Abstract
Objective
A large proportion of paediatric patients with attention-deficit/hyperactivity disorder (ADHD) have associated sleep problems which not only affect the child’s wellbeing but also impact family functioning. Management of sleep problems is consequently an important aspect of overall ADHD management in paediatric patients. Although some drugs are being used off-label for the management of paediatric insomnia, there is scant clinical evidence supporting their use. Our aim was to identify and assess the quality of published studies reporting the safety, tolerability and efficacy of drugs used for treating behavioural insomnia in children with ADHD.
Methods
After an initial screen to determine which drugs were most commonly used, we conducted a systematic review of English-language publications from searches of PubMed, EMBASE, PsycINFO and two trial register databases to February 2017, using keywords ‘clonidine’, ‘melatonin’, ‘zolpidem’, ‘eszopiclone’, ‘L-theanine’, ‘guanfacine’, ‘ADHD’, ‘sleep disorder’ and ‘children’. For quality assessment of included studies, we used the CONSORT checklist for randomised control trials (RCTs) and the Downs and Black checklist for non-RCTs.
Results
Twelve studies were included. Two case series for clonidine, two RCTs and four observational studies for melatonin and one RCT each for zolpidem, eszopiclone, L-theanine and guanfacine. Of the 12 included studies, only one on eszopiclone scored excellent for quality. The quality of the rest of the studies varied from moderate to low. For clonidine, melatonin and L-theanine, improvements in sleep-onset latency and total sleep duration were reported; however, zolpidem, eszopiclone and guanfacine failed to show any improvement when compared with placebo. Clonidine, melatonin, L-theanine, eszopiclone and guanfacine were well tolerated with mild to moderate adverse events; zolpidem was associated with neuropsychiatric adverse effects.
Conclusion
There is generally poor evidence for prescribing drugs for behavioural insomnia in children with ADHD. Further controlled studies are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There is a need to treat sleep problems associated with ADHD. |
This review examines the safety, effectiveness and efficacy of pharmacological treatments being used to treat behavioural insomnia in children with ADHD. |
There is limited evidence supporting the use of pharmacological treatments being used after sleep hygiene or behavioural interventions are rendered ineffective. |
Further research including randomised controlled trials is required to define the effects of pharmacological treatments in detail. |
1 Introduction
Attention-deficit/hyperactivity disorder (ADHD) is the most common neurodevelopmental disorder affecting children and adolescents. It is characterised by age-inappropriate and persistent symptoms of inattention and/or hyperactivity/impulsivity [1]. In a recent meta-analysis, the overall pooled prevalence of ADHD was estimated to be 7.2% [2]. Among the conditions associated with ADHD, sleep problems have generally received inadequate attention in the past but are currently the focus of increasing interest.
Corkum et al. [3] concluded that sleep problems had been reported in about 25–50% of children with ADHD. The sleep problems reported in children with ADHD include disturbances in sleep quality or quantity, restless leg syndrome (RLS), periodic limb movement (PLM) and sleep disordered breathing (SDB) [4]. Among these, the most common problems include difficulty initiating sleep, maintaining sleep (recurrent waking or restless sleep) and early morning awakening with inability to return to sleep [4–7]. There is a relationship between sleep and ADHD symptoms, which seem to overlap with very little separation. It is currently not clear whether sleep disturbances are elemental to ADHD or sleep disorders cause ADHD-like symptoms. Even though the causes of ADHD-associated sleep problems seem to be complex and multifactorial, possible factors include adverse effects of drugs taken for treating ADHD such as stimulant medication [8], clinical correlates stemming from core ADHD symptoms (e.g. hyperactivity at night leading to difficulty falling asleep), psychiatric comorbidities (e.g. bedtime behavioural issues arising from associated conduct disorder), or a combination of these factors [9–12].
In a meta-analysis of subjective and objective sleep studies, Cortese et al. [13] showed that children with ADHD were significantly more impaired than controls in most of the parent-reported (subjective) parameters such as bedtime resistance, sleep-onset difficulties and daytime sleepiness, and in some of the actigraphic/polysomnographic-measured (objective) sleep items such as sleep-onset latency and number of stage shifts in total sleep time. Using the subjective parameters, most of the studies have reported sleep disturbances such as early and middle insomnia, nocturnal awakening, short sleep time, restless sleep and daytime sleepiness in children with ADHD [14–16]. Even though there is poor understanding of the relationship between sleep and ADHD symptoms, from a clinical standpoint, sleep disturbances associated with ADHD are very relevant since they can cause worsening of ADHD symptoms, leading to an increase in disruptive behaviour [17]. Sleep disturbances can not only have a significant impact on the quality of life of the child with ADHD but can also cause parental stress, disturbed caregiver mental health and disorganised family functioning [16]. Because of these issues, treatment of comorbid sleep disturbances is often a very important aspect of ADHD management. There is increasing awareness of the importance of behavioural insomnia treatments in children with ADHD. In one of the Australian Paediatric Research Network Surveys to document the management practices by Australian paediatricians for paediatric sleep disturbances, 89.1% of paediatricians prescribed melatonin for paediatric sleep disturbance [18]. Out of these, 54.5% prescribed it for sleep problems in children with ADHD. Another study showed that almost one quarter (22%) of the children with ADHD were prescribed sleep medication, with 14 and 9% taking clonidine and melatonin, respectively [19]. An anonymous questionnaire survey of members of the British Association for Community Child Health (BACCH) and the British Academy of Childhood Disability (BACD) was carried out in the UK to examine prescribing practices for melatonin in children [20]. Responses to questionnaires showed that sleep-onset difficulties (39%) and night-waking (12%) were the most frequent indications reported for melatonin use, with autism (68%) and ADHD (44%) being the most frequent clinical diagnoses.
A panel of experts in ADHD and sleep concluded that non-pharmacological interventions, which include sleep hygiene and behavioural interventions, should be the first-line management [10, 21]. The National Institute for Health and Care Excellence (NICE) also recommends non-pharmacological interventions such as good sleep hygiene or behavioural therapy [22]. If non-pharmacological treatments fail, pharmacological treatments may need to be considered. Drugs that have been used in clinical practice include clonidine, melatonin, antidepressants such as trazodone and mirtazapine, hypnotics such as zolpidem, and antihistamines [11]. However, evidence supporting these treatments remains limited [23]. Furthermore, none of these drugs has been approved for treating sleep disturbances in children with ADHD [24]. In addition, a drug closely related to clonidine, guanfacine, which, like clonidine, is also an α-2 receptor agonist, is becoming more widely used for the treatment of ADHD. Somnolence is a major side effect of guanfacine; it remains to be seen what role this drug will have in the management of sleep problems in children with ADHD [25].
Clonidine and guanfacine have been approved by the FDA for ADHD treatment. However, there are no approved treatments, either prescribed or over-the-counter preparations, for managing sleep disturbances such as behavioural insomnia in these children, compared with those in general paediatric populations [4, 26, 27]. Despite the widespread use of these unapproved agents to aid sleep in children with ADHD, few data exist on their safety, tolerability and efficacy. Furthermore, the methodological quality of the limited information available has not been assessed.
This paper provides a systematic review and methodological quality assessment of published studies on the safety, tolerability and efficacy of the most commonly used drugs for treating behavioural insomnia associated with ADHD, focusing on sleep-onset insomnia (SOI), total sleep duration and number of awakenings during the night.
2 Methods
The systematic review was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [28].
2.1 Systematic Search
An initial search was performed in PubMed using the search terms (adhd [ti] OR attention deficit [ti]) (sleep [ti] OR insomnia [ti]) and similar searches were carried out in Embase and PsycINFO. This initial search identified reports on the following drugs having been used for the treatment of sleep problems in individuals with ADHD: clonidine [29], melatonin [30], eszopiclone [31], zolpidem [32], L-theanine [33] and guanfacine [34]. Clonidine and guanfacine are established drugs for treating ADHD [35–37]. Sedative effects of these drugs become advantageous for the management of sleep [38, 39]. Melatonin is currently the most commonly used medication (hormone) for sleep problems in children [40]. The ‘z-drugs’, such as eszopiclone and zolpidem are specifically indicated for sleep problems in adults [41–43]; however, their safety and efficacy in patients below the age of 18 have not been established. L-theanine (5-N-ethyl-l-glutamine) is a herbal remedy that is a constituent of teas, including both green and black tea; it is promoted as inducing relaxation, although the evidence for this appears to be limited [44, 45].
The search strategy was then refined to focus on these six drugs and extra databases were searched. A systematic literature search of PubMed, EMBASE and PsycINFO was conducted using keywords, MeSH and Emtree terms. The following search terms were used: (ADHD OR attention deficit hyperactivity disorder OR neurodevelopmental disorder) AND (sleep OR insomnia) AND (clonidine OR melatonin OR eszopiclone OR zolpidem OR L-theanine OR guanfacine) AND (child OR children OR youth OR adolescent OR paediatric). The US National Institutes of Health Trial Register (http://www.ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform (ICTRP) (http://www.who.int/ictrp/en/) were also searched to identify potentially relevant studies. All databases were searched from their inception to February 2017. Duplicates were removed. Titles, abstracts and the content of the articles were screened to determine suitability for inclusion. References in the retrieved articles were also searched to identify any additional studies missed in the electronic search.
2.2 Inclusion Criteria
Observational and interventional studies investigating the effects of clonidine, melatonin, zolpidem, eszopiclone, L-theanine and guanfacine on behavioural insomnia in children with ADHD were included. ADHD was defined in the papers under review according to the following criteria: Diagnostic and Statistical Manual (DSM) of Mental Disorders (version DSM-III-R, DSM-IV or DSM-IV-TR) or guidelines of the American Academy of Child and Adolescent Psychiatry or through the Diagnostic Interview Schedule for Children Version IV, and parents’ and teachers’ report on the child symptom inventories [46–52]. As recommended by the Cochrane group, in order to ensure high levels of methodological adequacy and to avoid the inevitable bias caused by dependence on investigators agreeing to provide data from unpublished studies, only published, peer-reviewed studies were included.
2.3 Exclusion Criteria
The following exclusion criteria were applied:
-
1.
Single case reports.
-
2.
Medications used in other medical conditions, including developmental disabilities or other neurodevelopmental disorders such as autism, unless the subjects also had ADHD.
-
3.
Mixed neurodevelopmental disorder subject groups; for example, autism spectrum disorder with and without ADHD, for which separate results for each subgroup were not provided.
-
4.
Publications in languages other than English.
-
5.
Non peer-reviewed publications (such as conference proceedings).
-
6.
Animal studies.
2.4 Data Extraction
Two authors selected the studies on the basis of the inclusion/exclusion criteria and extracted data including study design, ADHD medication use, patient age, drug, sleep hygiene information and outcome measures of safety, tolerability and efficacy. Any disagreement was resolved by consensus.
2.5 Assessment of Study Quality
The methodological quality for the included randomised controlled trials (RCTs) was assessed using the CONSORT statement [53, 54]. The checklist was divided into domains: title and abstract, introduction, methods, randomisation, results, discussion and other information. The scores for each domain were summed to obtain the overall score. The methodological quality for observational studies was assessed using the Downs and Black scale for observational studies [55].
Two authors appraised each RCT and observational study independently. Assessment was conducted independently and cross-checked. The discrepancies were resolved by consensus. A CONSORT score from a maximum score of 25 was calculated by analysing each item in the checklist. Some of the items in the checklist contain two parts: ‘a’ and ‘b’. Each CONSORT checklist item as a whole was scored as 1 if present in the appraised study or 0.5 if only one part of the item was addressed. For observational studies, the quality score was calculated from a maximum score of 28. The checklist is divided into different domains: reporting, external validity, internal validity and power. The scores for each domain are summed to obtain the overall score. The Downs and Black checklist has several domains: reporting, external validity, internal validity and power, containing 27 items. Each item was scored 1 if the answer was ‘yes’ and 0 if the answer was ‘no’ or ‘unable to determine’ (UTD), except for one of the reporting subscales which was scored as 0 or 2. The scores were then added for total quality score. We adopted the following quality levels based on previous literature: excellent (>20 items), good (13–19) and poor (≤12) for CONSORT [56] and excellent (26–28), good (20–25), fair (15–19) and poor (≤14) for Downs and Black assessment [57–59].
3 Results
3.1 Search Results and General Characteristics of Included Studies
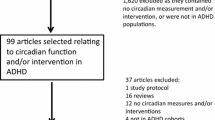
The PRISMA flow diagram of the review is shown in Fig. 1. The electronic database yielded a total of 702 records. Ten additional records were identified from the references. Titles and abstracts were screened, and the full texts of 23 articles were further screened. Twelve studies, either observational studies or RCTs, met the inclusion criteria for this systematic review.
For clonidine, two case series were identified [29, 60]. For melatonin, three RCTs [30, 61, 62], and three observational studies [63–65] met the inclusion criteria. Two studies [62, 66] had the same data for melatonin, hence only one was included. For zolpidem, eszopiclone, L-theanine and guanfacine, one RCT for each met the inclusion criteria [31–34]. Characteristics of the included studies are shown in Table 1.
3.2 Quality Assessment
3.2.1 Randomised Controlled Trials
The CONSORT checklist was used for quality assessment of RCTs as shown in online resource 1 (see electronic supplementary material). The scores for each study were as follows. For eszopiclone, Sangal et al. [31]: 21.5 (excellent quality). For melatonin, Weiss et al. [61]: 15.5 and Van der Heijden et al. [30]: 18.5 (both good quality) and Mohammadi et al. [62]: 10.5 (poor quality). For zolpidem, Blumer et al. [32]: 17.5 (good quality). For L-theanine, Lyon et al. [33]: 9.5 (poor quality). For guanfacine, Rugino [34]: 17.5 (good quality). The individual scores for each study are detailed in Table 2.
3.2.2 Observational Studies
The Downs and Black checklist was used for observational studies as shown in online resource 2. The scores for each study were as follows. For clonidine, Wilens et al. [29]: 4 and Prince et al. [60]: 13 (poor quality). For melatonin, Tjon Pian Gi et al. [63]: 11, Ayyash et al. [65]: 14 (both poor quality) and Hoebert et al. [64]: 22 (good quality). The quality of the results is detailed in Table 3.
The results from both RCTs and observational studies indicated that the quality of most of the available studies for the drugs treating behavioural insomnia in children with ADHD is not very high.
3.3 Efficacy of the Pharmacological Agents
Please note that, for all the studies in this section, where specific measures have been used or statistically significant differences have been found, these have been stated in the text that follows. Further information, for example on the quality of the studies, is available in the tables and elsewhere in the paper but, to avoid unnecessary duplication, has not been repeated here.
3.3.1 Clonidine
Based on a case series of >100 children with ADHD, Wilens et al. [29] stated that the beneficial effects of clonidine on sleep commenced within 30 min and persisted until morning. Both children and parents reported (subjective measure) favourable comments regarding clonidine treatment taken at bedtime, with overall improvement of sleep problems: less oppositional behaviour in the context of sleep activities, reduced sleep latency, less sleep restlessness, increased number of hours slept and improved morning awakening.
Prince et al. [60] carried out a systematic chart review of 62 children with ADHD and sleep problems, such as difficulty falling asleep, restless sleep and difficulty awakening. Subjective measures such as clinical global assessment of sleep severity (CGS) and of improvement (CGI) were used to rate sleep, with scores that ranged from 1 to 7. Fifty-three out of 62 (85%) of the children and adolescents had CGI values of 1 (very much improved; n = 19) or 2 (much improved; n = 34) after clonidine treatment.
3.3.2 Melatonin
Weiss et al. [61] evaluated the efficacy of sleep hygiene and melatonin for initial insomnia in children with ADHD in an RCT. Attention to sleep hygiene resulted in significant improvement in mean sleep-onset latency (SOL) from baseline (91.7 min reported subjectively by Somnolog, which were parent-completed sleep logs, and 98.1 min reported objectively by actigraphy) to 69.3 min by Somnolog and 73 min by actigraphy (in five subjects); that is, mean sleep-onset latency was improved (decreased) by 22.4 min by Somnolog and 15.1 min by actigraphy. For non-responders to sleep hygiene measures, the mean Somnolog SOL (documentation from parents for the amount of time between when the child was put to bed and when he/she fell asleep) for melatonin was 46.4 min [standard deviation (SD) 26.4] and for placebo was 62.1 min (SD 26.6). Two-sample t-tests comparing the mean period difference between sleep latencies and crossover treatment differences for melatonin versus placebo indicated a significant difference between these sleep latencies (p < 0.01) and a significant period effect (period difference in two crossover-treatment sequences) (p < 0.05). For total night-time sleep, more time asleep (15 min) was evident during melatonin treatment, (p < 0.01) on Somnolog monitoring, whereas actigraphic [67] analysis did not show a significant treatment difference. Open-label follow-up did not show a significant improvement in SOL; however, the improvement in sleep duration by 23 min continued (p < 0.01) with the melatonin treatment.
Van der Heijden et al. [30] investigated the efficacy of melatonin on sleep objectively with actigraphy and with dim-light melatonin onset (DLMO) using saliva samples, and also with assessments of behaviour, cognition and quality of life using different questionnaires in an RCT. There was an increase in mean total time asleep of 19.8 ± 61.9 min with melatonin and a decrease of 13.6 ± 50.6 min with placebo (p = 0.01). Compared with placebo, the melatonin group had a statistically significant decrease in sleep latency (p = 0.001), increase in sleep efficiency (p = 0.01) and decrease in nocturnal restlessness (p = 0.03). The saliva samples of melatonin-treated children showed an advance in DLMO of 44.4 ± 67.9 min compared with a delay of 12.8 ± 60.0 min in children receiving placebo (p < 0.0001). No statistically significant improvement was found in problem behaviour, cognitive ability or quality of life scores assessed on the different questionnaires.
Mohammadi et al. [62], in another RCT, subjectively studied the effects of melatonin on sleep and features of hyperactivity and attention deficit in children taking methylphenidate (Ritalin). The mean sleep latency (in minutes) decreased with melatonin. The mean latency at baseline for placebo was 21.37, and at 8 weeks was 26.37. The mean latency at baseline for melatonin was 23.15 and at 8 weeks was 17.96. The mean total sleep duration (in hours) increased with melatonin. The mean sleep duration at baseline for placebo was 8.77 and at 8 weeks was 8.27 (slight deterioration). The mean sleep duration at baseline for melatonin was 8.0 and at 8 weeks was 8.51 (improvement). The mean sleep latency and total sleep disturbance scores were reduced in the melatonin group, while the scores increased in the placebo group but no statistically significant differences were found for the two groups during the study period.
Tjon Pian Gi et al. [63] demonstrated the effect of melatonin on SOI in children with ADHD on methylphenidate in an observational study through subjective measures. Short-term (1–4 weeks) and long-term (>3 months) effects showed significant improvement in sleep latency, varying between 15–240 min and between 15–64 min, respectively. Relapse of SOI occurred when melatonin treatment was forgotten during the study and after the end of the study but improved when the melatonin was restarted.
In a follow-up study, Hoebert et al. [64] aimed to determine the long-term effectiveness and safety of melatonin therapy through subjective measures, along with the relapse rate of SOI after discontinuing melatonin treatment. Twenty-two children (23.4%) discontinued melatonin completely because of either total improvement of SOI (8), adverse events (3), initiative of treating physician (2), concerns about long-term treatment effects (2), refusal by child (1), lack of positive effect of therapy (3), melatonin therapy substituted by light therapy (1) and for an unknown reason (2). DLMO, as in the initial study, was assessed at baseline and on the first evening of the fourth week. The mean (±standard error of the mean) pre-treatment DLMO time for the eight children who discontinued melatonin completely because of improvement of SOI was 20:21 ± 0.25 h, while it was 20:41 ± 0.06 h in the remaining subjects who discontinued treatment due to other reasons [p = 0.413, effect size (ES) = −0.09]. The mean pre-treatment DLMO of the 11 children (20:11 ± 0.15 h) who used melatonin occasionally was earlier compared with DLMO in the 61 children (20:48 ± 0.007 h) who took melatonin daily (p = 0.037, ES = −0.26). Almost 90% of parents were satisfied with melatonin for the improvement of sleep-onset problems, 70.8% for improved daytime behaviours and 60.9% for improvement of mood. The authors concluded that melatonin improved chronic SOI in children with ADHD only as long as treatment was continued, but did not cure it.
Ayyash et al. [65] subjectively assessed the effects of melatonin on sleep latency and night-time awakening in children with neurodevelopmental disorders (ADHD, autism spectrum disorder or intellectual disability) in an observational study. The increase in the mean (±SD) for total sleep time (hours/night) in children with ADHD only was 2.68 ± 1.22, (p < 0.001), for sleep latency the mean decrease was 1.24 ± 1.20 h, (p < 0.02) and for awakening (number/night) the mean decrease was 0.23 ± 0.22, (p < 0.02). Significant improvement in all three sleep problems was observed via sleep diaries.
3.3.3 Zolpidem
Blumer et al. [32] evaluated the hypnotic efficacy of zolpidem compared with placebo in children with ADHD-associated insomnia in an RCT. No significant difference in latency to persistent sleep (LPS) between the zolpidem group (−20.28 min) and placebo (−21.27 min) was detected at week 4. For actigraphic (objective) measures at week 4, the baseline-adjusted least square (LS) mean difference ± standard error (SE) for total sleep time (i.e. total sleep time minus baseline total sleep time) was 2.77 ± 14.23 min (p = 0.8461), and for LPS was 1.55 ± 110.37 min, (p = 0.8884), indicating no significant difference between the groups. On the basis of Clinical Global Impression-Improvement (CGI-I) child assessments (subjective measure), the zolpidem group showed greater improvement in child score compared with the placebo group at week 4 with LS mean difference ±SE of 0.4 ± 0.200 (p = 0.0280). For Clinical Global Impression-Severity (CGI-S) child scores at week 4, the baseline-adjusted mean decrease was greater for zolpidem than for placebo, with an LS mean difference ± SE of −0.64 ± 0.230 (p = 0.0059). At weeks 4 and 8, CGI-I and CGI-S variables showed greater improvement with zolpidem for the 12- to 17-year-old age group but not for the 6- to 11-year-old age group.
3.3.4 L-theanine
Lyon et al. [33] investigated the efficacy of L-theanine on objective and subjective aspects of sleep quality in boys with ADHD in an RCT. The objective sleep-quality measure was actigraph watch data, and the subjective sleep measure was the Paediatric Sleep Questionnaire (PSQ). The actigraphy results indicated that the percentage of time spent in restful sleep was increased in the L-theanine group compared with the placebo group (p < 0.05) and there were fewer nocturnal activities in the L-theanine group compared with the placebo group (p < 0.05). A lower mean number of minutes spent awake after onset of sleep was found in the L-theanine group compared with placebo, although this did not quite reach statistical significance (p < 0.058). There was no significant difference between the groups for sleep latency or duration (p > 0.05). The authors did not present the details of the PSQ data but stated that this did not correlate significantly to the objective data gathered from actigraphy, suggesting that parents were not particularly aware of the quality of their child’s sleep.
3.3.5 Eszopiclone
Sangal et al. [31] found no significant differences between eszopiclone (high or low dose) groups and the placebo groups in the change from baseline to week 12 on polysomnography-measured LPS in an RCT: for high-dose eszopiclone versus placebo, p = 0.3749, and for low-dose eszopiclone versus placebo, p > 0.9999. Assessment of secondary subjective measures (patient/parent reports on sleep-onset latency, total sleep time, wake time after sleep onset (WASO), number of awakenings after sleep onset and sleep quality) revealed no statistically significant differences on hierarchical statistical analysis.
3.3.6 Guanfacine
Rugino [34] found that, in comparison with placebo, guanfacine worsened certain sleep parameters. The total sleep time for the treatment group decreased by 57.32 min (SD 89.17) in comparison with an increase by 31.32 min (SD 59.54) in the placebo group (p = 0.005), showing a statistically significant difference. The children in the treatment group were awake for a mean of 4.19 more minutes per hour of sleep, whereas the children receiving placebo were awake for a mean of 0.58 min less per hour of sleep, showing a significant difference. Later onset of persistent sleep by 10.54 ± 88.44 min was seen in the treatment group compared with 19.94 ± 54.12 min earlier with placebo; however, this difference did not reach statistical significance. No statistical significance was seen in time of persistent sleep and time of awakening between the two groups.
3.4 Tolerability/Safety of Pharmacological Agents
3.4.1 Clonidine
In the systematic chart review by Prince et al. [60], treatment-emergent adverse events (TEAEs) with clonidine were usually mild, occurring in 31% of patients, the most common being sedation and fatigue. In one child, clonidine was associated with depression, which resolved after discontinuation of the drug. In the case series reported by Wilens et al. [29], neither the cardiovascular nor central nervous system adverse reactions typical for clonidine were observed.
3.4.2 Melatonin
The TEAEs reported with melatonin have usually been mild and similar to those with placebo. Weiss and Salpekar [11] reported a single serious event of migraine. Van der Heijden et al. [30] reported no significant difference between the melatonin and placebo groups. Adverse events such as headache, hyperactivity, dizziness and abdominal pain were reported. Hoebert et al. [64] reported adverse events of sleep-maintenance insomnia, excessive morning sedation, decreased mood, headache, profuse perspiration and daytime laziness. Persistence of these events led to discontinuation of melatonin in three children. Mohammadi et al. [62] reported that there was no statistically significant difference between mean scores of adverse effects for melatonin and placebo (p = 0.686) based on a stimulant-drug side-effect questionnaire; however, the study was not powered adequately to allow any definitive comment on this issue. The most common adverse events reported were irritability, loss of appetite, sadness, weight loss, headache and difficulty falling asleep.
3.4.3 Zolpidem
In the study by Blumer et al. [32], one or more TEAEs were reported in 62.5% of the zolpidem-treated group and 47.7% of the placebo-treated group. The TEAEs included dizziness, headache and hallucination. Administration was discontinued permanently because of TEAEs in ten patients in the zolpidem group, compared with none in the placebo group. The main TEAE leading to discontinuation of zolpidem was hallucination, which occurred in ten of 136 patients.
3.4.4 L-theanine
Only one minor TEAE (facial tic) was observed for patients treated with L-theanine in the study by Lyon et al. [33]. The event causality was deemed unlikely by the principal investigator. No other TEAEs were noted.
3.4.5 Eszopiclone
In the study by Sangal et al. [31], TEAEs were reported for 61.0, 59.5 and 46.0% of the patients receiving high-dose eszopiclone, low-dose eszopiclone and placebo, respectively. The most commonly reported TEAEs with eszopiclone were headache, dysgeusia and dizziness. Reported TEAEs of special interest included skin reaction, hallucination and suicidality. The open-label extension for this RCT demonstrated that eszopiclone was generally well tolerated for up to 1 year. Several patients discontinued treatment due to hallucinations and suicidal ideation; the former was noted in 2.3% and the latter in 1% of eszopiclone-treated patients.
3.4.6 Guanfacine
The study by Rugino [34] reported treatment-emergent somnolence in 73% of children in the treatment group compared with 6% in the placebo group. No electrocardiographic, laboratory, growth or vital sign parameter was statistically significantly different between the two groups.
4 Discussion
To our knowledge, this is the first systematic review assessing the quality of studies of pharmacological treatments for behavioural insomnia in children with ADHD. Based on the results from the methodological quality assessment, only one high-quality study (an RCT of eszopiclone [31]) was identified. Except for the RCT on eszopiclone [31] and an observational study on melatonin [64], the rest of the studies scored moderate to low on quality, reflecting a number of issues including high risk of bias (due to poor methodological quality), inconsistency (due to the high degree of heterogeneity between studies) and inaccuracy/unreliability (due to the low numbers of participants).
In most of the studies, the determination of behavioural insomnia was based on small sample sizes using subjective measures (parental reports, Somnologs or questionnaires) rather than more precise objective measures, using actigraphy. The retrospective chart review on clonidine by Prince et al. [60] was subject to observer bias. The small research letter by Tjon Pian Gi et al. [63] on melatonin did not provide sufficient details on study methodology, diagnosis of sleep insomnia or patient characteristics. No randomisation or blinding was performed and consequently a placebo effect could not be excluded. In the Weiss et al. [61] study on melatonin, the effect of sleep hygiene could not be isolated from the effect of the melatonin. Although this is a relatively minor issue, the criterion for SOI in the study by Van der Heijden et al. [30] on melatonin was based on a Dutch child population and may not be generalisable to other population groups. A more important issue was that a considerable amount (31%) of data were missing, implying limitations to the data analysis and potential bias.
The Hoebert et al. [64] study on melatonin lacked a long-term placebo arm and the questionnaire lacked information regarding concomitant medication. In the Mohammadi et al. [62] study on melatonin, the confounding effect of methylphenidate could not be excluded. Lyon et al. [33] studied the effect of the drug L-theanine in boys only. The results for guanfacine cannot be generalised due to unequal sample sizes at baseline and early termination of the study [34].
Given the high prevalence and compelling impact of behavioural insomnia in these children, there is a need for effective pharmacological agents with strong evidence. There is currently insufficient evidence to allow firm recommendations to be made with regard to the prescription of these pharmacological agents, due to a lack of high-quality published studies; however, melatonin has showed consistent positive results. Zolpidem, eszopiclone and guanfacine showed unremarkable results by worsening different sleep parameters when compared with placebo. Although there are RCTs on the use of melatonin, zolpidem, eszopiclone, L-theanine and guanfacine for sleep-onset delay, the small number and the limitations of these RCTs imply that there is inadequate evidence on efficacy, effectiveness and safety. We note that a formal meta-analysis could not be performed due to the low quality and heterogeneous nature of the studies.
4.1 Additional Limitations
Sleep issues in children with ADHD can be affected by a number of additional variables which may be confounding factors in the assessment of efficacy or adverse effects of medication used to treat behavioural insomnia. These include the following:
First, ADHD is not a single condition but a group of conditions with certain core features in common; typically, poor concentration, over activity and impulsivity. Against this background it is not surprising that a drug that is effective in treating ADHD in one child may be ineffective in another; similarly, it would not be surprising if a drug that was effective in treating sleep in one child with ADHD was ineffective in another. In particular, there is a subgroup of children with ADHD in whom sleep onset is improved with an evening dose of methylphenidate whereas, in most children, an evening dose of methylphenidate would delay sleep onset [68].
Second, the medication used to treat ADHD may be a confounding factor when assessing drugs used to help with behavioural insomnia. Some medications that are frequently used to treat ADHD can delay sleep onset (e.g. methylphenidate or dexamfetamine, except in the subgroup referred to in the previous paragraph), whereas others are either sleep neutral or may improve sleep, such as clonidine [69–71]. This implies that the assessment of medications used to treat behavioural insomnia in children with ADHD should be adjusted for co-medication used to treat the ADHD, which may not be easy to achieve.
Third, ADHD is associated with a very high rate of comorbidities which may, in turn, be associated with a high rate of sleep problems, which could affect the response to sleep medication. For example, autism spectrum disorder is associated with a high rate of ADHD and is also associated with a high rate of sleep disorders. Medications used to treat the comorbidities can also have a major effect on sleep. For example, risperidone used to treat anxiety and behavioural disorders in children with autism spectrum disorder and ADHD can improve sleep [72].
Fourth, sleep is highly dependent on environmental factors [73]. Proper attention to sleep hygiene should minimise the confounding effects of such factors but may not eliminate them completely.
Finally, we limited our search to papers in English.
4.2 Implications
Our systematic review suggests that, with the possible exception of melatonin, there is generally an insufficient evidence base for the use of medications in treating sleep-related disturbances such as insomnia in ADHD. It was also seen that zolpidem, eszopiclone and guanfacine did not show significant improvement in different sleep parameters when compared with placebo. Considering that there are currently no FDA drugs approved for the treatment of sleep disturbance in children with ADHD, clinicians should discuss the limitations of available evidence carefully with the patient and the family, aiming for a short period of treatment should a trial with a pharmacological intervention be agreed upon.
Further high-quality research is required, as these medications appear to be widely used despite the lack of long-term data on benefits or risks. Future research should include RCTs with sufficient sample size, using both objective and subjective outcome measures. They should be powered adequately to yield statistically meaningful results of the measures of interest. These studies should evaluate the effect of pharmacological agents not only on the sleep-associated disturbances but also on long-term daytime function, health and well-being.
5 Conclusion
Although some of the included studies reported similar conclusions of having a positive effect in improving behavioural insomnia, because of their low quality, small sample sizes and heterogeneous designs, the results cannot be viewed as reliable. Incontrovertible evidence establishing the definitive values of clonidine, melatonin, zolpidem, eszopiclone, L-theanine and guanfacine in treating ADHD-related behavioural insomnia in children does not appear to be available. Further high-quality research and RCTs are required to evaluate the effectiveness and safety of these pharmaceutical agents in treating behavioural insomnia in children with ADHD.
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
Thomas, R, et al. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. 2015.
Corkum P, Tannock R, Moldofsky H. Sleep disturbances in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1998;37(6):637–46.
Barrett JR, Tracy DK, Giaroli G. To sleep or not to sleep: a systematic review of the literature of pharmacological treatments of insomnia in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2013;23(10):640–7.
Corkum P, et al. Better nights/better days-distance intervention for insomnia in school-aged children with/without ADHD: a randomized controlled trial. J Pediatr Psychol. 2016;41(6):701–13.
Yoon SY, Jain U, Shapiro C. Sleep in attention-deficit/hyperactivity disorder in children and adults: past, present, and future. Sleep Med Rev. 2012;16(4):371–88.
Chiang HL, et al. Association between symptoms and subtypes of attention-deficit hyperactivity disorder and sleep problems/disorders. J Sleep Res. 2010;19(4):535–45.
Ironside S, Davidson F, Corkum P. Circadian motor activity affected by stimulant medication in children with attention-deficit/hyperactivity disorder. J Sleep Res. 2010;19(4):546–51.
Harpin VA. The effect of ADHD on the life of an individual, their family, and community from preschool to adult life. Arch Dis Child. 2005;90(Suppl 1):i2–7.
Sciberras E, et al. Managing sleep problems in school aged children with ADHD: a pilot randomised controlled trial. Sleep Med. 2011;12(9):932–5.
Weiss MD, Salpekar J. Sleep problems in the child with attention-deficit hyperactivity disorder: defining aetiology and appropriate treatments. CNS Drugs. 2010;24(10):811–28.
Hunt RD, Minderaa RB, Cohen DJ. Clonidine benefits children with attention deficit disorder and hyperactivity: report of a double-blind placebo-crossover therapeutic trial. J Am Acad Child Psychiatry. 1985;24(5):617–29.
Cortese S, et al. Sleep in children with attention-deficit/hyperactivity disorder: meta-analysis of subjective and objective studies. J Am Acad Child Adolesc Psychiatry. 2009;48(9):894–908.
Li S, et al. Sleep problems in chinese school-aged children with a parent-reported history of ADHD. J Attend Disord. 2009;13(1):18–26.
Hvolby A, Jorgensen J, Bilenberg N. Parental rating of sleep in children with attention deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry. 2009;18(7):429–38.
Sung V, et al. Sleep problems in children with attention-deficit/hyperactivity disorder: prevalence and the effect on the child and family. Arch Pediatr Adolesc Med. 2008;162(4):336–42.
Dahl RE. The impact of inadequate sleep on children’s daytime cognitive function. Semin Pediatr Neurol. 1996;3(1):44–50.
Heussler H, et al. Pharmacological and non-pharmacological management of sleep disturbance in children: an Australian Paediatric Research Network survey. Sleep Med. 2013;14(2):189–94.
Efron D, Lycett K, Sciberras E. Use of sleep medication in children with ADHD. Sleep Med. 2014;15(4):472–5.
Waldron DL, Bramble D, Gringras P. Melatonin: prescribing practices and adverse events. Arch Dis Child. 2005;90(11):1206–7.
Cortese S, et al. Assessment and management of sleep problems in youths with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2013;52(8):784–96.
ESUOM2, N.a., http://www.nice.org.uk/advice/esuom2. January 2013.
Cortese S, et al. Assessment and management of sleep problems in youths with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2013;52(8):784–96.
Owens JA, et al. The use of pharmacotherapy in the treatment of pediatric insomnia in primary care: rational approaches. A consensus meeting summary. J Clin Sleep Med. 2005;1(1):49–59.
Spencer TJ, et al. Safety and effectiveness of coadministration of guanfacine extended release and psychostimulants in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(5):501–10.
Owens JA, Rosen CL, Mindell JA. Medication use in the treatment of pediatric insomnia: results of a survey of community-based pediatricians. Pediatrics. 2003;111(5 Pt 1):e628–35.
Tsai MH, Hsu JF, Huang YS. Sleep problems in children with attention deficit/hyperactivity disorder: current status of knowledge and appropriate management. Curr Psychiatry Rep. 2016;18(8):76.
Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Wilens TE, Biederman J, Spencer TJ. Clonidine for sleep disturbances associated with attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1994;33(3):424–6.
Van der Heijden KB, et al. Effect of melatonin on sleep, behavior, and cognition in ADHD and chronic sleep-onset Insomnia. J Am Acad Child Adolesc Psychiatry. 2007;46(2):233–41.
Sangal RB, et al. Eszopiclone for insomnia associated with attention-deficit/hyperactivity disorder. Pediatrics. 2014;134(4):e1095–103.
Blumer JL, et al. Controlled clinical trial of zolpidem for the treatment of insomnia associated with attention-deficit/hyperactivity disorder in children 6 to 17 years of age. Pediatrics. 2009;123(5):e770–6.
Lyon MR, Kapoor MP, Juneja LR. The effects of L-theanine (Suntheanine) on objective sleep quality in boys with attention deficit hyperactivity disorder (ADHD): a randomized, double-blind, placebo-controlled clinical trial. Altern Med Rev. 2011;16(4):348–54.
Rugino TA. Effect on primary sleep disorders when children with ADHD are administered guanfacine extended release. J Attend Disord. 2014.
Nair V, Mahadevan S. Randomised controlled study-efficacy of clonidine versus carbamazepine in children with ADHD. J Trop Pediatr. 2009;55(2):116–21.
Newcorn JH, et al. Extended-release guanfacine hydrochloride in 6–17-year olds with ADHD: a randomised-withdrawal maintenance of efficacy study. J Child Psychol Psychiatry. 2016;57(6):717–28.
Kollins SH, et al. Clonidine extended-release tablets as add-on therapy to psychostimulants in children and adolescents with ADHD. Pediatrics. 2011;127(6):e1406–13.
Blackmer AB, Feinstein JA. Management of sleep disorders in children with neurodevelopmental disorders: a review. Pharmacotherapy. 2016;36(1):84–98.
Huss M, Chen W, Ludolph AG. Guanfacine extended release: a new pharmacological treatment option in Europe. Clin Drug Invest. 2016;36:1–25.
van Geijlswijk IM, Korzilius HP, Smits MG. The use of exogenous melatonin in delayed sleep phase disorder: a meta-analysis. Sleep. 2010;33(12):1605–14.
Walsh JK, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30(8):959–68.
Roth T, et al. An evaluation of the efficacy and safety of eszopiclone over 12 months in patients with chronic primary insomnia. Sleep Med. 2005;6(6):487–95.
Nowell PD, et al. Benzodiazepines and zolpidem for chronic insomnia: a meta-analysis of treatment efficacy. JAMA. 1997;278(24):2170–7.
Vuong QV, Bowyer MC, Roach PD. L-theanine: properties, synthesis and isolation from tea. J Sci Food Agric. 2011;91(11):1931–9.
Nobre AC, Rao A, Owen GN. L-theanine, a natural constituent in tea, and its effect on mental state. Asia Pac J Clin Nutr. 2008;17(Suppl 1):167–8.
Dulcan M. Practice parameters for the assessment and treatment of children, adolescents, and adults with attention-deficit/hyperactivity disorder. American Academy of Child and Adolescent Psychiatry. J Am Acad Child Adolesc Psychiatry. 1997. 36(10 Suppl): 85S–121S.
Sheehan DV, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998. 59 Suppl 20:22–33; quiz 34–57.
Shaffer D, et al. NIMH diagnostic interview schedule for children version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28–38.
Psychiatry, A.A.O.C.A.A., Practice parameter for the assessment and treatment of children, adolescent and adults with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry, 1997. 36:85S–121S.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 3rd edition-revised (DSM-III-R). Washington, DC: American Psychiatric Association; 1987.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed., text rev.). Washington, DC: American Psychiatric Association; 2000.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM–IV). American Psychiatric Association, 1994.
Altman DG, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134(8):663–94.
Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357(9263):1191–4.
Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–84.
Wu W, Zhang L, Xue R. Lorazepam or diazepam for convulsive status epilepticus: a meta-analysis. J Clin Neurosci. 2016.
O’Connor SR, et al. Failure of a numerical quality assessment scale to identify potential risk of bias in a systematic review: a comparison study. BMC Res Notes. 2015;8:224.
Pas HI, et al. Efficacy of rehabilitation (lengthening) exercises, platelet-rich plasma injections, and other conservative interventions in acute hamstring injuries: an updated systematic review and meta-analysis. Br J Sports Med. 2015;49(18):1197–205.
Montane E, et al. Reporting randomised clinical trials of analgesics after traumatic or orthopaedic surgery is inadequate: a systematic review. BMC Clin Pharmacol. 2010;10:2.
Prince JB, et al. Clonidine for sleep disturbances associated with attention-deficit hyperactivity disorder: a systematic chart review of 62 cases. J Am Acad Child Adolesc Psychiatry. 1996;35(5):599–605.
Weiss MD, et al. Sleep hygiene and melatonin treatment for children and adolescents with ADHD and initial Insomnia. J Am Acad Child Adolesc Psychiatry. 2006;45(5):512–9.
Mohammadi MR, et al. Melatonin effects in methylphenidate treated children with attention deficit hyperactivity disorder: a randomized double blind clinical trial. Iran J Psychiatry. 2012;7(2):87–92.
Tjon Pian Gi CV, et al. Melatonin for treatment of sleeping disorders in children with attention deficit/hyperactivity disorder: a preliminary open label study. Eur J Pediatr. 2003;162(7–8):554–5.
Hoebert M, et al. Long-term follow-up of melatonin treatment in children with ADHD and chronic sleep onset Insomnia. J Pineal Res: Mol Biol Physiol Clin Aspects Melatonin. 2009;47(1):1–7.
Ayyash HF, et al. Melatonin for sleep disturbance in children with neurodevelopmental disorders: prospective observational naturalistic study. Expert Rev Neurother. 2015;15(6):711–7.
Mostafavi SA, et al. Dietary intake, growth and development of children with ADHD in a randomized clinical trial of Ritalin and Melatonin co-administration: through circadian cycle modification or appetite enhancement? Iran J Psychiatry. 2012;7(3):114–9.
Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Med Rev. 2002;6(2):113–24.
Kidwell KM, et al. Stimulant medications and sleep for youth with ADHD: a meta-analysis. Pediatrics, 2015.
Tirosh E, et al. Effects of methylphenidate on sleep in children with attention-deficient hyperactivity disorder: an activity monitor study. Am J Dis Child. 1993;147(12):1313–5.
Lee SH, et al. Effect of methylphenidate on sleep parameters in children with ADHD. Psychiatry Invest. 2012;9(4):384–90.
Stein MA, Weiss M, Hlavaty L. ADHD treatments, sleep, and sleep problems: complex associations. Neurotherapeutics. 2012;9(3):509–17.
Eapen V, Gururaj AK. Risperidone treatment in 12 children with developmental disorders and attention-deficit/hyperactivity disorder. Primary Care Companion J Clin Psychiatry. 2005;7(5):221–4.
Gregory AM, Sadeh A. Annual research review: sleep problems in childhood psychiatric disorders—a review of the latest science. J Child Psychol Psychiatry. 2016;57(3):296–317.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work has not been supported by any funding.
Conflict of interest
Shweta Anand, Henry Tong, Prof. Frank M.C. Besag, Dr. Esther W. Chan and Prof. Ian C.K. Wong declare no conflict of interest. Dr. Samuele Cortese has received grant or research support from the Solent National Health Service (NHS) Trust, UK. He has also received honorarium and travel expenses from the Association for Child and Adolescent Mental Health (ACAMH), UK, a non-profit organisation, all unrelated to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Anand, S., Tong, H., Besag, F.M.C. et al. Safety, Tolerability and Efficacy of Drugs for Treating Behavioural Insomnia in Children with Attention-Deficit/Hyperactivity Disorder: A Systematic Review with Methodological Quality Assessment. Pediatr Drugs 19, 235–250 (2017). https://doi.org/10.1007/s40272-017-0224-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-017-0224-6