Abstract
Treatment of type 1 diabetes mellitus (T1DM) requires lifelong administration of exogenous insulin. The primary goal of treatment of T1DM in children and adolescents is to maintain near-normoglycemia through intensive insulin therapy, avoid acute complications, and prevent long-term microvascular and macrovascular complications, while facilitating as close to a normal life as possible. Effective insulin therapy must, therefore, be provided on the basis of the needs, preferences, and resources of the individual and the family for optimal management of T1DM. To achieve target glycemic control, the best therapeutic option for patients with T1DM is basal-bolus therapy either with multiple daily injections (MDI) or continuous subcutaneous insulin infusion (CSII). Many formulations of insulin are available to help simulate endogenous insulin secretion as closely as possible in an effort to eliminate the symptoms and complications of hyperglycemia, while minimizing the risk of hypoglycemia secondary to therapy. When using MDI, basal insulin requirements are given as an injection of long- or intermediate-acting insulin analogs, while meal-related glucose excursions are controlled with bolus injections of rapid-acting insulin analogs. Alternatively, CSII can be used, which provides a 24-h preselected but adjustable basal rate of rapid-acting insulin, along with patient-activated mealtime bolus doses, eliminating the need for periodic injections. Both MDI treatment and CSII therapy must be supported by comprehensive education that is appropriate for the individual needs of the patient and family before and after initiation. Current therapies still do not match the endogenous insulin profile of pancreatic β-cells, and all still pose risks of suboptimal control, hypoglycemia, and ketosis in children and adolescents. The safety and success of a prescribed insulin regimen is, therefore, dependent on self-monitoring of blood glucose and/or a continuous glucose monitoring system to avoid critical hypoglycemia and glucose variability. Regardless of the mode of insulin therapy, doses should be adapted on the basis of the daily pattern of blood glucose, through regular review and reassessment, and patient factors such as exercise and pubertal status. New therapy options such as sensor-augmented insulin pump therapy, which integrates CSII with a continuous glucose sensor, along with emerging therapies such as the artificial pancreas, will likely continue to improve safe insulin therapy in the near future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Type 1 diabetes mellitus (T1DM) is characterized by insulin deficiency due to autoimmune destruction of pancreatic β-cells. In most western countries, T1DM accounts for over 90 % of childhood and adolescent diabetes [1], and in the USA, Europe, and Australia, its incidence has been increasing by 2–5 % over the past two decades [2–5].
Treatment of T1DM requires lifelong administration of exogenous insulin, along with monitoring of blood glucose levels (BGL) to prevent acute and late complications of diabetes. The Diabetes Control and Complications Trial and its follow-up Epidemiology of Diabetes Interventions and Complications Study demonstrated conclusively that intensive blood glucose control reduces and delays the progression of diabetes complications [6–8]. Thus, optimal glycemic control is the cornerstone of diabetes care. Unfortunately, almost a century after the discovery of insulin, the most common cause of death in a child with diabetes, from a global perspective, is lack of access to insulin [9].
The challenge in treating children and adolescents with T1DM is safely achieving and maintaining tight metabolic control as early as possible after disease onset, while supporting growth and development (Table 1). This update aims to provide an overview of insulin therapy in youth with T1DM in healthcare settings with access to current insulin therapy options, with an emphasis on current-day management and practical considerations for the providers of care to these children.
2 Insulin Formulations in Clinical Use
Since the discovery of insulin by Banting et al. in 1921 [10], insulin therapy has evolved considerably. Human insulin is now biosynthetically produced through recombinant techniques, and the availability of highly purified human insulin preparations has markedly reduced immunogenicity, virtually eliminating therapeutic complications such as insulin allergy, immune insulin resistance, and localized lipoatrophy at the injection site [11].
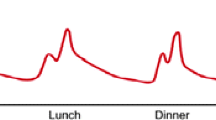
The aim of insulin therapy in children and adolescents with T1DM is to replicate the insulin secretion of a normally functioning pancreas. To achieve glucose homeostasis, the pancreas releases both (1) continuous, background insulin to help maintain euglycemia in the fasting state (basal insulin); and (2) short bursts of insulin in response to plasma glucose levels rising above 80–100 mg/dL (bolus insulin), such as those occurring shortly after a meal [12]. Thus, an insulin replacement regimen for T1DM consists of both basal and bolus insulin components, along with extra insulin required to lower serum glucose into the target range when hyperglycemia occurs (Fig. 1).
Current therapies, however, still do not match the endogenous insulin profile of pancreatic β-cells, and all still pose risks of suboptimal control, hypoglycemia, and ketosis [13]. Each insulin formulation differs with regard to the time of onset and duration of action (Table 2).
2.1 Short-Acting Insulin Preparations
Regular insulin is a soluble crystalline zinc insulin, which has historically been used as bolus insulin to approximate the response of endogenous insulin. However, regular insulin has delayed absorption because of its tendency to self-aggregate into large hexamers, resulting in an onset of action 30 min after administration, a late peak effect at close to 2–4 h, and a longer duration of action (5–8 h) [14]. While its use in daily insulin regimens has diminished in youth with T1DM, regular insulin is well suited to intravenous insulin therapy; thus, it plays an important role in the management of diabetic ketoacidosis (DKA) or during perioperative management of patients with T1DM [15].
Rapid-acting insulin analogs differ from regular insulin in that they dissociate quickly into monomers because of a structurally altered β-insulin chain that impedes self-aggregation. As a result, they are absorbed more rapidly, with an onset of action 10–15 min after subcutaneous injection and peak physiologic effects between 90 and 100 min after the bolus dose, and they have a shorter duration of action (3–5 h) than regular human insulin [16, 17]. Currently, three rapid-acting insulin analogs (aspart, lispro, and glulisine) are commercially available for children, all of which have similar pharmacokinetic and pharmacodynamic properties [18, 19]. Each has a more consistent duration of action than regular insulin, and all have been shown to have less variability in absorption at the injection site [20]. Rapid-acting insulin analogs are now widely used in mealtime insulin replacement and are the insulins used in insulin pump therapy.
2.2 Long-Acting Insulin Preparations
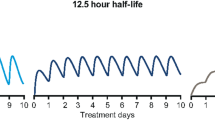
Neutral protamine Hagedorn (NPH) consists of a protaminated form of recombinant regular insulin in a neutral pH solution. It was introduced in the 1940s in an attempt to prolong the biological action of regular insulin preparations, to help reduce insulin dosing frequency, and to mimic basal release of insulin from the pancreas [21]. NPH, however, has an onset of action at 2–4 h and reaches a pronounced peak between 4 and 8 h. Its pharmacologic effect subsequently falls off rapidly for a duration of action that is less than 24 h (usually 12–18 h in clinical practice), making it most suitable for a twice-daily regimen [22]. A notable drawback of NPH is that both inter- and intra-individual variability are high [23].
Insulin glargine was the first long-acting analog developed to better approximate a basal insulin profile. Glargine differs from human insulin by three amino acids, resulting in a molecule that is less soluble in neutral pH at the injection site. After administration, it forms a precipitate that acts as a depot from which insulin is slowly released into the circulation [24, 25]. Unlike NPH, glargine is without any pronounced peaks and is generally given once daily to provide basal coverage [26, 27].
Insulin detemir is the other long-acting insulin analog in common clinical use for basal insulin replacement. Its modifications cause it to self-associate into hexamers and reversibly bind to albumin, which delays its absorption and gives it both a flat and protracted pharmacodynamic profile and lower within-subject variability than glargine [28, 29]. An important consideration is that lower doses result in a shorter duration of action; thus, twice-daily injections are usually employed, especially in children and adolescents with T1DM, to allow for complete 24-h basal insulin coverage [30].
Insulin degludec is an ultra-long-acting insulin analog which, after subcutaneous injection, forms long chains of multi-hexamers, resulting in a soluble depot in the subcutaneous tissue [31]. The gradual separation of insulin degludec monomers from the multihexamers leads to prolonged, stable release of insulin degludec from the subcutaneous depot, with a glucose-lowering profile that is ultra-long, flat, and without apparent peaks [32]. Insulin degludec has been shown to have a duration of action >42 h and four times less within-subject variability in its glucose-lowering effect than insulin glargine [33]. Thus, it has the potential to broaden the options for current diabetes treatment, with a flexible three-times-weekly dosing regimen that can be administered at any time of the day, but it is currently only approved in the European Union and Japan.
3 Initiation of Insulin Therapy
Insulin doses must be individualized and may vary by time of day, by relationship to meals, and by age. The metabolic derangements seen at the time of diagnosis of T1DM are accompanied by a significant deterioration in insulin sensitivity. Thus, when BGL normalize, the required insulin doses may rapidly decline, reflecting improved insulin sensitivity and, perhaps, early recovery of endogenous insulin secretion [34]. Early normalization or near-normalization of BGL with intensive insulin therapy after a new diagnosis of T1DM may be associated with improved long-term glycemic control and higher endogenous insulin production 1 year after diagnosis [35].
3.1 Clinical and Patient Factors
Insulin should be commenced shortly after diagnosis. If ketosis is present, insulin should be initiated within 6 h to prevent DKA. The prevalence of DKA at the time of diagnosis of T1DM in children is high, especially in very young children [36, 37]. It is well recognized by most clinicians that insulin sensitivity is lower in the immediate aftermath of DKA; thus, starting insulin doses may need to be higher than those in children presenting without DKA [38].
In practice, total insulin doses of 0.5–0.75 U/kg/day are typically chosen at T1DM onset, and the dose is then adjusted on a daily basis to achieve target glycemia [9]. The obese [39] or pubertal child typically requires more insulin, as both conditions exacerbate insulin resistance (see Sect. 4.5). Younger children, on the other hand, require reduced amounts of insulin because of lower body weight, higher insulin sensitivity, and the risk of hypoglycemia. In the very young child, total daily doses (TDD) may be ≤0.5 U/kg/day, and if carbohydrate intake is low, diluted rapid acting insulin analogs may even be required. For example, it is not uncommon at the authors’ institution to use U10 insulin (10 U/mL) instead of the usual U100 for rapid-acting insulin in the very young to facilitate more accurate mealtime dosing. This is not required when pump therapy is utilized; however, an insulin pump is rarely commenced at the time of insulin initiation.
3.2 Basal Insulin
Basal insulin is started at the time of diagnosis of diabetes or at the time of transition from an intravenous insulin infusion, if this has been necessary for resolution of DKA. At the authors’ institution, since the tissue half-life of insulin is longer than that of intravenous insulin, the first dose of subcutaneous basal insulin is given 30 min prior to stopping the intravenous insulin infusion.
In addition to adjusting the dose on the basis of the patient and clinical factors delineated above, the initial basal insulin dose depends upon which long-acting insulin preparation is chosen. While the use of intermediate insulins such as NPH persists in clinical practice, basal-bolus regimens that utilize long-acting analogs are increasingly preferred. Glargine is generally given once daily in the evening or at bedtime but can be given in the morning before breakfast, where it may be associated with lower rates of nocturnal hypoglycemia [34]. If subsequent monitoring suggests a duration of action in the individual patient of less than 24 h, it may be given twice daily [40]. Detemir is typically given twice daily because it has a shorter duration of action than glargine.
With modern rapid-acting analogs now in widespread use for multiple daily injections (MDI), the proportion of the total daily dose that is given as a long-acting basal analog is typically 40–50 % (in some individuals, this may be as much as 60 % if carbohydrate intake is low) [15]. If regular insulin is chosen as the mealtime insulin rather than a rapid-acting analog, then the basal dose as a proportion of the TDD will be lower, as regular short-acting insulin has a longer duration of action; thus, it may provide some basal coverage. NPH insulin may be chosen where basal analogs are not available and will require twice-daily dosing when combined with rapid-acting analogs. In most children, this option is not preferred.
3.3 Bolus Insulin
Initiation of bolus insulin is determined by practical considerations and generally coincides with the introduction of basal insulin. A majority of children are now prescribed one of the three available rapid-acting analogs, which are given multiple times per day to coincide with meals, significant snacks, and the need for additional doses to correct hyperglycemia between meals. Their advantages, as detailed previously, include a quicker onset of action and shorter duration, with a resultant reduced risk of insulin “stacking” (overlap of two consecutive rapid-acting doses) and, thus, hypoglycemia. If glargine or detemir is chosen as the basal insulin, the proportion of the TDD given as rapid-acting insulin is generally 50–60 %.
The bolus insulin dose is based on the intended carbohydrate intake, with a set ratio of insulin to carbohydrates utilized (or insulin per “exchanges” where one exchange is the equivalent of 15 g of carbohydrate). This insulin:carbohydrate ratio is individualized but, in general, it can be calculated using the TDD and the “rule of 500” as a starting point, with the resulting number being equivalent to the approximate ratio required to adequately maintain target blood glucose despite a given carbohydrate load (Table 3). Insulin:carbohydrate ratios may vary by time of day. For example, insulin dosing early in the day may need to be more aggressive than that used at lunch and/or dinner, because of the diurnal variation in insulin sensitivity.
In general, only the carbohydrate content of a given meal is considered when calculating the prandial bolus insulin dose. However, there are limitations to this approach, as meals high in protein and fat have been shown to increase glucose excursions in youth using intensive insulin therapy 3–5 h post-meal and have an additive impact on the delayed postprandial glycemic rise [41]. Continuous subcutaneous insulin infusion (CSII) (see Sect. 4.2) may be one option to tailor prandial insulin delivery to the composition of a meal and anticipated glycemic effects. The dual-wave bolus option, for example, is a tool implemented in insulin pumps, which delivers a combination of an instant standard pre-meal insulin bolus followed by a square bolus infused over several hours to extend bolus insulin delivery for a given meal [42]. Formulas for increasing meal-time insulin doses and bolus calculators that include the fat and protein content of the food have yet to be validated, and further research is required.
In addition, an initial “correction factor” to lower the glucose level into the target range is implemented and adjusted as required. If a patient is found to be hyperglycemic on the basis of the pre-meal blood glucose level, the “correction factor” dose is usually added to the rapid-acting insulin being given to cover carbohydrates consumed. Where glucose is measured in milligrams per deciliter, the “rule of 1,800” (or the “rule of 100” for glucose levels measured in millimoles per liter) may be used to calculate a reasonable initial correction dose of rapid-acting insulin.
3.4 Diabetes Education
At the time of diagnosis, the child or adolescent (depending upon age, maturity, and social/family circumstances) and the patient’s family must be taught diabetes self-management skills. A structured program has been shown to be superior to unstructured new-onset diabetes teaching and will initially include survival skills such as the pathogenesis of diabetes, types of insulin used, insulin dose calculation, and how to detect, treat, and prevent both ketosis and hypoglycemia [9].
The diabetes education program may be based in the inpatient setting once the patient is medically stable or in an ambulatory setting, if appropriate facilities exist, as several studies have suggested that outpatient-based education is equally effective and cheaper than inpatient-based education [43]. For patients receiving education in the inpatient setting, it may be reasonable to provide somewhat more aggressive insulin dosing than in those who are being taught new-onset survival skills on an outpatient basis. When an ambulatory setting is chosen for education, an initial total daily dose of 0.3–0.5 U/kg/day has been shown to be safe and effective, since some of the insulin administration and glucose monitoring will be performed at home by the patient and/or the family [44].
4 Maintenance Insulin Therapy
4.1 Self-Monitoring of Blood Glucose
The goal of maintenance insulin therapy is to eliminate the symptoms and complications of hyperglycemia while minimizing the risk of hypoglycemia secondary to therapy. Thus, the safety and success of a prescribed insulin regimen is dependent on some form of self-monitoring of blood glucose (SMBG).
The frequency of SMBG is individualized depending on the type of insulin regimen, the ability of the child to identify hypoglycemia, and the available technology. Measuring BGL at different times of the day allows for better insulin adjustment for food consumption, correction of out-of-target glucose values, unnoticed overnight hypoglycemia and hyperglycemia, and changes with physical activity [45]. Modern glucometers are inexpensive, accurate, and easy-to-use, and children and adolescents are recommended to check BGL four or more times daily [46]. Frequent testing has been repeatedly associated with improved glycated hemoglobin (HbA1c) levels in both youth and adults with T1DM [47–49].
Even when performed correctly, multiple BGL checks only provide information about a single point in time and do not sufficiently capture the wide fluctuations in blood glucose that occur during a 24-h period in many patients with diabetes. Continuous glucose monitoring (CGM) systems, which measure interstitial fluid glucose semi-continuously, have the potential to provide information on trends in the decline or rise of BGL that would not be identified in conventional monitoring; thus, they are being increasingly utilized in the overnight setting to avoid critical hypoglycemia [50, 51].
A recent Cochrane review demonstrated that CGM combined with MDI lowered HbA1c levels by 0.2 %, while those patients on insulin pump therapy augmented by CGM had, on average, a 0.7 % improvement in HbA1c levels across all age groups, compared with patients using MDI and SMBG [52]. CGM is offered as an adjunct to SMBG to all children and adolescents who would benefit on the basis of the available evidence, particularly those with T1DM who have hypoglycemia unawareness, experience frequent hypoglycemia, and/or are highly motivated to improve their glycemic control. The disadvantages of CGM include the lag time between the blood glucose value and the interstitial glucose value, and the need for routine calibration; thus, patients must confirm the sensor glucose measurement with a fingerstick measurement before making any treatment decisions.
4.2 Insulin Pump Therapy
As insulin pump devices have become more reliable, smaller, and safer, insulin pump treatment is of increasing interest in children and adolescents with T1DM as an alternative to MDI. By delivering rapid-acting insulin continuously as an infusion into the subcutaneous tissue throughout the day, using a small self-inserted catheter, insulin pump therapy replaces the need for periodic injections. Pump devices mimic physiologic insulin release by administration of a 24-hour preselected but adjustable basal rate, along with patient-activated mealtime bolus doses [53]. Potential adverse events that may arise include a pump malfunction, which could lead to DKA because of insufficient insulin delivery, and catheter-site infection or discomfort [54].
CSII represents an important advancement in diabetes technology to help children with type 1 diabetes achieve near-normal BGL. Pump therapy is commonly used in preschool children—particularly in infants and toddlers because of their frequent snacking patterns and the ability to deliver small amounts of insulin—and in children who suffer from needle fear, by helping to reduce the number of “needle sticks” [55]. In addition, insulin pumps have the capability to deliver varying basal rates at different times of the day. The ability to establish a basal profile tailored to a child or adolescent is particularly helpful in those children who are prone to frequent hypoglycemia, as well as in managing children with early-morning hyperglycemia due to a pronounced dawn phenomenon secondary to increased tissue insulin resistance [56]. Further, the additional flexibility of modern insulin pumps allows for easier adaptation of insulin dosing for current physiologic needs (e.g., cessation of insulin delivery during exercise or reduced temporary basal rates); thus, pumps may be preferred for athletes. Compared with MDI, CSII has been found to yield better satisfaction with overall quality of life in children with T1DM [57].
Recent meta-analyses have demonstrated that, compared with multiple daily insulin injections, the use of insulin pump therapy reduces the HbA1c level without an increase in hypoglycemia [58, 59], though the data are inconsistent in younger children [57, 60]. At the authors’ institution, we offer insulin pump therapy to any interested patient/family where adequate self-monitoring of BGL, competent carbohydrate-counting skills, and reliable communication with the medical team regarding issues with frequent hypoglycemia or significant hyperglycemia have all been demonstrated. There is no age-related limit to pump use. Children who are experiencing suboptimal diabetes control with MDI or microvascular complications and/or have risk factors for macrovascular complications are strongly considered [54]. Like MDI treatment, CSII therapy must be supported by comprehensive education appropriate for the individual needs of the patient and family before and after initiation.
Of additional promise is the development of sensor-augmented insulin pump therapy, which integrates CSII with a continuous glucose sensor. The CGM sensor gives the patient predictive alerts of impending hyper- or hypoglycemia, based on the trending glucose levels. This information can then be used by the patient (or the insulin pump itself) to adjust the rate of insulin delivery or to temporarily suspend it altogether [51, 61]. The efficacy and clinical utility of this technology have been established in the STAR 3 study, in which better glycemic control was achieved safely in CSII therapy, compared with MDI [62].
4.3 Hypoglycemia
Several studies have shown that the fear of hypoglycemia, and especially the fear of nocturnal hypoglycemia, is the most prominent barrier to target glycemic control. This may be patient or parental fear, or both [63]. Severe hypoglycemia can cause seizures or a significantly altered mental status, while mild hypoglycemia is associated with altered cognitive function with respect to learning and attention. One episode of hypoglycemia is associated with recurrent near-term hypoglycemia due to changes in the glucose threshold required to trigger an autonomic response [64].
Exercise is strongly associated with hypoglycemia in children with T1DM, both during the event itself and afterward. Indeed, nocturnal hypoglycemia is particularly common after exercise, in part because of the greater insulin sensitivity after exercise [65] and also because sleep itself is a state of impaired hormonal counterregulation in the presence of hypoglycemia [66]. Other risk factors for hypoglycemia include younger age, a longer duration of diabetes, and a lower HbA1c level [67], which must be considered when managing the diabetic child or adolescent with insulin.
Modern insulin analogs display distinct advantages, compared with their older-generation counterparts, when considering the risk of hypoglycemia. While the evidence is mixed regarding improvement in overall glycemic control with the use of long-acting basal analogs [15], rates of hypoglycemia are routinely lower, and this is perhaps their most significant advantage over older regimens using NPH, premixed insulin, or a combination thereof. This may be particularly true of nocturnal hypoglycemia, given the relative absence of a “peak” in the middle of the night and less day-to-day variability when compared with NPH. Rapid-acting analogs also appear to mostly improve rates of hypoglycemia rather than overall control as defined by the HbA1c level [68]. Similarly, pump therapy is associated with lower rates of hypoglycemia when compared with MDI regimens using NPH [69, 70] and glargine [71] as the long-acting insulin.
4.4 Exercise
While physical activity and exercise are cornerstones of the management of type 1 diabetes, the management of insulin dosing around exercise deserves special consideration [72]. In particular, exercise increases the risk of hypoglycemia during and after exercise, in part because of increased insulin sensitivity and insulin-dependent glucose disposal [73]. Those without diabetes are able to compensate for this by decreasing insulin secretion and increasing counterregulatory hormone responses, which together increase hepatic glucose output to balance skeletal muscle uptake. However, in T1DM, insulin is not tied to physical activity or glucose levels; thus, mindful and manual adjustment to delivered insulin is necessary to attenuate the risk of hypoglycemia. This may be best achieved with the flexibility of insulin pump therapy.
While the glycemic effects vary by type and intensity of exercise, it has been demonstrated that suspending basal insulin during exercise decreases the rate of hypoglycemia [74]. In addition, it has recently been shown that following afternoon exercise, a further 20 % reduction in the basal insulin rate between 9 p.m. and 3 a.m. is safe and effective in reducing the rate of nocturnal hypoglycemia [75]. It may be that in some patients and some situations, simple avoidance of hypoglycemia during activity by suspending insulin delivery is effective also in preventing delayed hypoglycemia because there is less depletion of counterregulatory responses.
The modern rapid-acting insulins all have a similar onset and peak action [76]; thus, a bolus of these insulins should ideally be avoided in the 60–90 min prior to exercise to prevent the peak effect of the bolus coinciding with the hypoglycemic stimulus of exercise itself. For this reason, it is recommended that the pre-exercise meal be consumed 3–4 h prior to exercise to facilitate appropriate insulin dosing and time for glucose uptake and energy storage. Where this is impractical, or the exercise is likely to be particularly prolonged or intense, a reduction in the pre-exercise meal bolus dose by as much as 75 % may be effective [77]. It is important, though, that a reduction in the post-exercise bolus is also considered along with adjustments to basal insulin delivery. If a meal is consumed in the early post-exercise period, a 50 % reduction may be safest but is still associated with delayed nocturnal hypoglycemia without a basal insulin reduction.
4.5 Puberty
Puberty is a time of physiologic insulin resistance. Insulin resistance increases at pubertal onset, peaks at mid-puberty in both boys and girls (Tanner stage 3), and generally returns to near pre-pubertal levels at the completion of puberty [78]. In longitudinal studies, insulin sensitivity has been shown to fall by about one third by mid-puberty, when compared with the pre-pubertal state [79]. It is, therefore, not surprising that in youth with type 1 diabetes, higher insulin doses are required to maintain target glycemic control, with doses as much as 1.5 U/kg/day being required, or more. While the aforementioned pubertal increases in insulin resistance drive some of the absolute increases in the insulin dose, some may also be driven by increased appetite, more frequent snacking, and larger meal sizes. One study, however, found that on a per kilogram basis, the amount of bolus insulin delivered on a daily basis in adolescents is no different than that in younger children [80].
5 Emerging Therapies
Optimal glycemic control through intensive insulin therapy and modern insulin delivery methods may come at the expense of hypoglycemia, indicating the need for improved treatments to achieve target glycemic control more safely [64]. To address this, new insulin analogs with improved safety profiles, such as insulin degludec, are being investigated [81, 82].
The most promising therapy for T1DM, however, is the closed-loop artificial pancreas, which mimics the physiology of the pancreas by coupling automated delivery of insulin with continuous glucose measurements to achieve target BGL under the control of a computer algorithm [83]. Early proof-of-concept studies have shown that closed-loop systems, with minimal patient involvement, can provide adequate glycemic control in short-term, inpatient, clinical research center studies [84, 85]. A recent trial in a small group of critically ill adult patients randomized to receive fully automated closed-loop therapy for 48 h saw a fourfold increase in time spent in the target glucose range and reduced time spent at higher glucose levels, suggesting that fully automated closed-loop control based on subcutaneous glucose measurements is feasible and has the potential to be more efficacious [86].
6 Conclusion
The primary goal of treatment of T1DM in children and adolescents is to maintain near-normoglycemia through intensive insulin therapy, avoid acute complications, and prevent long-term microvascular and macrovascular complications, while facilitating as close to a normal life as possible. Effective insulin therapy must, therefore, be available for optimal management of T1DM.
The choice of insulin regimen should consider the needs, preferences, and resources of the individual and the family. Treatment innovations—including MDI regimens, new and more physiologic insulin analogs, insulin delivery through CSII, and sophisticated blood glucose monitoring—have all contributed to improvement in the management of T1DM. Emerging therapies and, in particular, the artificial pancreas, will likely continue to improve safe insulin therapy in the near future.
References
Craig ME, Hattersley A, Donaghue KC. Definition, epidemiology and classification of diabetes in children and adolescents. Pediatr Diabetes. 2009;10(Suppl 12):3–12.
Liese AD, D’Agostino RB, Hamman RF, et al. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118(4):1510.
The DIAMOND Project Group. Incidence and trends of childhood type 1 diabetes worldwide 1990–1999. Diabetic Med. 2006;23(8):857–66.
Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G, Group TES. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027–33.
Taplin CE, Craig ME, Lloyd M, et al. The rising incidence of childhood type 1 diabetes in New South Wales, 1990–2002. Med J Aust. 2005;183(5):243–6.
Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. J Pediatr. 1994;125(2): 177–188
The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86.
White NH, Cleary PA, Dahms W, et al. Beneficial effects of intensive therapy of diabetes during adolescence: outcomes after the conclusion of the Diabetes Control and Complications Trial (DCCT). J Pediatr. 2001;139(6):804–12.
International Society for Pediatric and Adolescent Diabetes. 2011 global IDF/ISPAD guideline for diabetes in childhood and adolescence [Internet]. ISPAD.org. 2011 [cited 2012 Jan 9]. 1–132. Available from: https://www.ispad.org/sites/default/files/idf-ispad_diabetes_in_childhood_and_adolescence_guidelines_2011.pdf.
Banting FG, Best CH, Collip JB, et al. Pancreatic extracts in the treatment of diabetes mellitus. Can Med Assoc J. 1922;12:141–6.
Hirsch IB. Insulin analogues. N Engl J Med. 2005;352(2):174–83.
Ferrannini E. Physiology of glucose homeostasis and insulin therapy in type 1 and type 2 diabetes. Endocrinol Metab Clin N Am. 2012;41(1):25–39.
Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group, Nathan DM, Zinman B, et al. Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications and Pittsburgh Epidemiology of Diabetes Complications experience (1983–2005). Arch Inter Med. 2009;169(14):1307–16.
Heinemann L, Richter B. Clinical pharmacology of human insulin. Diabetes Care. 1993;16(Supplement 3):90–100.
Bangstad H-J, Danne T, Deeb L, Jarosz-Chobot P, Urakami T, Hanas R. Insulin treatment in children and adolescents with diabetes. Pediatr Diabetes. 2009;10:82–99.
Howey DC, Bowsher RR, Brunelle RL, Woodworth JR. [Lys(B28), Pro(B29)]-human insulin: a rapidly absorbed analogue of human insulin. Diabetes. 1994;43(3):396–402.
Mudaliar SR, Lindberg FA, Joyce M, et al. Insulin aspart (B28 asp-insulin): a fast-acting analog of human insulin. Absorption kinetics and action profile compared with regular human insulin in healthy nondiabetic subjects. Diabetes Care. 1999;22(9):1501–6.
Homko C, Deluzio A, Jimenez C, Kolaczynski JW, Boden G. Comparison of insulin aspart and lispro pharmacokinetic and metabolic effects. Diabetes Care. 2003;26(7):2027–31.
Danne T, Aman J, Schober E, et al. A comparison of postprandial and preprandial administration of insulin aspart in children and adolescents with type 1 diabetes. Diabetes Care. 2003;26(8):2359–64.
Miles HL, Acerini CL. Insulin analog preparations and their use in children and adolescents with type 1 diabetes mellitus. Paediatr Drugs. 2008;10(3):163–76.
Borgoño CA, Zinman B. Insulins: past, present, and future. Endocrinol Metab Clin N Am. 2012;41(1):1–24.
Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000;23(5):644–9.
Jehle PM, Micheler C, Jehle DR, Breitig D, Boehm BO. Inadequate suspension of neutral protamine Hagendorn (NPH) insulin in pens. Lancet. 1999;354(9190):1604–7.
Bolli GB, Di Marchi RD, Park GD, Pramming S, Koivisto VA. Insulin analogues and their potential in the management of diabetes mellitus. Diabetologia. 1999;42(10):1151–67.
Bolli GB, Owens DR. Insulin glargine. Lancet. 2000;356(9228):443–5.
Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49(12):2142–8.
Owens DR, Coates PA, Luzio SD, Tinbergen JP, Kurzhals R. Pharmacokinetics of 125I-labeled insulin glargine (HOE 901) in healthy men: comparison with NPH insulin and the influence of different subcutaneous injection sites. Diabetes Care. 2000;23(6):813–9.
Plank J, Bodenlenz M, Sinner F, et al. A double-blind, randomized, dose-response study investigating the pharmacodynamic and pharmacokinetic properties of the long-acting insulin analog detemir. Diabetes Care. 2005;28(5):1107–12.
Danne T, Datz N, Endahl L, et al. Insulin detemir is characterized by a more reproducible pharmacokinetic profile than insulin glargine in children and adolescents with type 1 diabetes: results from a randomized, double-blind, controlled trial. Pediatr Diabetes. 2008;9(6):554–60.
Robertson KJ, Schoenle E, Gucev Z, Mordhorst L, Gall M-A, Ludvigsson J. Insulin detemir compared with NPH insulin in children and adolescents with type 1 diabetes. Diabet Med. 2007;24(1):27–34.
Jonassen I, Havelund S, Hoeg-Jensen T, Steensgaard DB, Wahlund P-O, Ribel U. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res. 2012;29(8):2104–14.
Heise T, Nosek L, Bøttcher SG, Hastrup H, Haahr H. Ultra-long-acting insulin degludec has a flat and stable glucose-lowering effect in type 2 diabetes. Diabetes Obes Metab. 2012;14(10):944–50.
Heise T, Hermanski L, Nosek L, Feldman A, Rasmussen S, Haahr H. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes Metab. 2012;14(9):859–64.
Thisted H, Johnsen SP, Rungby J. An update on the long-acting insulin analogue glargine. Basic Clin Pharmacol Toxicol. 2006;99(1):1–11.
Shah SC, Malone J, Simpson NE. A randomized trial of intensive insulin therapy in newly diagnosed insulin-dependent diabetes mellitus. N Engl J Med. 1989;320(9):550–4.
Lévy-Marchal C, Patterson CC, Green A, EURODIAB ACE Study Group, Europe and Diabetes. Geographical variation of presentation at diagnosis of type I diabetes in children: the EURODIAB study. European and Dibetes. Diabetologia. 2001;44(Suppl 3):B75–80.
Rewers A, Klingensmith G, Davis C, et al. Presence of diabetic ketoacidosis at diagnosis of diabetes mellitus in youth: the Search for Diabetes in Youth Study. Pediatrics. 2008;121(5):e1258–66.
Wolfsdorf J, Craig ME, Daneman D, et al. Diabetic ketoacidosis in children and adolescents with diabetes. Pediatr Diabetes. 2009;10:118–33.
Liu LL, Lawrence JM, Davis C, et al. Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth Study. Pediatr Diabetes. 2010;11(1):4–11.
Hamann A, Matthaei S, Rosak C, Silvestre L. A randomized clinical trial comparing breakfast, dinner, or bedtime administration of insulin glargine in patients with type 1 diabetes. Diabetes Care. 2003;26(6):1738–44.
Smart CEM, Evans M, O’Connell SM, et al. Both dietary protein and fat increase postprandial glucose excursions in children with type 1 diabetes, and the effect is additive. Diabetes Care. 2013;36(12):3897–902.
Pańkowska E, Szypowska A, Lipka M, Szpotańska M, Błazik M, Groele L. Application of novel dual wave meal bolus and its impact on glycated hemoglobin A1c level in children with type 1 diabetes. Pediatr Diabetes. 2009;10(5):298–303.
Jasinski CF, Rodriguez-Monguio R, Tonyushkina K, Allen H. Healthcare cost of type 1 diabetes mellitus in new-onset children in a hospital compared to an outpatient setting. BMC Pediatr. 2013;13:55.
Srinivasan S, Craig ME, Beeney L, et al. An ambulatory stabilisation program for children with newly diagnosed type 1 diabetes. Med J Aust. 2004;180(6):277–80.
Rewers M, Pihoker C, Donaghue K, Hanas R, Swift P, Klingensmith GJ. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr Diabetes. 2009;10(Suppl 12):71–81.
Pihoker C, Badaru A, Anderson A, et al. Insulin regimens and clinical outcomes in a type 1 diabetes cohort: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2013;36(1):27–33.
Evans JM, Newton RW, Ruta DA, MacDonald TM, Stevenson RJ, Morris AD. Frequency of blood glucose monitoring in relation to glycaemic control: observational study with diabetes database. BMJ. 1999;319(7202):83–6.
Karter AJ, Ackerson LM, Darbinian JA, et al. Self-monitoring of blood glucose levels and glycemic control: the Northern California Kaiser Permanente Diabetes Registry. Am J Med. 2001;111(1):1–9.
Haller MJ, Stalvey MS, Silverstein JH. Predictors of control of diabetes: monitoring may be the key. J Pediatr. 2004;144(5):660–1.
Buckingham B, Chase HP, Dassau E, et al. Prevention of nocturnal hypoglycemia using predictive alarm algorithms and insulin pump suspension. Diabetes Care. 2010;33(5):1013–7.
Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369(3):224–32.
Langendam M, Luijf YM, Hooft L, DeVries JH, Mudde AH, Scholten RJPM. Continuous glucose monitoring systems for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2012;(1):CD008101.
Pickup J, Keen H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care. 2002;25(3):593–8.
Phillip M, Battelino T, Rodriguez H, et al. Use of insulin pump therapy in the pediatric age-group: consensus statement from the European Society for Paediatric Endocrinology, the Lawson Wilkins Pediatric Endocrine Society, and the International Society for Pediatric and Adolescent Diabetes, endorsed by the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2007;30(6):1653–62.
Shalitin S, Phillip M. The use of insulin pump therapy in the pediatric age group. Horm Res. 2008;70(1):14–21.
Edge JA, Matthews DR, Dunger DB. The dawn phenomenon is related to overnight growth hormone release in adolescent diabetics. Clin Endocrinol (Oxf). 1990;33(6):729–37.
Yeh H-C, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med. 2012;157(5):336–47.
Pańkowska E, Błazik M, Dziechciarz P, Szypowska A, Szajewska H. Continuous subcutaneous insulin infusion vs. multiple daily injections in children with type 1 diabetes: a systematic review and meta-analysis of randomized control trials. Pediatr Diabetes. 2009;10(1):52–8.
Misso ML, Egberts KJ, Page M, O’Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2010;(1):CD005103.
Monami M, Lamanna C, Marchionni N, Mannucci E. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in type 1 diabetes: a meta-analysis. Acta Diabetol. 2010;47(Suppl 1):77–81.
Davis SN, Horton ES, Battelino T, Rubin RR, Schulman KA, Tamborlane WV. STAR 3 randomized controlled trial to compare sensor-augmented insulin pump therapy with multiple daily injections in the treatment of type 1 diabetes: research design, methods, and baseline characteristics of enrolled subjects. Diabetes Technol Ther. 2010;12(4):249–55.
Slover RH, Welsh JB, Criego A, et al. Effectiveness of sensor-augmented pump therapy in children and adolescents with type 1 diabetes in the STAR 3 study. Pediatr Diabetes. 2012;13(1):6–11.
Gonder-Frederick L, Nyer M, Shepard JA, Vajda K, Clarke W. Assessing fear of hypoglycemia in children with type 1 diabetes and their parents. Diabetes Manag (Lond). 2011;1(6):627–39.
Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57(12):3169–76.
Tsalikian E, Mauras N, Beck RW, et al. Impact of exercise on overnight glycemic control in children with type 1 diabetes mellitus. J Pediatr. 2005;147(4):528–34.
Jones TW, Porter P, Sherwin RS, et al. Decreased epinephrine responses to hypoglycemia during sleep. N Engl J Med. 1998;338(23):1657–62.
Clarke W, Jones T, Rewers A, Dunger D, Klingensmith GJ. Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes. 2009;10(Suppl 12):134–45.
Siebenhofer A, Plank J, Berghold A, et al. Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus. Cochrane Database Syst Rev. 2006;(2):CD003287.
DiMeglio LA, Pottorff TM, Boyd SR, France L, Fineberg N, Eugster EA. A randomized, controlled study of insulin pump therapy in diabetic preschoolers. J Pediatr. 2004;145(3):380–4.
Pickup J, Mattock M, Kerry S. Glycaemic control with continuous subcutaneous insulin infusion compared with intensive insulin injections in patients with type 1 diabetes: meta-analysis of randomised controlled trials. BMJ. 2002;324(7339):705.
Doyle EA, Weinzimer SA, Steffen AT, Ahern JAH, Vincent M, Tamborlane WV. A randomized, prospective trial comparing the efficacy of continuous subcutaneous insulin infusion with multiple daily injections using insulin glargine. Diabetes Care. 2004;27(7):1554–8.
Robertson K, Adolfsson P, Scheiner G, Hanas R, Riddell MC. Exercise in children and adolescents with diabetes. Pediatr Diabetes. 2009;10(s12):154–68.
Riddell MC, Iscoe KE. Physical activity, sport, and pediatric diabetes. Pediatr Diabetes. 2006;7(1):60–70.
Diabetes Research in Children Network (DirecNet) Study Group, Tsalikian E, Kollman C, et al. Prevention of hypoglycemia during exercise in children with type 1 diabetes by suspending basal insulin. Diabetes Care. 2006;29(10):2200–4.
Taplin CE, Cobry E, Messer L, McFann K, Chase HP, Fiallo-Scharer R. Preventing post-exercise nocturnal hypoglycemia in children with type 1 diabetes. J Pediatr 2010;157(5):784–8.e1.
Becker RHA, Frick AD, Burger F, Potgieter JH, Scholtz H. Insulin glulisine, a new rapid-acting insulin analogue, displays a rapid time-action profile in obese non-diabetic subjects. Exp Clin Endocrinol Diabetes. 2005;113(8):435–43.
Campbell MD, Walker M, Trenell MI, et al. Large pre- and postexercise rapid-acting insulin reductions preserve glycemia and prevent early- but not late-onset hypoglycemia in patients with type 1 diabetes. Diabetes Care. 2013;36(8):2217–24.
Moran A, Jacobs DR, Steinberger J, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48(10):2039–44.
Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50(11):2444–50.
Danne T, Battelino T, Kordonouri O, et al. A cross-sectional international survey of continuous subcutaneous insulin infusion in 377 children and adolescents with type 1 diabetes mellitus from 10 countries. Pediatr Diabetes. 2005;6(4):193–8.
Heller S, Buse J, Fisher M, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379(9825):1489–97.
Keating GM. Insulin degludec and insulin degludec/insulin aspart: a review of their use in the management of diabetes mellitus. Drugs. 2013;73(6):575–93.
Thabit H, Hovorka R. Closed-loop insulin delivery in type 1 diabetes. Endocrinol Metab Clin N Am. 2012;41(1):105–17.
Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55(12):3344–50.
Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31(5):934–9.
Leelarathna L, English SW, Thabit H, et al. Feasibility of fully automated closed-loop glucose control utilizing continuous subcutaneous glucose measurements in critical illness: a randomised controlled trial. Crit Care. 2013;17(4):R159.
American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(Suppl 1):S14–80.
Conflicts of Interest and Funding
Dr. Malik and Dr. Taplin declare no conflicts of interest that are relevant to the content of this article. No funding was received for the preparation of the article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malik, F.S., Taplin, C.E. Insulin Therapy in Children and Adolescents with Type 1 Diabetes. Pediatr Drugs 16, 141–150 (2014). https://doi.org/10.1007/s40272-014-0064-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-014-0064-6