer, myocardial infarction [MI] and stroke) are determined and the corresponding costs and changes in mortality and quality of life are estimated. The Smoking Cessation Treatment and Outcomes Patterns (STOP) simulation was programmed in ARENA v12 (Rockwell Software, Inc., Warrendale, PA, USA). Fig. 1 provides the conceptual framework for the model, while Fig. 2 is a representation of the model flow. At the beginning of the simulation, a cohort of smokers is created by sampling from subject-level data with individual patient characteristics collected at baseline of five varenicline trials [17–21]. Individuals then start the simulation by either immediately making a quit attempt or being assigned a time for their next quit attempt.
Based on the individual patient’s characteristics and assigned quitting method, the outcome of the initial quit attempt is predicted. If the quit attempt is a failure (i.e., the individual fails to achieve abstinence at the end of the 12-week quit attempt), the time to next attempt and the cessation strategy to support the subsequent attempt are assigned. If successful (i.e., the individual achieves abstinence at the end of the 12-week quit attempt), individuals are at risk of relapsing, and a time to relapse is assigned. Upon relapse, individuals are assigned a time to next quit attempt.
Individualized predictions for treatment success, sequences of smoking cessation strategies, intervals between quit attempts, and relapse are applied throughout the simulation with outcomes being based on the current value of the subject’s pertinent characteristics (e.g., age, smoking history). That is, during the simulation, depending on patient characteristics, smoking history, prior quitting behaviour and medical history, each individual carries risks of events which are calculated as a unique time for each possible event (e.g., smoking-related disease, quitting, relapse, quit attempt, death) based on random sampling from appropriate probability distributions. These risks and the accrual of costs and quality-adjusted life years (QALYs) are updated over time as the individual passes through the model and their characteristics (e.g., smoking status) get modified.
2.2 Data Sources/Population
Each simulated individual has a profile that includes age, sex, race, medical history (history of COPD or cardiovascular disease [CVD]), smoking history (years smoked, age started, reported average number of cigarettes per day in the past month, reported average number of cigarettes per day since start of smoking), Fagerström Test for Nicotine Dependence (FTND) score, and whether each subject has frequent contact with a smoker. In addition, each profile includes details of their prior quit attempts comprising the longest period of abstinence in the past year, number of previous quit attempts and the smoking cessation strategy used in each attempt. Table 1 contains baseline characteristics of the patients from the clinical trial according to medical history (i.e., COPD history alone, CVD history alone, COPD and CVD history, no history of COPD or CVD). Strategies that can be assessed in the simulation are unassisted quit attempts, brief counselling alone, behaviour modification therapy (BMT), NRT, bupropion and varenicline.
STOP creates individual smokers for the simulation by sampling from patient-level data from five 52-week varenicline clinical trials [17–21]:
-
Two double-blind trials comparing varenicline with placebo and bupropion [17, 18].
-
A randomized, open-label trial comparing varenicline with NRT [19].
-
A double-blind trial comparing varenicline with placebo in smokers with stable cardiovascular disease [20].
-
A double-blind trial comparing varenicline with placebo in patients with mild to moderate COPD [21].
All interventions in each of the trials were accompanied by brief counselling.
Each of the clinical trials comprised a 12-week treatment period and a subsequent 40-week non-treatment follow-up period. A quit attempt was considered to be successful if the subject was abstinent during weeks 9–12 of the treatment period.
In order to reflect the distribution of smokers with a history of COPD and CVD in the US, sampling weights were assigned to each individual in the simulation according to estimated age and sex-specific prevalence of these diseases in US smokers [22–26]. For males and females respectively, the proportion of the simulated population with a history of COPD alone was 5.0 % and 1.7 %, for CVD alone 28.5 % and 24.8 %, and for both diseases, 1.5 % and 0.4 %.
The clinical trials contained some information on the nature of previous quit attempts (prior unassisted, prior non-medically assisted, and prior medical quit attempts), but did not have information on the actual strategy employed in the five most recent quit attempts, a significant variable in the predictive equation for the assignment of interventions to be used in subsequent quit attempts (described later). This information had to be imputed from another source. A US-based web survey of current and former smokers containing this information was utilized [10]; random sampling from these patient profiles was conducted while ensuring that the quit attempt classification matched that of the clinical trials. For example, if an individual had only a prior unassisted quit attempt according to the clinical trial profile, random sampling from the survey profiles is restricted to those profiles that match this quit attempt history.
2.3 Smoking Cessation Patterns
2.3.1 Smoking Cessation Strategies
Smokers can initiate a quit attempt with one of six cessation strategies: unassisted, brief counselling alone, BMT, NRT, bupropion, or varenicline. In the analyses presented here, the first quit attempt intervention is assigned using data from a 2010 US market survey [9]: unassisted quit attempts (46 %), NRT (27.6 %), BMT alone (9.9 %), varenicline (9.5 %), or bupropion (7 %).
Brief counselling, which best represents the placebo arm in the head-to-head comparisons of the clinical trials, was not included as a category in the market survey, and is therefore not included in these analyses except for the purposes of validation (see Sect. 3 for a description of the validation analyses).
The interventions used for subsequent quit attempts are predicted by a multinomial logistic regression developed by analysis of data collected from the US-based web survey [10]. The regression includes terms for interventions used in the five most recent quit attempts, as well as the number of previous quit attempts, and race (white vs. other) (parameter estimates are provided in Supplement Table S1 [see electronic supplementary material (ESM)]). Individuals were more likely to use pharmacological interventions if they had used them in the past; also, the greater the number of previous quit attempts, the more likely it was that individuals would use pharmacological interventions. The simulation also allows for assignment of interventions based on user-defined probability distributions for subsequent quit attempts. If this option is selected, quit attempt interventions are still assigned probabilistically, but subject characteristics are no longer considered when making the assignment.
2.3.2 Quit Attempt Outcome
Individualized probabilities (i.e., probabilities that account for patient characteristics) of abstinence are applied to determine the outcome of each quit attempt using a logistic regression (Table 2) derived from the varenicline clinical trials [17–21]. The probability of success is a function of the cessation aid being utilized, nicotine dependence, demographics, smoking history and medical history. Because the NRT trial [19] was designed as an open-label trial, the regression included a dummy variable for open-label versus double-blind study design. In the equation shown in Table 2, this term is absorbed into the intercept.
The logistic regression results indicate that subjects on varenicline have the highest success rates. While age is associated with a higher probability of success, this is partially counteracted by the lower success associated with number of years smoked. Greater nicotine dependence, frequent previous quit attempts, and female sex were associated with lower success rates. Success rates were also influenced by race. Somewhat surprisingly, individuals with COPD were also less likely to successfully quit, especially if attempting without the assistance of pharmacologic therapies.
As no data from the trials were available for unassisted quit attempts and BMT, odds ratios from the literature were used to modify the logistic regression results for quit attempt success using these strategies. For unassisted quit attempts, an odds ratio of 0.097 versus varenicline was derived based on a study which provided a success rate of 10.9 % at 12 weeks with unassisted quit attempts. [4, 19] For BMT, an odds ratio of 1.524 versus brief counselling was derived using information from each of the trials included in a meta-analyses that assessed quit attempt success with individual behavioural counselling versus minimal contact (i.e., up to 10 min of advice, with or without the provision of self-help materials) [27].
2.3.3 Relapse
For those individuals who make a successful quit attempt (i.e., remain abstinent after 12 weeks), the individualized risk of relapse within the first year is estimated using data from the five varenicline trials [17–21]. Parametric distributions failed to capture the observed distributions of abstinence duration. Thus, a series of interconnecting segments of Kaplan–Meier curves were fitted, with distinct segments specified for weeks 0–13 and weeks 14 or later. The following equation for the underlying risk of relapse was fitted:
where the time variables and coefficients were defined as follows:
where t represents time after a successful quit attempt. The observed probability of relapse across the five trials for weeks 0–13 and weeks 14 or later after a successful quit attempt is 36.6 % and 23.3 %, respectively.
A Cox proportional hazards model was then fit to factor in the predictors of risk of relapse (Table 3). Significant predictors included age, measures of smoking history (e.g., number of years smoked), frequent contact with smokers, and a history of COPD (Table 3). Older individuals were at lower risk of relapse, although individuals who started smoking at an older age were more likely to relapse, as were those who had smoked longer and at a higher frequency. Again, subjects with COPD were more likely to relapse. Treatment was not a significant predictor for the risk of relapse. The annual probability of relapse following the first year from the start of a successful quit attempt was derived from a published study that examined sustained abstinence among former smokers [28], with the annual probability of relapsing assigned as follows:
-
year 1–2: 14.5 %
-
years 2–5: 2.8 %
-
>5 years: 2.1 %
2.3.4 Time Until Next Quit Attempt
The time from relapse to the initiation of a subsequent quit attempt is estimated from the US-based web survey [10]. The prediction, which assumes an exponential distribution for time to next quit attempt, is individualized by incorporating an individual smoker’s number of previous quit attempts, sex, and race (parameter estimates are provided in Supplement Table S2 [see ESM]).
2.4 Smoking-Related Diseases
STOP captures the impact of smoking cessation on the incidence of four smoking-related diseases (COPD, lung cancer, MI, stroke) as a function of age and also the duration of abstinence. Each simulated individual is categorized as either a current smoker or abstinent (considered as a former smoker) throughout their lifetime.
The annual probability (p) of an individual developing each of the smoking-related diseases (COPD, lung cancer, MI and stroke) is determined assuming an exponential distribution:
This equation provides the probability of developing a smoking-related disease in a given year (t = 1) for an individual with annual hazard equal to λ, which is assigned conditional on their current smoking status.
The hazards for developing these four smoking-related diseases for current and former smokers (λ current and λ former) are determined as follows:
where, λ never is the hazard among never smokers, RRcurrent is the relative risk among current smokers, RRformer is the relative risk among former smokers.
RRformer during the simulation are determined for each of the four smoking-related diseases as a function of the individual’s age and duration of abstinence using the model published by Hoogenveen et al. [29] (disease-specific coefficient estimates are provided in Supplement Table S3 [see ESM]). The derived relative risk estimates for former smokers decrease with longer abstinence, thereby allowing the simulation to appropriately capture the cumulative benefits of sustained abstinence.
Age- and gender-stratified relative risks for current versus non-smokers were derived for each of the four smoking-related diseases from the event rates observed in the Cancer Protection Study (CPS) II [22]. Age- and gender-specific λ never estimates were derived by combining the relative risks calculated from CPS II, with data on the incidence of lung cancer, COPD, MI and stroke obtained from published sources [23, 24, 26, 30–33]. Estimates of the population size and age- and sex-dependent smoking prevalence statistics were obtained from the US census [25]. Specifically, the overall incidence (i overall), of smoking-related diseases (for any sex and age category) can be decomposed as follows:
where P refers to the proportion of individuals in each smoking category. This equation can be further broken down to incorporate the relative risks, as follows:
This equation can be manipulated algebraically to yield the age- and sex-specific annual incidence in never-smokers (i.e., λ never).
Patients without a history of COPD or CVD at baseline can experience each of the smoking-related diseases once during the simulation. Patients that enter the model with COPD or CVD (MI, stroke, or both) history will not be subject to the risk of developing the particular smoking-related disease over the course of the simulation.
2.4.1 Mortality
For individuals without a history of COPD, lung cancer, stroke or MI, a time of death is assigned at the start of the simulation as a function of each individual’s age and gender and is modelled using Gompertz functions derived from US life tables (parameter estimates are provided in Supplement Table S4 [see ESM]) [34]. Individuals entering the simulation with a history of smoking-related disease are assigned a time to death based on disease-specific mortality functions. When a smoking-related disease develops during the simulation, the time of death is updated with the minimum of the previously estimated time of death and the new predicted time.
Post-MI exponential survival functions were fitted to published Kaplan–Meier curves [35]. The probability of death at the time of the event was obtained from the same publication (44 % for males and 45 % for females). The case fatality rate applied in the model is independent of all other patient characteristics.
Parametric survival distributions failed to capture the observed distributions in published Kaplan–Meier curves for COPD [36], lung cancer [37] and stroke [38]. Linear models were fit to log-log survival versus log-time. For COPD and stroke, a series of interconnecting segments of the Kaplan–Meier curves were fitted. Additional details on the equations used to predict time to death are provided in the supplement along with the coefficient estimates for each of the models (Supplement Table S5 [see ESM]).
2.5 Health Utilities
During the simulation, an individual’s utility is set to the minimum of the individual’s age-dependent utility, and the utility associated with any of the smoking-related diseases that the individual has experienced up to that point in time. This ensures that the model has accounted for the impact of aging and all smoking-related diseases on the individual’s utility. Age-dependent health utility values were assigned using the following equation based on reported European Quality of Life-5 Dimensions (EQ-5D) index scores of a nationally representative sample of US adults participating in the 2000–2002 Medical Expenditure Panel Survey [39].
The equation used in the simulation was:
The values associated with smoking-related diseases were consistent with an existing cost-effectiveness model of smoking cessation, with the values for coronary heart disease used as estimates for MI [40]. These were 0.76 for COPD [41, 42], 0.61 for the first year following lung cancer [43] and 0.50 in subsequent years, 0.76 for MI [44, 45], and 0.74 for stroke [46, 47].
2.6 Costs
Direct medical costs were assessed from the perspective of a comprehensive US healthcare payer (i.e., all direct medical costs considered), and reported in $US, 2012 values. All reported costs were inflated to 2012 values using the medical care component of the US consumer price index [48]. The following costs were used in the STOP simulation: MI (acute $US37,591, annual year 1 $US24,538, subsequent $US10,630) [49], COPD (annual $US4,385) [50], stroke (acute $US16,217, annual year 1 $US11,628, subsequent $US1,324) [49], and lung cancer (annual year 1 $US67,701, subsequent $US8,192) [51]. Acute costs are applied immediately at the time of onset of the smoking-associated disease, while annual costs accrue continuously.
The cost of a quit attempt with varenicline ($US569) or bupropion sustained release ($US304) consists of the cost of a single physician visit ($US70.00) [52] plus the cost of the medication based on average wholesale price (AWP) required to cover the 12-week quit attempt [53]. The cost of a quit attempt with NRT ($US226) is a weighted average of the cost of the available formulations according to the distribution observed in the US-based web survey (51.4 % patch, 34.9 % gum and 13.8 % lozenges) [10]. Medication costs for NRT were derived from AWP [53] and recommendations for use for each presentation [54–57]. The cost per BMT quit attempt ($US82) was derived from the 2012 Medicare coverage fees for smoking cessation counselling; each attempt is assumed to comprise a maximum of four intermediate or intensive counselling sessions [52, 58].
3 Analyses
Several sets of analyses were conducted to confirm that the simulation accurately replicates the results of the clinical trials. Specifically, the analyses were structured to analyze the proportion of the simulated population that was abstinent at 12 months for individuals initiating a single quit attempt using varenicline, bupropion, NRT, or brief counselling versus the proportions observed in the clinical trials.
We then evaluated outcomes when patients are limited to a single quit attempt versus being allowed to initiate multiple quit attempts over a lifetime time horizon.
Additionally, in order to evaluate how results change in different populations, subgroup analyses were run allowing for multiple quit attempts for the following populations: individuals 45 years of age or younger, individuals with a high degree of nicotine dependence (FTND >5), 100 % males, and 100 % females.
For each analysis, 500,000 subjects were run through the simulation. Probabilistic sensitivity analyses were not conducted as the focus was on assessing the impact of varying individual drivers of outcomes, although a series of one-way sensitivity analyses were performed to establish the importance of key variables on predicted outcomes. QALYs and cost outcomes are discounted at 3 % per annum.
3.1 Results
One-year abstinence rates for the first quit attempt were compared with the five clinical trials individually for each of the trials’ treatment arms (Table 4) and generally showed strong concordance with the observed trial results. The one exception was in the trial evaluating varenicline in individuals with stable CVD, where the simulation predicted a higher abstinence rate for individuals on varenicline and placebo. Given that almost 50 % of all patients with a history of CVD were participants in other trials (i.e., not the trial conducted in patients with a history of CVD only), analyses were run on all the subjects with a history of CVD regardless of trial. In these analyses, there was closer alignment between simulated and observed abstinence rates—23.0 % of subjects assigned to varenicline were abstinent at 52 weeks, compared with the observed 21.5 %. For placebo, estimates were 9.2 % and 8.2 % for the simulated and observed subjects, respectively.
The reference analysis comparing predicted outcomes allowing for single versus multiple quit attempts showed a dramatic difference in predictions over a lifetime time horizon. Fig. 3 shows the proportion of patients abstinent over time under both scenarios. Whereas STOP predicts a steady increase in the proportion abstinent, growing from 15 % after 1 year to 68 % by the end of the simulation, with no opportunity to make further quit attempts, the single quit attempt scenario showed a steady decrease, with only 5 % abstinent at the end of the simulation. The divergence occurs even within the first year of the simulation and becomes dramatic by year 5 (30 % abstinence versus 8 %). On average, subjects in the multiple quit attempt scenario made just over nine quit attempts over the course of the simulation with only 9 % of subjects making only one attempt, and 16 % making over 15 attempts.
When looking at cumulative abstinence time at the individual level (Fig. 4), mean cumulative abstinence time in the single quit attempt scenario parallels the course of the bottom quartile of subjects’ accumulation of abstinence time in the multiple quit attempt scenario. Fig. 4, which is based on a simulated population of 5,000 subjects, also shows the substantial spread in outcomes across individuals, with the bottom quartile accumulating less than 1 year of abstinence time over their lifetimes, and the top quartile almost 22 years (mean of 13.3 years).
The impact of single versus multiple quit attempts on other outcomes is less dramatic, but nevertheless substantial (Table 5). The predicted number of smoking-related diseases begins to diverge after 5 years, and results in a 19 % difference by the end of the simulation. Similarly, there is a 7 % difference in mortality predictions by year 20, and total discounted QALYs over the course of the simulation differ by 0.6 per patient. Not surprisingly, the costs associated with smoking-related diseases are higher in the single quit attempt scenario, while the costs associated with smoking cessation aids and related monitoring are lower.
3.2 Sensitivity and Scenario Analyses
Outcomes are sensitive to variations in initial treatment response, relapse risk, and the interval between quit attempts (Table 6). Even when reference case parameters were varied by 25 % or even 50 % in a negative direction, estimated QALYs and time spent abstinent were well above predictions with the single quit attempt scenario. Abstinence times were most sensitive to changes in inputs, whereas QALYs and costs showed much less variance. Of note, analyses on time to next quit attempt showed almost no variation in predicted total costs. This is because, as the frequency of quit attempts increases, abstinence time, and hence the cost of smoking-related diseases decreases, whereas the cost of smoking-related interventions increases. The opposite, of course, is true when the frequency of quit attempts is reduced: smoking-related diseases and their associated costs increase, but this is offset by a decrease in intervention costs.
The most striking subgroup analysis results were those run on younger patients (i.e., 45 years of age or younger). Not surprisingly, given the younger age of the population, abstinence times and QALYs were higher in the younger population. What is striking is that total costs are substantially lower in the subgroup despite the longer time they have over which to accumulate costs. This is a direct result of the lower frequency of smoking-related diseases in this population, where the reduction in the incidence of smoking-related diseases can be as much as 19 % compared with the base case considering the overall population. Results in more nicotine-dependent populations (i.e., FTND scores above 5) were, not surprisingly, less favourable, with less time spent abstinent, lower QALYs, and higher total costs. Results in males are also worse, as this population is older (mean age of 48.8 years at baseline versus 45.2 years in females), has higher nicotine dependence (mean FTND scores of 5.6 and 5.3 in males and females, respectively), and has a higher prevalence of smoking-related diseases at baseline.
4 Discussion
Models assessing the impact of smoking cessation interventions that limit the analytic framework to a single quit attempt lead to substantial mischaracterization of health and economic outcomes in smokers, and may be producing biased estimates of the cost effectiveness of smoking cessation strategies. The analyses presented in this paper demonstrate that differences in predicted time spent abstinent are large, even only 5 years into the modelling time horizon, and the consequences of these differences become apparent early on, and are dramatic over a lifetime horizon.
The STOP model we have described not only represents an effort to overcome some of the limitations of previous decision tree and Markov cohort models of smoking cessation by simulating at the individual level, it also enables meaningful analyses of outcomes in population subgroups. For instance, the benefits of initiating quit attempts at earlier ages can be more accurately captured. By accounting for subject characteristics when predicting quit attempt success, relapse, the timing of future quit attempts and the selection of smoking cessation aids, if any, the simulation provides a powerful tool to evaluate the health and economic impact of alternative strategies for improving quit rates, and reducing the risk of relapse. Although the current analyses focus on how predictions of health and economic outcomes change with the adoption of a multiple quit attempt framework, and not on how cost-effectiveness estimates might be modified under alternative cessation strategies, the multiple quit attempt framework should provide a more reliable basis for these estimates.
One of the most widely cited economic models in this area, the BENESCO (Benefits of Smoking Cessation on Outcomes) model [13], adopts a single quit attempt framework. BENESCO also adopts a Markov cohort modelling framework, rather than individual level modelling. While comparisons of predictions between our model and BENESCO are difficult given differences in model inputs and assumptions, a recent application of the model in the US [40] does provide some basis for comparison of outcomes over time. For example, while our model predicts higher overall mortality (49 % over 20 years when all individuals are initially assigned to varenicline, versus 36 % in the BENESCO model varenicline arm), the differential in mortality projections over time narrows substantially, with a 65 % difference in year 5, dropping to a 35 % difference in year 20. This narrowing is explained by the lower long-term abstinence rates one would expect in the BENESCO model given that subjects who relapse are assumed to remain smokers for life. Other differences are more obvious. The BENESCO model assumes a one-time cost for smoking cessation assistance, whereas, in our reference analysis, the costs associated with smoking cessation medications or other forms of support are projected to increase from an average of about $US260 per patient in year 1, to $US1,850 after 20 years, and $US2,160 over a lifetime.
Our model is not without its limitations. For one, only four smoking-related diseases are considered: lung cancer, COPD, MI and stroke. Furthermore, the simulation does not currently account for any benefits associated with quitting smoking once these conditions occur. For example, subjects developing COPD over the course of the simulation are subject to the same mortality risk, regardless of their smoking status. The model also relies on a number of different predictive equations derived from various data sources, including surveys, clinical trials, and other public data sources. While the model projections seem reasonable, and certainly more in line with what one would expect than models assuming a single quit attempt, validation against an external dataset, and possible refinement of the equations would be of great value. For example, a report based on data from the National Health Interview Surveys showed that the mean number of quit attempts over a lifetime for current smokers aged 18 and over is around 6 and 9 attempts for males and females, respectively [59]. The STOP model predicts an average of just over nine lifetime quit attempts over the course of the simulation, closer to the upper bound of the cited range. The reliance on clinical trial data to simulate success and relapse rates in the year following initiation of a quit attempt is also a limitation of the current analyses, although the simulation allows for alternative success rates and short as well as long-term relapse rate inputs. Finally, the analyses presented in this manuscript are based largely on inputs derived for the US, which limits the generalizability of projections to other countries. In particular, patterns of smoking cessation strategies, the frequency of quit attempts, which were based on US survey data, as well as the profile of smokers, are likely to vary significantly across countries.
5 Conclusion
Despite the limitations addressed above, we believe the STOP simulation represents a significant advancement in the modelling of smoking cessation strategies, and provides a solid platform for evaluating not only individual, one-off interventions, but also more complex strategies, including policies which may encourage earlier or more frequent quit attempts. Given that the vast majority of previous models have restricted evaluations to a single quit attempt [13], the multiple quit attempt aspect of STOP by itself provides a better evaluation framework. By simulating at the individual level, the model produces a rich dataset of simulated patient trajectories, allowing for a better understanding of the experience of subgroups of subjects, as well as the timing and consequences of various events.
References
Centers for Disease Control and Prevention (CDC). Annual smoking-attributable mortality, years of potential life lost, and economic costs—United States, 1995–1999. MMWR Morb Mortal Wkly Rep. 2002;14:300–3.
Centers for Disease Control and Prevention (CDC). State-specific smoking-attributable mortality and years of potential life lost—United States, 2000–2004. MMWR Morb Mortal Wkly Rep. 2009;58(2):29–33.
Agaku I, King B, Dube SR. Current cigarette smoking among adults – United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(44):889–94.
Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38.
Hyland A, Borland R, Li Q, et al. Individual-level predictors of cessation behaviours among participants in the International Tobacco Control (ITC) Four Country Survey. Tob Control. 2006;15 Suppl. 3:iii83–94.
Hymowitz N, Cummings KM, Hyland A, et al. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tob Control. 1997;6(Suppl. 2):S57–62.
Fiore M, Jaen C, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Rockville: US Department of Health and Human Services, US Public Health Service; 2008
Fix BV, Hyland A, Rivard C, et al. Usage patterns of stop smoking medications in Australia, Canada, the United Kingdom, and the United States: findings from the 2006–2008 International Tobacco Control (ITC) Four Country Survey. Int J Environ Res Public Health. 2011;8(1):222–33.
Chantix U.S. Consumer Attitudes, Trends, and Usage Research—Wave 8 Report—April 2010; conducted by Opinion Research Corporation Guideline, Inc., Princeton NJ and Pfizer, Inc. New York (data on file)
Yeomans K, Payne KA, Marton JP, et al. Smoking, smoking cessation and smoking relapse patterns: a web-based survey of current and former smokers in the US. Int J Clin Pract. 2011;65(10):1043–54.
Ferguson J, Bauld L, Chesterman J, et al. The English smoking treatment services: one-year outcomes. Addiction. 2005;100(Suppl. 2):59–69.
Fiore M, Jaén C, Baker T, et al. Treating tobacco use and dependence: 2008 update. Quick reference guide for clinicians. Rockville: US Department of Health and Human Services, US Public Health Service; 2009
Bolin K. Economic evaluation of smoking-cessation therapies: a critical and systematic review of simulation models. Pharmacoeconomics. 2012;30(7):551–64.
Olsen KR, Bilde L, Juhl HH, et al. Cost-effectiveness of the Danish smoking cessation interventions: subgroup analysis based on the Danish Smoking Cessation Database. Eur J Health Econ. 2006;7(4):255–64.
Levy DT, Friend K. A simulation model of policies directed at treating tobacco use and dependence. Med Decis Making. 2002;22(1):6–17.
Xenakis JG, Kinter ET, Ishak KJ, et al. A discrete-event simulation of smoking-cessation strategies based on varenicline pivotal trial data. Pharmacoeconomics. 2011;29(6):497–510.
Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55.
Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56–63.
Aubin HJ, Bobak A, Britton JR, et al. Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial. Thorax. 2008;63(8):717–24.
Rigotti NA, Pipe AL, Benowitz NL, et al. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation. 2010;121(2):221–9.
Tashkin DP, Rennard S, Hays JT, et al. Effects of varenicline on smoking cessation in patients with mild to moderate COPD: a randomized controlled trial. Chest. 2011;139(3):591–9.
Thun MJ. Data from Cancer Prevention Study II, 1982–1988 [provided by author – Michael J. Thun]
Shibuya K, Mathers C, Lopez A. Chronic obstructive pulmonary disease (COPD): consistent estimates of incidence, prevalence, and mortality by WHO region [DRAFT] [online]; 2001. http://www.who.int/healthinfo/statistics/bod_copd.pdf (Accessed 8 Mar 2010)
Centers for Disease Control and Prevention (CDC). Prevalence of heart disease—United States 2005 [online]; 2007. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5606a2.htm#fig (Accessed 8 Mar 2010)
Bureau of Labor Statistics (BLS). United States Bureau of Labor Statistics website [online]; 2010. http://www.bls.gov (Accessed May 2010)
Lloyd-Jones D. Prevalence of stroke by age and sex (NHANES: 2003–2006) [online]; 2010. http://circ.ahajournals.org/cgi/content/full/121/7/e46/FIG36192671 (Accessed 8 Mar 2010)
Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev. 2005;(2):CD001292
Wetter DW, Kenford SL, Welsch SK, et al. Prevalence and predictors of transitions in smoking behavior among college students. Health Psychol. 2004;23(2):168–77.
Hoogenveen RT, van Baal PH, Boshuizen HC, et al. Dynamic effects of smoking cessation on disease incidence, mortality and quality of life: the role of time since cessation. Cost Eff Resour Alloc. 2008;6:1.
SEER. SEER Cancer Statistics Review 1975–2006. Cancer of the lung and bronchus (invasive) Table 15.9. SEER incidence and U.S. death rates, age-adjusted and age-specific rates, by race and sex, years 2002–2006 [online]; 2006. http://seer.cancer.gov/csr/1975_2006/results_single/sect_15_table.09.pdf (Accessed 8 Mar 2010)
Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–215.
Williams GR, Jiang JG, Matchar DB, et al. Incidence and occurrence of total (first-ever and recurrent) stroke. Stroke. 1999;30(12):2523–8.
SEER. SEER Cancer Statistics Review 1975–2006. U.S. complete prevalence counts, invasive cancers only, January 1, 2006 by age at prevalence. Table 1.22 [online]; 2006. http://seer.cancer.gov/csr/1975_2006/results_single/sect_01_table.22_2pgs.pdf (Accessed 8 Mar 2010)
University of California Berkeley (USA), Max Planck Institute for Demographic Research (Germany). Human Mortality Database [online]; 2010. http://www.mortality.org or http://www.humanmortality.de (Accessed 8 Mar 2010)
Bronnum-Hansen H, Jorgensen T, Davidsen M, et al. Survival and cause of death after myocardial infarction: the Danish MONICA study. J Clin Epidemiol. 2001;54(12):1244–50.
de Torres JP, Cote CG, Lopez MV, et al. Sex differences in mortality in patients with COPD. Eur Respir J. 2009;33(3):528–35.
Tammemagi CM, Neslund-Dudas C, Simoff M, et al. Smoking and lung cancer survival: the role of comorbidity and treatment. Chest. 2004;125(1):27–37.
Wolfe CD, Smeeton NC, Coshall C, et al. Survival differences after stroke in a multiethnic population: follow-up study with the South London stroke register. BMJ. 2005;331(7514):431.
Sullivan PW, Ghushchyan V. Mapping the EQ-5D index from the SF-12: US general population preferences in a nationally representative sample. Med Decis Making. 2006;26(4):401–9.
Howard P, Knight C, Boler A, et al. Cost-utility analysis of varenicline versus existing smoking cessation strategies using the BENESCO Simulation model: application to a population of US adult smokers. Pharmacoeconomics. 2008;26(6):497–511.
Mannino DM, Buist AS, Petty TL, et al. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax. 2003;58(5):388–93.
Spencer M, Briggs AH, Grossman RF, et al. Development of an economic model to assess the cost effectiveness of treatment interventions for chronic obstructive pulmonary disease. Pharmacoeconomics. 2005;23(6):619–37.
Trippoli S, Vaiani M, Lucioni C, et al. Quality of life and utility in patients with non-small cell lung cancer. Quality-of-life Study Group of the Master 2 Project in Pharmacoeconomics. Pharmacoeconomics. 2001;19(8):855–63.
Fryback DG, Dasbach EJ, Klein R, et al. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making. 1993;13(2):89–102.
Hay JW, Sterling KL. Cost effectiveness of treating low HDL-cholesterol in the primary prevention of coronary heart disease. Pharmacoeconomics. 2005;23(2):133–41.
Duncan PW, Lai SM, Keighley J. Defining post-stroke recovery: implications for design and interpretation of drug trials. Neuropharmacology. 2000;39(5):835–41.
Tengs TO, Lin TH. A meta-analysis of quality-of-life estimates for stroke. Pharmacoeconomics. 2003;21(3):191–200.
Bureau of Labor Statistics (BLS). United States Bureau of Labor Statistics: Consumer Price Index website [online]; 2012. http://www.bls.gov/cpi/home.htm (Accessed Jun 2012)
O’Sullivan AK, Rubin J, Nyambose J, et al. Cost estimation of cardiovascular disease events in the US. Pharmacoeconomics. 2011;29(8):693–704.
Dalal AA, Christensen L, Liu F, et al. Direct costs of chronic obstructive pulmonary disease among managed care patients. Int J Chron Obstruct Pulmon Dis. 2010;5:341–9.
Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–28.
MAG Mutual Healthcare Solutions. Physicians’ Fee and Coding Guide: 2012. MAG Mutual Healthcare Solutions Incorporated; 2012.
Red Book Online. New York: Thomson-Reuters; 2012. http://www.redbook.com/redbook/online/ (Accessed 8 Jun 2012)
Nicoderm CQ website [online]; 2012. http://www.nicodermcq.com/Quit_Place_Dose.aspx (Accessed 8 Jun 2012)
Nicorette. Frequently asked questions [online]; 2012. http://www.nicorette.com/Faqs.aspx#lnk16 (Accessed 1 Jun 2012)
Nicotrol® NS (nicotine nasal spray) prescribing information [online]; 2012. http://media.pfizer.com/files/products/uspi_nicotrol.pdf (Accessed 5 Jun 2012)
Nicorette® Lozenge website [online]; 2012. http://www.commitlozenge.com/Quit_Place_Dose.aspx (Accessed 5 Jun 2012)
Department of Health and Human Services: Centers for Medicare and Medicaid Services. Tobacco-Use Cessation Counseling Services. Baltimore: Centers for Medicare and Medicaid Services; 2012. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/smoking.pdf (Accessed Feb 2012)
Office on Smoking and Health (US). Women and smoking: a report of the surgeon general. Atlanta: Centers for Disease Control and Prevention (US); 2001. http://www.ncbi.nlm.nih.gov/books/NBK44303/ [Accessed Jun 2013]
Acknowledgments
Funding
Funding for this project was provided by Pfizer Inc.
Role of the funding source
Employees of Pfizer Inc. (Jenő P. Marton, Richard J. Willke and Dale Rublee) were involved in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review and approval of the manuscript. Jenő P. Marton was an employee of Pfizer Inc. at the time the model was being designed and developed.
Conflicts of interest
Denis Getsios, Nikhil Revankar, Alexandra J. Ward, and K. Jack Ishak are employees of United BioSource Corporation, who were paid consultants to Pfizer Inc. in connection with the development of the manuscript at the time of study conduct. James G. Xenakis was an employee of United BioSource Corporation at the time the model was being designed and developed. Jenő P. Marton, Dale Rublee, and Richard J. Willke were employees of Pfizer Inc. at the time of study conduct.
Author contributions
DG, JPM, NR, AW, RW, DR, KJI, and JX participated in the design of the model, identification of data sources, conduct of data analyses, and implementing the design. Each author also contributed to the interpretation of data and results, drafting the manuscript, and has approved the final version. Denis Getsios will serve as a guarantor for the overall content of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Getsios, D., Marton, J.P., Revankar, N. et al. Smoking Cessation Treatment and Outcomes Patterns Simulation: A New Framework for Evaluating the Potential Health and Economic Impact of Smoking Cessation Interventions. PharmacoEconomics 31, 767–780 (2013). https://doi.org/10.1007/s40273-013-0070-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-013-0070-5