Abstract
Introduction
Reducing postoperative opioid consumption is a priority given its impact upon recovery, and the efficacy of ketamine as an opioid-sparing agent in children is debated. The goal of this study was to update a previous meta-analysis on the postoperative opioid-sparing effect of ketamine, adding trial sequential analysis (TSA) and four new studies.
Materials and Methods
A comprehensive literature search was conducted to identify clinical trials that examined ketamine as a perioperative opioid-sparing agent in children and infants. Outcomes measured were postoperative opioid consumption to 48 h (primary outcome: postoperative opioid consumption to 24 h), postoperative pain intensity, postoperative nausea and vomiting and psychotomimetic symptoms. The data were combined to calculate the pooled mean difference, odds ratios or standard mean differences. In addition to this classical meta-analysis approach, a TSA was performed.
Results
Eleven articles were identified, with four added to seven from the previous meta-analysis. Ketamine did not exhibit a global postoperative opioid-sparing effect to 48 postoperative hours, nor did it decrease postoperative pain intensity. This result was confirmed using TSA, which found a lack of power to draw any conclusion regarding the primary outcome of this meta-analysis (postoperative opioid consumption to 24 h). Ketamine did not increase the prevalence of either postoperative nausea and vomiting or psychotomimetic complications.
Conclusions
This meta-analysis did not find a postoperative opioid-sparing effect of ketamine. According to the TSA, this negative result might involve a lack of power of this meta-analysis. Further studies are needed in order to assess the postoperative opioid-sparing effects of ketamine in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ketamine has been found ineffective as a postoperative opioid-sparing effect. |
Using two statistical methodologies, this updated systemic review found the number of studies insufficient to draw any conclusion about the opioid-sparing effect of ketamine or its side effects. |
Further studies are needed in order to assess the postoperative opioid-sparing effects of ketamine in children. |
1 Introduction
Postoperative pain management in children has evolved in the context of increased use of regional analgesia and of non-opioid analgesics, particularly nonsteroidal anti-inflammatory drugs (NSAIDs) [1].
Both pain and opioid consumption are of major concern for long-term outcomes in children. Many studies have found pain to cause functional and structural central nervous system sensitization resulting in higher pain perception during future painful stimulation [2, 3]. Moreover, the opioids commonly used for pain relief have themselves been shown to cause postoperative pain sensitization [4]. These findings led to the development of the concept of opioid-free anesthesia (OFA), aiming both to prevent opioid-induced hyperalgesia and to improve postoperative pain management.
Ketamine has been found to produce analgesic, anti-hyperalgesia, and opioid-sparing effects in adult patients [5]. Interestingly, these postoperative effects, especially the opioid-sparing effect, have been observed for up to 24 postoperative hours even when ketamine is administered as a single intraoperative bolus. These effects have been hypothesized to involve an intraoperative opioid-sparing effect with a reduction of opioid-related hyperalgesia and inhibition of the major mechanism of this phenomenon, namely NMDA-receptor stimulation [6–8]. However, these effects in children are still debated. One meta-analysis performed in 2010 found ketamine to be ineffective in reducing postoperative opioid administration in children [9]. Recently, a new statistical technique called trial sequential analysis (TSA) has been strongly recommended as a complement to meta-analysis [10, 11]. It allows for correction of type I risk and heterogeneity, and furthermore determines the number of patients required in future trials for adequately powered meta-analyses.
Thus, the goal of the present work was to update the 2010 meta-analysis on the opioid-sparing effect of ketamine, perform meta-analysis with recent additional trials, and perform TSA—the latter to confirm results and determine number of patients to include in future adequately powered trials, if indicated.
2 Materials and Methods
2.1 Bibliographic Search and Analysis
We conducted this meta-analysis according to the Cochrane Handbook for Systematic Reviews guidelines [12] and the PRISMA statements [13].
Literature database searches included PubMed, Embase, the Cochrane Central Register of Controlled Trials, clinical trial registers and open access journals not indexed in major databases (Directory of Open Access Journals, Open Journal of Anesthesiology, Anesthesiology Research and Practice, Journal of Anesthesia & Clinical Research, Journal of Anesthesiology & Clinical Science, Journal of Anesthesiology and Critical Care Medicine). The following queries were used: ‘ketamine or ketalar’ and ‘children or infant’. In addition, a manual search of the references from the selected articles (including reviews and meta-analyses) was also performed. Congress abstracts were not included. The date of the most recent search was June 2016. Given that seven articles were identified in our previous 2010 ketamine meta-analysis on the opioid-sparing effect of ketamine, we extended our searches from January 2010 to June 2016 for PubMed and Embase databases and included all dates for others sources [5].
Articles meeting the following criteria were included in our analysis: randomized controlled studies, double blinded, absence of neurological and/or psychiatric diseases, standardized anesthesia protocols, postoperative opioid administration, standardized analgesic protocols (including rescue analgesics), presence of a control group with no active treatment (placebo), and administration of ketamine via the systemic route (oral, intravenous, sublingual or rectal). These criteria led to the selection of articles with lowest methodological bias. Readers assessed article quality and the presence of potential bias using the following criteria: randomization and allocation concealment (clear, sufficiently detailed description of methodology demonstrating whether intervention allocations could have been foreseen before or during enrollment), double blinding, incomplete data report statements (concerning excluded patients and data), and selective reporting (presence of studied outcomes report verified). Extracted data consisted of patient ages, performed surgery, ketamine administration (route, doses, and timing), hypnotic agents used, intraoperative analgesia, postoperative analgesia, and meta-analysis outcomes. The primary outcome of this study was opioid consumption during the first 24 postoperative hours; secondary outcomes were postoperative opioid requirements in the post-anesthesia care unit (PACU) and during the first 48 postoperative hours, postoperative pain in PACU and during the first 24 and 48 postoperative hours, the occurrence of postoperative nausea and vomiting (PONV) during the first 24 postoperative hours, and the occurrence of psychotomimetic symptoms such as nightmares or hallucinations (percentage of patients) during the postoperative period to 24 h.
The articles were independently analyzed (double-check analysis) by four anesthesiologists (Julie Hilly, Alia Skhiri, Rachida Abdat, Thierno Diallo) and when conflicting results were found, the article was checked again by two anesthesiologists.
2.2 Statistical Analysis
Classical meta-analysis procedures were performed using the Review Manager 5 software (RevMan 5.3, The Cochrane Collaboration, Oxford, UK). Further analyses were also performed using TSA software (Copenhagen Trial Unit’s Trial Sequential Analysis Software, hereafter: TSA Software, Copenhagen, Sweden). When original data were expressed as continuous variables, meta-analysis was performed using the standardized mean difference (SMD). This method allowed aggregation of outcomes measured using different tools, particularly pertinent to pediatric pain which is assessed using multiple different pain scales such as OPS (Objective Pain Scale), FLACC (Face, Legs, Activity, Cry, Consolability scale) or EDIN (Echelle Douleur Inconfort Nouveau-Né) [14, 15]. Opioid consumption outcomes were computed using mean difference (MD) and converted to morphine equivalents as follows: 1 mg of intravenous morphine = 1 mg of nalbuphine = 10 µg of fentanyl = 7.5 mg of pethidine = 0.5 mg of oxycodone. In all other cases, outcome incidence analysis was performed using the risk ratio (RR). In order to include a maximum number of appropriate studies, incomplete data were managed by contacting the corresponding author or estimation of the mean and the standard deviation (SD) on the basis of the sample size, median, and range according to the method described by Hozo and colleagues [16]. The absence of a validated method to convert medians and interquartile ranges to means and SDs led to all involved data being discarded. Where outcomes were expressed as continuous and dichotomous values, a partial standardized mean ratio (SMR) was initially computed for each study, than transformed to partial odds ratios (ORs) using Chinn’s formulae [17]: LnOR = 1.814 × SMR (Ln: logarithm). Data were entered as Ln(OR) and SD(LnOR) in the software. Overall MD, SMD, or RR (and its 95 % confidence interval [CI]) was then calculated using the inverse variance method.Footnote 1
Heterogeneity was assessed for the primary outcome of this meta-analysis (opioid consumption during the first 24 postoperative hours) using I 2 statistics. This describes the percentage of the variability in effect estimates (MD) that is due to heterogeneity rather than sampling error. According to the Cochrane review guidelines,Footnote 2 an I 2 > 40 % and a p < 0.1 were considered as the threshold for heterogeneity and indicated the use of a random effects model. In addition, subgroup analyses were performed (when at least two studies included the considered outcome) according to the type of surgery, time of administration (intraoperative vs concomitant intraoperative and postoperative), and the use of postoperative non-opioid analgesics in addition to the opioid agent. Postoperative non-opioid agents considered in the analysis were paracetamol, NSAIDs, nefopam, and dexamethasone. Comparison between subgroups (called the interaction test) consisted of subgroup heterogeneity determination. In studies with more than one intervention arm, each arm was considered as a study and compared with the control group so as to take all data into account. In this particular case, the number of participants in the control group was distributed equally (with a tolerance of one participant difference in case of an odd number of participants) between analyses in order to avoid unit of analysis error (the number of total participants must always be included in individual trials). Finally, to avoid calculation failure related to zero values, a 1 or 0.001 was added to all groups when the number of events was equal to 0 in any group (for RevMan and TSA, respectively).
Statistical methods are available to assess the effects of unpublished studies on the results of meta-analysis (publication bias). This type of bias is assessed by plotting the OR, or the logarithm of the OR, against a measurement of the precision of the OR such as the standard error of the OR. This plot is named the funnel plot and its asymmetry may indicate studies left unpublished because of negative resultsFootnote 3 [18]. Such asymmetry may also indicate data heterogeneity or poor methodology in included studies [18, 19]. Some studies, due to design, may also produce strongly positive results that lead to funnel plot asymmetry interpreted as ‘publication bias’. Methodological bias may also lead to strongly positive results creating funnel plot asymmetry [18, 20]. According to the Cochrane collaborative guideline,Footnote 4 publication bias can be assessed when aggregating at least 10 studies for analysis.
Results of intervention effects were expressed as OR, MD, or SMD (95 % CI) I 2, and p value for I 2 statistics. Interaction tests between paired subgroups were expressed as X 2 value, df, I 2, and p value for I 2. Difference between subgroups was considered significant when p < 0.05.
In order to confirm or infirm primary outcome results, a second set of analyses were performed using the trial sequential method [10, 11]. This method has been found to be more reliable when analyzing cumulative heterogeneous results (the computation of overall results takes into account the heterogeneity of results using Biggerstaff and Tweedie’s method [21]) and decreases type I error. TSA provides three important supplementary data points when compared with traditional meta-analysis. Firstly, it combines results and provides a cumulated sample size of included trials using an approximate semi-Bayes procedure with an adjusted threshold for statistical significance and adjusted alpha risk in order to decrease type I error. The result is considered as significant when the boundary of significance is crossed.
Secondly, TSA considers the effect of previous meta-analyses on overall results. Considering that the alpha risk must be adjusted to the number of comparisons, classical meta-analysis does not take into account this correction, leading to 20–30 % false-positive results [10], while TSA can perform corrections according to studies previously included in meta-analyses.
Thirdly, TSA allows the description of further trial requirements, through a procedure known as trial sequential monitoring boundaries. When the cumulative z score for the studied outcome crosses the trial sequential monitoring boundary, the level of evidence for the intervention is considered reached and no further trials are needed. TSA also determines the futility area, indicating that no significant result would be found with additional trials. Finally the O’Brien-Fleming approach in TSA (the number of patients to be included in the meta-analysis in order to reach the desired level of significance) predicts the sample size to be included in future trials in order to achieve proof of the desired effect according to current results. In our study, information size was computed for the primary study outcome (opioid consumption in the first postoperative 24 h) assuming an alpha risk of 5 %, a beta risk of 20 %, and an MD equal to the one computed on overall available results of the classical meta-analysis method.
3 Results
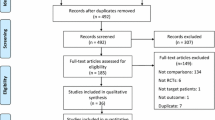
Using the above criteria, 323 additional articles were identified, of which four were assessed as relevant. They were added to the seven studies examining postoperative opioid consumption in our previously published meta-analysis, and analyses were performed on 11 studies [22–32]. No study included in this meta-analysis used the S isomer of ketamine (S-ketamine). The details of the selection process are summarized in Fig. 1. The description of included studies is displayed in Table 1. Two hundred and sixty-nine patients received ketamine and 239 received placebo. All studies employed intravenous ketamine. All studies employed ketamine boluses (range 0.15–0.7 mg/kg). Five of eleven studies employed intraoperative ketamine infusions (range 0.08–0.25 mg/kg/h). Four studies employed postoperative ketamine infusions (range 0.08–1 mg/kg/h), of which three also employed intraoperative infusions.
3.1 Overall Results
Overall, ketamine was found to be ineffective in decreasing opioid consumption in PACU (Fig. 2a), during the first 24 postoperative hours (Fig. 2b), or during the first 48 h (Fig. 2c) (MD = 0.2 mg/kg morphine [−0.59 to 1.00], I 2 = 77 %, p = 0.0005; MD = 0.00 [−0.06 to 0.05], I 2 = 40 %, p = 0.13; MD = 0.11 [−0.06 to 0.28], I 2 = 0 %, p = 0.89, respectively). Postoperative pain scores were also unaffected by ketamine administration in PACU (Fig. 3a), during the first 24 postoperative hours (Fig. 3b), or during the first 48 h (Fig. 3c) (SMD = 0.22 [−0.89 to 1.33], I 2 = 93 %, p < 0.00001; SMD = −0.75 [−2.32 to 0.82], I 2 = 96 %, p < 0.00001; SMD = −0.30 [−0.63 to 0.03], I 2 = 0 %, p = 0.91, respectively).
Forest plot of meta-analysis of the effects of intravenous (IV) ketamine on postoperative morphine consumption in the postoperative acute care unit (2 h) (a), in the 24 postoperative hours (b), and in the 48 postoperative hours (c). The square in front of each study (first author and year of publication) is the mean difference (MD) for individual trials, and the corresponding horizontal line is the 95 % confidence interval (CI). The diamond at the bottom of the figure is the pooled MD with the 95 % CI. *, **Studies with more than one intervention group were named ‘Author, year of publication_1’ and ‘Author, year of publication_2’. Assessment of bias: green circle low risk of bias, uncolored circle undetermined bias, red circle high risk of bias. SD standard deviation
Forest plot of meta-analysis of the effects of intravenous (IV) ketamine on postoperative pain intensity (postoperative pain scores) in the postoperative acute care unit (2 h) (a), in the 24 postoperative hours (b), and in the 48 postoperative hours (c). The square in front of each study (first author and year of publication) is the standard mean difference (SMD) for individual trials, and the corresponding horizontal line is the 95 % confidence interval (CI). The diamond at the bottom of the figure is the pooled SMD with the 95 % CI. *, **Studies with more than one intervention group were named ‘Author, year of publication_1’ and ‘Author, year of publication_2’. Assessment of bias: green circle low risk of bias, uncolored circle undetermined bias, red circle high risk of bias. SD standard deviation
Ketamine did not affect PONV incidence during PACU stay (N = 5, RR = 1.71 [0.82–3.53], I 2 = 0 %, p = 0.61) or during the first 24 postoperative hours (N = 7, RR = 1.48 [0.97–2.26], I 2 = 15 %, p = 0.13). The incidence of psychotomimetic symptoms was also found to be equivalent (N = 6, RR = 1.86 [0.83–4.14], I 2 = 0 %, p = 0.94). Detailed results of PONV and psychotomimetic manifestations are displayed in Table 2.
3.2 Subgroup Analyses
Subgroup analyses for the primary outcome are displayed in Table 3. These subgroups did not influence results.
3.3 Trial Sequential Analysis
TSA analysis demonstrated a statistically significant effect of ketamine on opioid consumption during the first 24 postoperative hours and introducing correction for the previously published meta-analysis found this result to be still significant (Fig. 4a).
a Trial sequential analysis graph with correction for previous meta-analyses (x axis: studies effect, y axis: cumulative z-scores). The upper full curve represents the actual z-score analysis without correction and the lower dotted curve the corrected z-scores, taking into account previous analyses. Horizontal full grey lines indicate the boundaries of significance (results in the region within these boundaries are nonsignificant). b Trial sequential analysis graph with monitoring boundaries (x axis: studies effect, y axis: cumulative z-scores). The curved full line displays the cumulative z-score, the horizontal full grey lines indicate the boundaries of significance (results in the region within these boundaries are nonsignificant). The vertical dotted line indicates the meta-analysis information size (number of patients to be included in order to show a significant outcome: 498). The dotted curve lines: the upper inward-sloping dotted line represents the trial sequential monitoring boundary and the lower outward-sloping dotted line represents the futility region. Given the evolution of the z-score outside the futility region without crossing the monitoring boundary curve, the opioid-sparing effect of ketamine still requires more data
Given that heterogeneity was taken into account in computing the MD (Biggerstaff and Tweedie’s method [21]), results were different from the ones obtained using the classical approach (MD = −0.04 mg/kg of morphine consumption during the first 24 postoperative hours [−0.06 to −0.01]). Trial sequential monitoring boundaries indicated the possible benefit of including more trials to confirm this result (Fig. 4b). The current meta-analysis was found to be underpowered to confirm the effect of ketamine on opioid consumption when analyzed for informative size using the O’Brien-Fleming approach (number of patients to be included in the meta-analysis in order to reach the desired level of significance, Fig. 4b). In order to reach 80 % power with an adjusted alpha risk of 5 % and with an MD of morphine consumption of 0.03 mg/kg (determined according to actual data) during the first 24 postoperative hours, 445 patients would be required, whereas only 257 patients were analyzed in this meta-analysis for the primary outcome (Fig. 2b).
3.4 Study Bias Analyses
Analyses for bias found a low or undetermined probability of bias across all studies included in this meta-analysis [1]. As a result, no subgroup analyses for bias effect were performed.
3.5 Publication Bias
Given that all outcomes included fewer than 10 studies, publication bias could not be assessed.
4 Discussion
The main finding of this meta-analysis can be summarized as follows: using either the classical or the TSA approach, ketamine did not exhibit an opioid-sparing effect during the first 24 postoperative hours. Even when performing subgroup analyses according to the timing of administration, the surgery performed, or the co-administration of non-opioid analgesics, results remained negative. Ketamine did not increase the incidence of PONV (or in PACU either during the first 24 postoperative hours) or the incidence of psychotomimetic manifestations.
Using the classical approach, and despite the addition of four studies to reach a total of eleven, overall results resemble those previously published in 2010 [9]. Ketamine was shown to be ineffective in decreasing postoperative opioid consumption. From a theoretical point of view, the absence of a morphine-sparing effect may be explained by the pharmacokinetics and pharmacodynamics of this compound in children. Previous studies have found altered ketamine pharmacokinetics in children in comparison with adults. For comparable ketamine infusion rates, the context-sensitive half-time in children (10 kg) was 30 min at 1 h and 55 min at 5 h, whilst in adults the context-sensitive half-time was 23 min at 1 h and 83 min at5 h [33]). Children also appear to be less sensitive to ketamine than adults; published wakening ketamine concentrations in children are 0.9–3.8 mg L−1 and in adults 0.5 mg L−1 [34, 35]). It is also of note that a wide variety of ketamine doses were employed with ranges of 4.7× for boluses (0.15–0.7 mg/kg), 3.1× for intraoperative infusions (0.08–0.25 mg/kg/h), and 12.5× for postoperative ketamine infusions (0.08–1 mg/kg/h). As such, insufficient dosage may also be a factor in negative and clinically irrelevant results.
Another hypothesis was that the opioid-sparing effects of other analgesics such as paracetamol, NSAIDs or dexamethasone could blunt the opioid-sparing effect of ketamine. However, subgroup analyses did not demonstrate any opioid-sparing effect of ketamine either when selecting for major surgery (scoliosis correction) or studies in which no non-opioid analgesics were administered. Finally, the negative overall result for reduction in opioid consumption may purely be due to the lack of power of this meta-analysis [10]. This hypothesis was bolstered by TSA results indicating that the information size has not been reached and an additional 241 patients are required from future trials in order to reach adequate size (445 patients) for 80 % power and an expected MD of 0.04 mg/kg of cumulative morphine reduction over 24 h. As a reminder, the primary outcome of this meta-analysis was computed on 257 patients (Fig. 2b). Consequently, any future trial should consider including at least 188 patients with an expected reduction of morphine consumption of 0.04 mg/kg over the first 24 postoperative hours.
Concerning the PONV outcome, results found that ketamine was not associated with an increased occurrence of this complication. However, this outcome was neither the primary outcome of the current meta-analysis nor the primary outcome of individual trials; consequently, this result must be interpreted cautiously because of the probable lack of power to detect it. In addition, the occurrence of PONV is known to depend upon many other factors such as anesthetics and opioids administered (opioids, N2O), patient characteristics (female) or surgery factors (surgery performed, duration of surgery). Consequently, further studies are also needed to confirm this result and explore potential confounding factors.
From a practical point of view, data are still lacking to form a definitive conclusion about the anti-hyperalgesic and opioid-sparing effect of ketamine in children. In addition, according to the TSA, performing more studies would provide a statistical result consisting of a reduction of morphine consumption by 0.04 mg/kg over 24 h. By comparison in adults, previous meta-analyses have shown ketamine to decrease 24-h postoperative opioid consumption by a mean of 16 mg, or 0.23 mg/kg for a presumed mean weight of 70 kg [36, 37]. Consequently, planning more studies must take into account this expected opioid-sparing effect (0.04 mg/kg decrease in morphine consumption over the first 24 postoperative hours) and the possible increase of ketamine adverse effects (especially PONV).
This meta-analysis suffers some limitations. Only 11 trials were included and only four new trials since the previous meta-analysis was performed in 2010. Consequently, analysis of the primary outcome still exhibits insufficient power to form a conclusion about the primary outcome. In addition, publication bias could not be explored because all outcomes included fewer than 10 studies. Second, results were heterogeneous concerning dose and duration of ketamine administration (postoperative infusion or not). This latter limitation is strongly supported by subgroup analysis results demonstrating more homogenous results when including trials involving intraoperative ketamine administration or postoperative non-opioid agent administration.
In conclusion, this meta-analysis found the absence of a clinically relevant ketamine opioid-sparing effect during the perioperative period. However, the heterogeneous results concerning this endpoint and the evident lack of power of statistics did not allow any conclusion about the primary outcome of this meta-analysis. This study also illustrated the benefit of using TSA as a complement to the classical approach when performing meta-analysis: further studies including at least 188 patients would be informative for a future meta-analysis and might allow a conclusion to be made about the opioid-sparing effect of ketamine in pediatrics.
Notes
http://www.cochrane-handbook.org/ (Section 9.4.6). Last accessed June 2016.
http://www.cochrane-handbook.org/ (Section 9.5.2). Last accessed June 2016.
http://www.cochrane-handbook.org/ (Section 10.4.3.1). Last access June 2016.
http://www.cochrane-handbook.org/ (Section 10.4.3.1). Last access June 2016.
References
Michelet D, Andreu-Gallien J, Bensalah T, Hilly J, Wood C, Nivoche Y, et al. A meta-analysis of the use of nonsteroidal antiinflammatory drugs for pediatric postoperative pain. Anesth Analg. 2012;114(2):393–406.
Taddio A, Katz J, Ilersich AL, Koren G. Effect of neonatal circumcision on pain response during subsequent routine vaccination. Lancet. 1997;349(9052):599–603.
Hohmeister J, Kroll A, Wollgarten-Hadamek I, Zohsel K, Demirakca S, Flor H, et al. Cerebral processing of pain in school-aged children with neonatal nociceptive input: an exploratory fMRI study. Pain. 2010;150(2):257–67.
Lavand’homme P. Perioperative pain. Curr Opin Anaesthesiol. 2006;19(5):556–61.
Elia N, Tramer MR. Ketamine and postoperative pain–a quantitative systematic review of randomised trials. Pain. 2005;113(1–2):61–70.
Ramasubbu C, Gupta A. Pharmacological treatment of opioid-induced hyperalgesia: a review of the evidence. J Pain Palliat Care Pharmacother. 2011;25(3):219–30.
Tawfic QA. A review of the use of ketamine in pain management. J Opioid Manag. 2013;9(5):379–88.
Weinbroum AA. Non-opioid IV adjuvants in the perioperative period: pharmacological and clinical aspects of ketamine and gabapentinoids. Pharmacol Res. 2012;65(4):411–29.
Dahmani S, Michelet D, Abback PS, Wood C, Brasher C, Nivoche Y, et al. Ketamine for perioperative pain management in children: a meta-analysis of published studies. Paediatr Anaesth. 2011;21(6):636–52.
Afshari A, Wetterslev J. When may systematic reviews and meta-analyses be considered reliable? 20141224 DCOM- 20150904(1365-2346 (Electronic)).
Higgins JP, Whitehead A Fau, Simmonds M, Simmonds M. Sequential methods for random-effects meta-analysis. 20110407 DCOM-20110727(1097-0258 (Electronic)).
Cochrane C. Cochrane Handbook. http://www.cochrane-handbook.org. Accessed 30 Aug 2016
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34.
Hannallah RS, Broadman LM, Belman AB, Abramowitz MD, Epstein BS. Comparison of caudal and ilioinguinal/iliohypogastric nerve blocks for control of post-orchiopexy pain in pediatric ambulatory surgery. Anesthesiology. 1987;66(6):832–4.
Debillon T, Zupan V, Ravault N, Magny JF, Dehan M. Development and initial validation of the EDIN scale, a new tool for assessing prolonged pain in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2001;85(1):F36–41.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13.
Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19(22):3127–31.
Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323(7304):101–5.
Sutton AJ, Higgins JP. Recent developments in meta-analysis. Stat Med. 2008;27(5):625–50.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Biggerstaff BJ, Tweedie RL. Incorporating variability in estimates of heterogeneity in the random effects model in meta-analysis. Stat Med. 1997;16(7):753–68.
Dix P, Martindale S, Stoddart PA. Double-blind randomized placebo-controlled trial of the effect of ketamine on postoperative morphine consumption in children following appendicectomy. Paediatr Anaesth. 2003;13(5):422–6.
O’Flaherty JE, Lin CX. Does ketamine or magnesium affect posttonsillectomy pain in children? Paediatr Anaesth. 2003;13(5):413–21.
Tarkkila P, Viitanen H, Mennander S, Annila P. Comparison of remifentanil versus ketamine for paediatric day case adenoidectomy. Acta Anaesthesiol Belg. 2003;54(3):217–22.
Batra YK, Shamsah M, Al-Khasti MJ, Rawdhan HJ, Al-Qattan AR, Belani KG. Intraoperative small-dose ketamine does not reduce pain or analgesic consumption during perioperative opioid analgesia in children after tonsillectomy. Int J Clin Pharmacol Ther. 2007;45(3):155–60.
Engelhardt T, Zaarour C, Naser B, Pehora C, de Ruiter J, Howard A, et al. Intraoperative low-dose ketamine does not prevent a remifentanil-induced increase in morphine requirement after pediatric scoliosis surgery. Anesth Analg. 2008;107(4):1170–5.
Inanoglu K, Ozbakis Akkurt BC, Turhanoglu S, Okuyucu S, Akoglu E. Intravenous ketamine and local bupivacaine infiltration are effective as part of a multimodal regime for reducing post-tonsillectomy pain. Med Sci Monit. 2009;15(10):CR539–43.
Bazin V, Bollot J, Asehnoune K, Roquilly A, Guillaud C, De Windt A, et al. Effects of perioperative intravenous low dose of ketamine on postoperative analgesia in children. Eur J Anaesthesiol. 2010;27(1):47–52.
Elshammaa N, Chidambaran V, Housny W, Thomas J, Zhang X, Michael R. Ketamine as an adjunct to fentanyl improves postoperative analgesia and hastens discharge in children following tonsillectomy—a prospective, double-blinded, randomized study. Paediatr Anaesth. 2011;21(10):1009–14.
Javid MJ, Hajijafari M, Hajipour A, Makarem J, Khazaeipour Z. Evaluation of a low dose ketamine in post tonsillectomy pain relief: a randomized trial comparing intravenous and subcutaneous ketamine in pediatrics. Anesth Pain Med. 2012;2(2):85–9.
Perello M, Artes D, Pascuets C, Esteban E, Ey AM. Prolonged perioperative low-dose ketamine does not improve short and long-term outcomes after pediatric idiopathic scoliosis surgery. Spine (Phila Pa 1976). 2016. (Epub ahead of print)
Pestieau SR, Finkel JC, Junqueira MM, Cheng Y, Lovejoy JF, Wang J, et al. Prolonged perioperative infusion of low-dose ketamine does not alter opioid use after pediatric scoliosis surgery. Paediatr Anaesth. 2014;24(6):582–90.
Dallimore D, Anderson BJ, Short TG, Herd DW. Ketamine anesthesia in children–exploring infusion regimens. Paediatr Anaesth. 2008;18(8):708–14.
Idvall J, Ahlgren I, Aronsen KR, Stenberg P. Ketamine infusions: pharmacokinetics and clinical effects. Br J Anaesth. 1979;51(12):1167–73.
Grant IS, Nimmo WS, McNicol LR, Clements JA. Ketamine disposition in children and adults. Br J Anaesth. 1983;55(11):1107–11.
Wang L, Johnston B, Kaushal A, Cheng D, Zhu F, Martin J. Ketamine added to morphine or hydromorphone patient-controlled analgesia for acute postoperative pain in adults: a systematic review and meta-analysis of randomized trials. Can J Anaesth. 2016;63(3):311–25.
Bell RF, Dahl JB, Moore RA, Kalso E. Perioperative ketamine for acute postoperative pain. Cochrane Database Syst Rev. 2006;1:CD004603.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Daphnée Michelet, Julie Hilly, Alia Skhiri, Rachida Abdat, Thierno Diallo, Christopher Brasher, and Souhayl Dahmani declare no conflict of interest.
Funding
This study was not funded.
Author contributions
D Michelet conceptualized and designed the study, selected articles, evaluated articles, collected data, corrected the manuscript and approved the final manuscript as submitted. J. Hilly selected articles, evaluated articles, collected data, corrected the manuscript and approved the final manuscript as submitted. A. Skhiri selected articles, evaluated articles, collected data, corrected the manuscript and approved the final manuscript as submitted. T. Diallo selected articles, evaluated articles, collected data, corrected the manuscript and approved the final manuscript as submitted. R. Abdat selected articles, evaluated articles, collected data, corrected the manuscript and approved the final manuscript as submitted. C. Brasher verified statistics, corrected the manuscript and approved the final manuscript as submitted. S. Dahmani conceptualized and designed the study, designed the data collection instruments, carried out the initial analyses and verified statistics, drafted the initial manuscript, corrected the manuscript and approved the final manuscript as submitted.
Rights and permissions
About this article
Cite this article
Michelet, D., Hilly, J., Skhiri, A. et al. Opioid-Sparing Effect of Ketamine in Children: A Meta-Analysis and Trial Sequential Analysis of Published Studies. Pediatr Drugs 18, 421–433 (2016). https://doi.org/10.1007/s40272-016-0196-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-016-0196-y