Abstract
Background
To protect children from harm, clinicians, educators, and patient safety champions need information to direct improvement efforts. Critical incident data could provide this but are often disregarded as a source of evidence because under-reporting makes them an inaccurate measure of error rates.
Objective
Our aim was to identify key targets for pediatric healthcare quality improvement. The objective was to evaluate the types, characteristics, and areas of risk within reported medication errors in pediatric patients.
Methods
We conducted a retrospective study of a large regional dataset of 1522 pediatric medication errors reported from secondary care between 2011 and 2015, including all hospitals and community pediatric settings in Northern Ireland. The following characteristics were included: error severity, patient age, drug involved, error type, and area of practice. Two academic pediatricians, a senior medicines governance pharmacist, a Reader in Pharmacy Practice, and a Professor of Medical Education analyzed the data. Validity checks included comparing the findings against key published literature and discussion by a practitioner panel representing five multidisciplinary stakeholder groups.
Results
Neonates, particularly in intensive care, were implicated in 19% of all errors. The medications most represented in risk were antimicrobials, paracetamol, vaccines, and intravenous fluids. The error types most implicated were dosing errors (32%) and omissions (21%).
Conclusions
Incident reports identified neonates, a shortlist of drugs, and specific error types, associated with modifiable behaviors, as priority improvement targets. These findings direct further study and inform intervention development, such as specific training in calculations to prevent dosing errors. Involving experienced practitioners both endorsed the findings and engaged the practice community in their future implementation. The utility of incident reports to direct improvement efforts may offset the limitations in their representativeness.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Critical incident reports can support medication safety by providing information on characteristics and nature of errors. |
Discussion with stakeholders and review of selected literature can offset incidents’ lack of representativeness and support their validity. |
In pediatric medication safety, factors associated with risk of error included neonatal care, dosing, and timely administration, and use of common drugs such as antimicrobials, paracetamol, intravenous fluids and vaccines. |
1 Introduction
In their third Global Patient Safety Challenge, Medication Without Harm, the World Health Organization (WHO) urged quality improvement (QI) initiatives to target “high-risk situations” [1]. Using medication in children, in whom errors are more common and more likely to cause harm than in adults [2, 3], is a case in point. Improvements are “long overdue” [1] but have been hampered by a lack of pertinent information about key characteristics of errors [4]. Most studies of pediatric medication safety provide evidence about the overall prevalence of errors and the efficacy of specific interventions [3, 5,6,7] rather than the specific drugs and error types that cause harm. More specific information of this nature could break down this complex problem and direct improvement efforts toward high-risk areas that offer the greatest potential for benefit.
Adult studies provide this type of information, but extrapolation is of limited use because medication use in children differs. For example, many drugs used routinely in adult practice are rarely given to children, and pediatric practice more often needs individualized dosing and off-license medication use [8]. Moreover, children frequently receive liquid medicines, which often require extemporaneous preparation as “specials,” and whose lack of standardization may complicate use. Error patterns also differ; for example, dosing errors, particularly potentially lethal tenfold errors, are more common in children [3, 9]. An alternative to learning from adult errors is to make better use of routinely reported critical incidents. This source of information, often maligned, has important strengths. Incident reports are directly related to real clinical practice, contemporaneously reported, and readily available. They show who was affected, where errors occurred, and what drugs were involved. Staff members choose to report errors and provide rich narrative information precisely because this will help prevent future harm.
Despite these strengths, the potential of incident reports to improve pediatric medication safety has not been fully realized. In risk management, their use is usually at a local level and focuses on detailed analysis of small numbers of incidents. In published research, most work has been conducted in specific settings (e.g., neonatal intensive care units [NICU] [10, 11]), with specific medication groups (e.g., sedation [12]), or on specific error types (e.g., tenfold dosing errors [9]). The few studies carried out in general pediatric settings have typically been on a small scale [13] compared with research in adults [14] and have estimated the prevalence of errors and their response to interventions. Critics have pointed out the shortcomings of this approach, arguing that using incident reports for epidemiological purposes or as a measure of changes is inappropriate [15]. In support of this, they show that error rates derived from incident data are lower than rates derived from drug chart review or direct observation [5, 16, 17]. These critiques rightly contend that under-reporting, incomplete data, and potential bias error compromise the representativeness of incident reports [18, 19]. The unintended consequence of criticizing the reliability of incident analysis is that this has obscured the potential utility of reported incidents to improve medication safety. Specifically, identifying risks rather than measuring rates [15] would make incident reports useful, particularly if information from reports was triangulated against other sources of information to increase confidence in findings.
Following a lead from the UK Royal College of Physicians [20], we reasoned that incident data could make an important contribution to setting QI priorities and set out to conduct a retrospective observational analysis of a large regional dataset of reported pediatric medication errors. Our aim was to identify priority targets for QI by analyzing error types, characteristics, and areas of risk and triangulating our findings against published evidence and the informed opinion of an advisory panel of experienced clinicians.
2 Methods
To identify key targets for pediatric healthcare QI, our objective was to evaluate the types, characteristics, and areas of risk within reported medication errors in pediatric patients.
2.1 Study Design
We analyzed a large set of errors reported from a geographically defined region, part of the UK national health service, whose error reporting is coordinated between all healthcare providers, making regional analysis of incident reports possible.
2.2 Setting
In collaboration with a regional medication safety group, the Northern Ireland (NI) Medicines Governance Team, we obtained all reported medication incidents occurring in pediatric patients aged 0–16 years between 2011 (when electronic reporting was first established in NI) and 2015 in all five NI Health and Social Care (HSC) Trusts. These organizations administer health and social care across NI, to a population of 1.8 million, including 380,000 children aged 0–16 years. Children receive care in various settings. There is a large regional children’s hospital, with a dedicated pediatric emergency department and a regional neonatal unit. There are seven district general hospitals with children’s wards, maternity services, and neonatal units as well as several others with pediatric outpatient clinics, ambulatory pediatric care, midwifery-led units, general emergency departments, and other services that treat patients of all ages (e.g., ophthalmology, orthopedics). Children are also looked after in community settings such as community pediatric centers, school health services, and respite and care facilities. Hospitalized young people usually receive care on adult wards from age 14 or 15 years.
Staff employed by HSC Trusts voluntarily report critical incidents for local risk management purposes, primarily via an electronic database on hospital computers. Staff are encouraged to report all adverse events where harm, or the potential for harm, occurs. Incidents are held within individual HSC Trusts. Reports contain categorical information (incident type, harm, location, etc.), and a free-text description of what happened and the action taken in response. Forms contain guidance on describing incidents, but staff decide on the level of detail to include.
All medication incident reports are reviewed by medicines governance pharmacists (MGPs) who are trained to process, extract, and analyze incidents. Consistent procedures are used across all HSC Trusts. They routinely check the stage of medication delivery (prescribing/administration/dispensing/monitoring/other), error type (wrong dose/wrong medicine, etc.), drug involved, and level of actual and potential harm for all incidents. When necessary, they seek further information from staff or check patient records.
Incident type labels applied by MGPs are similar to the WHO Conceptual Framework for the International Classification of Patient Safety [21]. We used its terminology where possible but, as this was analysis of routinely collected data, it was not possible to change existing category labels.
2.3 Data Extraction
MGPs within each Trust extracted all medication incidents relating to pediatric patients aged 0–16 years in all NI hospitals and community settings from July 2011 to July 2015. This was intended to include all children who were patients, not just those cared for in dedicated pediatric settings. To include reports that might have been missed because patient age was not recorded, MGPs also carried out a second extraction of medication incidents coded as occurring in specific pediatric settings. The final dataset included community settings but excluded primary care.
For the purpose of this study, MGPs used a protocol provided by us to extract incident data into a Microsoft Excel spreadsheet. They removed identifiable details, applied pseudonyms to Trust and site names, and double checked that incidents were appropriately categorized. A Medicines Governance Team administrator combined these proformas into a single regional dataset.
2.4 Data Processing
RLC reviewed all 1552 extracted incidents. Accuracy of classification was checked and drug categories (e.g., antimicrobials, anticonvulsants) were applied. Intravenous fluid errors were included within medication incidents. The focus of this research was medication error at the individual patient level. We therefore excluded incidents not relating to individual patients (e.g., a medication cabinet being left unlocked), adverse drug reactions where no error had taken place, errors occurring in primary care but reported in secondary care, and errors relating to medical devices or equipment. Where incident reports referred to multiple errors at more than one stage of medication delivery (typically, errors in both prescribing and administration), these incidents were duplicated and classified at both applicable stages.
2.5 Analysis and Identification of Risk
We defined risk as probability of occurrence of error combined with the potential severity of resultant harm [22, 23]. We deemed that “high-risk” aspects of practice—in terms of error types, patient groups, clinical areas, or medications—could represent QI targets.
We used descriptive statistics to summarize characteristics of reported medication errors (type, subtype, harm, age of patient, area of practice, reporter group, drug involved) in order of frequency. Because incident severity contributes to risk, we planned to analyze incidents that led to severe harm or death separately, but none were reported within the study period. We chose to focus on errors in prescribing and administration because they were commonest, and we judged that they would make relevant targets for QI in frontline clinical settings.
By reflecting on and discussing the reported error characteristics, RLC and AC agreed on preliminary areas of risk. In a process of triangulation [24], we then assessed these findings against two other sources of evidence. First, RLC discussed the results of the analysis (Tables 1, 2, 3, 4) with five stakeholder advisory groups, asking them to reflect on the commonly occurring incident types and, based on their experience, advise on their validity and importance. These groups were as follows: pediatric teams in two hospitals, a hospital drug and therapeutics committee, a regional QI body, and the medication safety subgroup of the statutory body responsible for health and social care in NI. Second, we reviewed other key sources of peer-reviewed evidence and gray literature—including prospective observational studies, other critical incident studies, and patient safety alerts—to establish prior knowledge about the prevalence and severity of provisionally identified areas of risk. Combining information from incident data and the two validation steps, the entire multidisciplinary research team—made up of two academic pediatricians, a senior medicines governance pharmacist, a Reader in Pharmacy Practice, and a Professor of Medical Education—agreed the final analysis.
2.6 Ethics
The research was deemed eligible for Proportionate Review by the first available committee. It was approved by the Proportionate Review Subcommittee of the East Midlands – Nottingham 2 Research Ethics Committee (reference 15/EM/0353).
3 Results
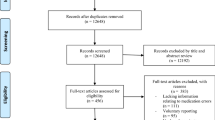
Figure 1 summarizes the processing of incidents. Of 1552 extracted incidents, 85 were excluded. Reasons were not an individual patient error (n = 37), adverse drug reaction without error (n = 12), error occurring in primary care but reported in secondary care (n = 12), incident relating to equipment (n = 7), and other reason (n = 17). In total, 55 incidents contained errors at more than one stage of medication delivery and were duplicated. This resulted in a final dataset of 1522 medication errors, from 1467 incident reports, for analysis.
3.1 Characteristics
Error characteristics are summarized in Table 1. The majority (88%) occurred during administration (822 [54%]) or prescribing (517 [34%]). Most reported errors led to insignificant (1130 [74%]) or minor (375 [24%]) harm; 17 (1%) were classified as moderate, and none caused severe harm or death within the 5-year study period. In contrast, 277 (18%) reported errors had potential to cause moderate harm, 111 (7%) major harm, and 19 (1%) catastrophic harm. Many did not cause harm because they were intercepted before reaching patients; staff reporting incidents often judged that significant harm could have occurred had they not been detected. Most were reported by nurses (682 [45%]). Pharmacists and doctors reported 163 (11%) and 151 (9%), respectively.
Neonates (291 [19%]) and infants (230 [15%]) were most likely to be involved in reported errors. Almost half occurred on pediatric medical wards (750 [49%]); 235 (15%) were reported in community settings and 208 (14%) in neonatal units (Table 2). Dosing errors occurred in 451 (32%) of reported errors overall and in over half of prescribing errors (54.1%). Other common error types were omitted/delayed doses (288 [20%]), wrong frequency (191 [14%]), and wrong medicine (174 [12%]) (Table 3). The drugs most frequently reported in errors were antimicrobials (329 [21%]), paracetamol (135 [9%]), intravenous fluids (102 [7%]), and vaccines (93 [6%]) (Table 4).
3.2 Areas of Particular Risk
Table 5 identifies the high-risk patient groups, drugs, settings, and error types that represent QI targets. The highest number of reported errors occurred in neonates, often in NICU settings. High-risk drugs were antimicrobials, paracetamol, intravenous fluids, and vaccines. Dosing errors were commonly reported and often associated with significant potential harm; medication omissions were also common. Table 5 also shows how the validation steps of discussing with stakeholders and reviewing published evidence helped to confirm identification of QI targets. Box 1 shows two example incidents relating to areas of risk.

4 Discussion
This study identified high-risk areas of practice that represent potential starting points for QI initiatives, which include neonates, NICU settings, drugs such as antimicrobials, paracetamol, intravenous fluids, and vaccines, and medication dosing and omissions. The validity of these targets derives from analysis of a comprehensive dataset of reported medication errors in pediatric patients in all secondary care settings, aggregated across an entire geographic region, triangulated against published evidence and the informed opinion of expert stakeholders, who are also potential improvers. Pending confirmation, we suggest that these targets may apply in other locations and that others wishing to improve pediatric medication safety may find our methodology useful.
Breaking down pediatric medication error into areas of risk enables clinicians, faced with finite time and resources, to prioritize QI efforts. Moreover, certain areas of risk are associated with specific behaviors that lead to errors. Dosing errors, for example, commonly arise from miscalculations and confusion around individualized dosing [8]. Armed with information that this type of error is frequently reported, clinicians involved in QI might respond by offering specific training in dose calculations.
Use of critical incident data can go further still: after areas of risk are identified, causes of errors can be investigated by analyzing free-text descriptions of what went wrong [35, 42, 43]. Answering the “why” question can guide development of interventions and make them more likely to be effective. Box 2 presents a worked example of how in-depth analysis of errors in prescribing and administering intravenous paracetamol informed potential solutions.
This approach addresses the limitations of using incident data in research and also goes beyond their typical use in risk management. In that context, learning from incident reporting is usually from single cases that point to critical, rectifiable safety hazards [15] and detailed investigation of incidents leading to severe harm [26]. While necessary, analyzing single cases is resource intensive and insufficient to completely address pediatric medication errors, which are highly variable in type. It also fails to maximize learning from errors that do not lead to harm, despite evidence showing that near misses offer important insights [22, 27]. We recognize that incidents cannot be considered representative and that numbers are affected by reporting rates and clinical activity levels. However, that a type of incident is reported frequently is an indicator of a clinically important problem that can be validated with other evidence. We suggest that our approach, summarized in Fig. 2, of aggregating a large number of locally collected incidents and using them to identify QI targets offers added benefits beyond traditional use of incidents.
A strength of our work is that we obtained data from an entire region, across the full spectrum of secondary care, meaning that this is among the largest studies of reported medication errors in children. We chose to include children treated in all secondary care settings (both adult and pediatric), not just those on dedicated pediatric wards, as we deemed that children looked after in nonspecialist areas may have been at risk of error.
Our work also has important limitations. First, unlike studies using prospective reporting, we made use of existing incidents [44]. Reports can be incomplete or inaccurate, leading to incomplete data capture or missing parameters. We minimized this by extracting data using both patient age and location where the incident occurred. Moreover, medication incidents are less likely to be incomplete because they are routinely vetted by MGPs after reporting. Second, we extracted incidents from all secondary care settings. This may limit direct comparison with other critical incident datasets, though it broadened our scope to identify risks. Third, not all risks can be identifying using incident data. For example, we found no reports about incomplete prescriptions or incorrect use of abbreviations, errors frequently seen in prospective studies [3]. Incident reporting should therefore be used alongside other forms of data collection, such as drug chart audit [45]. Fourth, most errors within our dataset were detected before reaching patients or led to only minor harm. This affected identification of risk, which depends on error severity. However, our triangulation steps offset this limitation by providing information on severity of error types. Fifth, our validation steps helped to offset the limitations of critical incidents but did not use research-level systematicity; instead, they were intended to mirror what a clinician could reasonably do in practice.


5 Recommendations and Conclusion
Our research recommendation is for further study to clarify the specific types and underlying causes of medication errors in children. Multiple methods of study—including prospective designs, critical incident studies, and qualitative approaches—could help build a more complete picture than any single method alone. Research to evaluate interventions that address the improvement targets identified is also needed.
Curricula should prioritize high-risk areas of practice. For example, this might involve emphasis on calculations at undergraduate level, to prevent dosing errors, or specific induction in the use of high-risk medications such as aminoglycoside antimicrobials during postgraduate induction.
Our practice recommendation is that clinicians consider the areas of risk identified here as potential starting points for QI. We recommend, too, that clinicians consider using aggregated incident reports at local or regional levels to guide their own QI priorities and provide insights into the underlying causes of errors. While critical incidents are not a panacea [15], this study suggests they can play an important role in combating pediatric medication error.
References
Donaldson LJ, Kelley ET, Dhingra-Kumar N, Kieny MP, Sheikh A. Medication without harm: WHO’s third global patient safety challenge. Lancet. 2017;389(10080):1680–1. https://doi.org/10.1016/S0140-6736(17)31047-4.
Kaushal R, Bates DW, Landrigan C, et al. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285(16):2114–20. https://doi.org/10.1097/00132586-200206000-00041.
Ghaleb MA, Barber N, Franklin BD, Wong ICK, Chi I, Wong K. The incidence and nature of prescribing and medication administration errors in paediatric inpatients. Arch Dis Child. 2010;95:113–8. https://doi.org/10.1136/adc.2009.158485.
Cass H. Reducing paediatric medication error through quality improvement networks; where evidence meets pragmatism. Arch Dis Child. 2016;101(5):26–9. https://doi.org/10.1136/archdischild-2015-309007.
Ghaleb MA, Barber N, Franklin BD, Yeung VWS, Khaki ZF, Wong ICK. Systematic review of medication errors in pediatric patients. Ann Pharmacother. 2006;40(10):1766–76. https://doi.org/10.1345/aph.1G717.
Wong ICK, Ghaleb MA, Franklin BD, Barber N. Incidence and nature of dosing errors in paediatric medications: a systematic review. Drug Saf. 2004;27(9):661–70. https://doi.org/10.2165/00002018-200427090-00004.
Rinke ML, Bundy DG, Velasquez CA, et al. Interventions to reduce pediatric medication errors: a systematic review. Pediatrics. 2014;134:338–60. https://doi.org/10.1542/peds.2013-3531.
Wong ICK, Wong LYL, Cranswick NE. Minimising medication errors in children. Arch Dis Child. 2009;94(2):161–4. https://doi.org/10.1136/adc.2007.116442.
Lesar TS. Tenfold medication dose prescribing errors. Ann Pharmacother. 2002;36:1833–9. https://doi.org/10.1345/aph.1C032.
Suresh G, Horbar JD, Plsek P, et al. Voluntary anonymous reporting of medical errors for neonatal intensive care. Pediatrics. 2004;113(6):1609–18. https://doi.org/10.1542/PEDS.113.6.1609.
Simpson JH, Lynch R, Grant J, Alroomi L. Reducing medication errors in the neonatal intensive care unit. Arch Dis Child Fetal Neonatal Ed. 2004;89(6):F480–2. https://doi.org/10.1136/adc.2003.044438.
Cote CJ, Notterman DA, Karl HW, Weinberg JA, McCloskey C. Adverse sedation events in pediatrics: a critical incident analysis of contributing factors. Pediatrics. 2000;105(4):805–14. https://doi.org/10.1542/peds.105.4.805.
Ross LM, Wallace J, Paton JY, Wallace J. Medication errors in a paediatric teaching hospital in the UK: five years operational experience. Arch Dis Child. 2000;83(6):492–7. https://doi.org/10.1136/adc.83.6.492.
Cousins DH, Gerrett D, Warner B. A review of medication incidents reported to the National Reporting and Learning System in England and Wales over 6 years (2005–2010). Br J Clin Pharmacol. 2012;74:597–604. https://doi.org/10.1111/j.1365-2125.2011.04166.x.
Pham JC, Girard T, Pronovost PJ. What to do with healthcare incident reporting systems. J Public health Res. 2013;2(3):27. https://doi.org/10.4081/jphr.2013.e27.
Kozer E, Scolnik D, Jarvis AD, Koren G. The effect of detection approaches on the reported incidence of tenfold errors. Drug Saf. 2006;29(2):169–74. https://doi.org/10.2165/00002018-200629020-00007.
Sari AB, Sheldon TA, Cracknell A, Turnbull A. Sensitivity of routine system for reporting patient safety incidents in an NHS hospital: retrospective patient case note review. BMJ. 2007;334:79. https://doi.org/10.1136/bmj.39031.507153.AE.
Macrae C. The problem with incident reporting. BMJ Qual Saf. 2016;25(2):71–5. https://doi.org/10.1136/bmjqs-2015-004732.
Noble DJ, Pronovost PJ. Underreporting of patient safety incidents reduces health care’s ability to quantify and accurately measure harm reduction. J Patient Saf. 2010;6:247–50. https://doi.org/10.1097/PTS.0b013e3181fd1697.
Royal College of Physicians. Supporting junior doctors in safe prescribing. London: RCP; 2017. https://www.rcplondon.ac.uk/projects/outputs/supporting-junior-doctors-safe-prescribing. Accessed 25 Mar 2020.
World Health Organization. Conceptual Framework for the International Classification of Patient Safety. Geneva: WHO; 2009. https://www.who.int/patientsafety/taxonomy/icps_full_report.pdf. Accessed 25 Mar 2020.
Battles JB, Lilford RJ. Organizing patient safety research to identify risks and hazards. BMJ Qual Saf. 2003;12(Suppl 2):ii2–7. https://doi.org/10.1136/qhc.12.suppl_2.ii2.
Department of Health. An organisation with a memory—report of an expert group on learning from adverse events in the NHS. London: TSO; 2000. https://www.webarchive.nationalarchives.gov.uk/20130105144251/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4065086.pdf. Accessed 25 Mar 2020.
Walsh K. When I say Triangulation. Med Educ. 2013;47(9):866. https://doi.org/10.1111/medu.12241.
Agency National Patient Safety. Review of patient safety for children and young people. London: NPSA; 2009.
Subhedar NV, Parry HA. Critical incident reporting in neonatal practice. Arch Dis Child Fetal Neonatal Ed. 2010;95(5):F378–82. https://doi.org/10.1136/adc.2008.137869.
Shaw KN, Lillis KA, Ruddy RM, et al. Reported medication events in a paediatric emergency research network: Sharing to improve patient safety. Emerg Med J. 2013;30(10):815–9. https://doi.org/10.1136/emermed-2012-201642.
Medicines and Healthcare products Regulatory Agency. Intravenous paracetamol (Perfalgan): risk of accidental overdose, especially in infants and neonates. Drug Saf Updat. 2010;3:2–3.
National Patient Safety Agency. Overdose of intravenous paracetamol in infants and children | Signal 1293F. London: NPSA; 2010. http://www.webarchive.nationalarchives.gov.uk/20171030130125/http://www.nrls.npsa.nhs.uk/resources/clinical-specialty/paediatrics-and-child-health/?entryid45=83757. Accessed 25 Mar 2020.
National Patient Safety Agency. Safer use of intravenous gentamicin for neonates. London: NPSA; 2010. https://www.sps.nhs.uk/wp-content/uploads/2018/02/2010-NRLS-1085-Safer-use-of-inonates-2010.02.03-v1.pdf. Accessed 25 Mar 2020.
Armon K, Riordan A, Playfor S, Millman G, Khader A. Hyponatraemia and hypokalaemia during intravenous fluid administration. Arch Dis Child. 2008;93(4):285–7. https://doi.org/10.1136/adc.2006.093823.
Hyponatraemia Inquiry Team. The inquiry into hyponatraemia-related deaths report. 2018. http://www.ihrdni.org/Full-Report.pdf. Accessed 25 Mar 2020.
Feikema SM, Klevens RM, Washington ML, Barker L. Extraimmunization among US children. J Am Med Assoc. 2000;283(10):1311–7. https://doi.org/10.1001/jama.283.10.1311.
World Health Organization. Global manual on surveillance of adverse events following immunization. 2014. http://www.who.int/vaccine_safety/publications/Global_Manual_revised_12102015.pdf?ua=1. Accessed 25 Mar 2020.
Rees P, Edwards A, Powell C, et al. Pediatric immunization-related safety incidents in primary care: A mixed methods analysis of a national database. Vaccine. 2015;33(32):3873–80. https://doi.org/10.1016/j.vaccine.2015.06.068.
Kaushal R, Goldmann DA, Keohane CA, et al. Medication errors in paediatric outpatients. Qual Saf Health Care. 2010;19(6):e30. https://doi.org/10.1136/qshc.2008.031179.
Sutcliffe K, Stokes G, O’Mara-Eves A, et al. Paediatric medication error: a systematic review of the extent and nature of the problem in the UK and international interventions to address it. London: EPPI-Centre; 2014. https://www.eppi.ioe.ac.uk/cms/Portals/0/PDF%20reviews%20and%20summaries/Paediatric%20medication%20error%202014%20Sutcliffe%20report.pdf?ver=2014-11-20-161504-377. Accessed 25 Mar 2020.
Huynh C, Wong ICK, Correa-West J, et al. Paediatric patient safety and the need for aviation black box thinking to learn from and prevent medication errors. Pediatr Drugs. 2017;19:99–105. https://doi.org/10.1007/s40272-017-0214-8.
Koren G, Barzilay Z, Greenwald M. Tenfold errors in administration of drug doses: a neglected iatrogenic disease in pediatrics. Pediatrics. 1986;77:848–9.
Keers RN, Williams SD, Cooke J, Ashcroft DM. Prevalence and nature of medication administration errors in health care settings: a systematic review of direct observational evidence. Ann Pharmacother. 2013;47(2):237–56. https://doi.org/10.1345/aph.1R147.
Poder TG, Maltais S. Systemic analysis of medication administration omission errors in a tertiary-care hospital in Quebec. Health Inf Manag J. 2018. https://doi.org/10.1177/1833358318781099(ePub Jun 18).
Williams H, Edwards A, Hibbert P, et al. Harms from discharge to primary care: mixed methods analysis of incident reports. Br J Gen Pract. 2015;65(641):e829–37. https://doi.org/10.3399/bjgp15X687877.
Rees P, Edwards A, Powell C, et al. Patient safety incidents involving sick children in primary care in england and wales: a mixed methods analysis. PLoS Med. 2017;14:e1002217. https://doi.org/10.1371/journal.pmed.1002217.
Snijders C, van Lingen RA, Klip H, et al. Specialty-based, voluntary incident reporting in neonatal intensive care: description of 4846 incident reports. Arch Dis Child Fetal Neonatal Ed. 2009;94(3):F210–5. https://doi.org/10.1136/adc.2007.135020.
Olsen S, Neale G, Schwab K, et al. Hospital staff should use more than one method to detect adverse events and potential adverse events: incident reporting, pharmacist surveillance and local real-time record review may all have a place. Qual Saf Heal Care. 2007;16(1):40–4. https://doi.org/10.1136/qshc.2005.017616.
Acknowledgements
The authors thank the Royal Belfast Hospital for Sick Children, whose Research Fellowship supported Richard Conn in conducting this work. We also thank Professor Karen Mattick for comments on a draft of this article.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Richard Conn and Angela Carrington. The first draft of the manuscript was written by Richard Conn. All authors commented on previous versions of the manuscript and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Richard Conn, Mary Tully, Michael Shields, Angela Carrington, and Tim Dornan have no conflicts of interest that are relevant to the content of this article.
Availability of data and material
Data were obtained with full NHS Ethics and Trust Governance approval. Data were fully anonymized prior to being made available to the research team. As data contain narrative information about patients and hospital staff, we have not openly published the dataset; access to data is available on request.
Code availability
Not applicable.
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Ethics approval
The research was deemed eligible for Proportionate Review by the first available committee. It was approved by the Proportionate Review Subcommittee of the East Midlands—Nottingham 2 Research Ethics Committee (reference 15/EM/0353).
Consent to participate/consent for publication
As this study used aggregated, anonymized incident data only, individual consent from patients and practitioners described in reports was not sought.
Rights and permissions
About this article
Cite this article
Conn, R.L., Tully, M.P., Shields, M.D. et al. Characteristics of Reported Pediatric Medication Errors in Northern Ireland and Use in Quality Improvement. Pediatr Drugs 22, 551–560 (2020). https://doi.org/10.1007/s40272-020-00407-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-020-00407-1