Abstract
Nivolumab plus relatlimab (nivolumab and relatlimab-rmbw; Opdualag™) is a fixed-dose, combination immunotherapy treatment being developed by Bristol Myers Squibb for the treatment of multiple types of advanced cancers. Both drugs are immunoglobulin G4 (IgG4) monoclonal antibodies developed to target immune checkpoints, with nivolumab targeting the programmed cell death protein 1 (PD-1) receptor and relatlimab being a newly developed, first-in-class drug targeting the lymphocyte-activation gene 3 (LAG-3) protein. In March 2022, nivolumab plus relatlimab received its first approval in the USA for the treatment of unresectable or metastatic melanoma in adult patients and paediatric patients aged ≥ 12 years who weigh ≥ 40 kg. This article summarizes the milestones in the development of this combination therapy leading to this first approval for unresectable or metastatic melanoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.19624245 |
A fixed-dose combination of nivolumab (a PD-1 inhibitor) and relatlimab (a LAG-3 blocking antibody) is being developed by Bristol Myers Squibb for the treatment of advanced cancer |
Received its first approval on 18 March 2022 in the USA |
Approved for use in adult patients and paediatric patients aged ≥ 12 years who weigh ≥ 40 kg with unresectable or metastatic melanoma |
1 Introduction
Nivolumab plus relatlimab (nivolumab and relatlimab-rmbw; Opdualag™) is a fixed-dose, combination immunotherapy being developed by Bristol Myers Squibb for the treatment of cancer [1]. The targeting of immune checkpoints, including those of immune cell or tumour cell receptors, to regulate the immune system in the tumour microenvironment has become an important cancer treatment strategy [2]. Such checkpoints in melanoma include programmed cell death protein 1 (PD-1) receptor, which is involved in the inhibition of immune responses to tumours and is targeted by the monoclonal blocking antibody, nivolumab [3]. Although immune checkpoint monotherapy is effective under certain circumstances, combination treatments have been associated with greater response rates and may be more effective in a broader range of patient populations [4]. There is an unmet need of immuno-oncological treatments with a high benefit to risk ratio, and dual immunotherapy with checkpoint inhibitors has become of interest to both extend the duration of response in those responding to treatment, as well as to improve patient outcomes in those with disease progression despite treatment [3].
Relatlimab is a newly developed, first-in-class blocking antibody of lymphocyte-activation gene 3 (LAG-3), a protein that is expressed on the surfaces of lymphocytes and is involved in the suppression of T-cell proliferation and promotion of T-cell exhaustion in the tumour immune microenvironment [5].

Key milestones and clinical t rials in the development of nivolumab plus relat limab. BLA Biologics License Application, GC gast ric adenocarcinoma, GEJ gast roesophageal adenocarcinoma, MAA Marketing Authorization Application, MM malignant melanoma, mUM metastatic uveal melanoma, NSCLC non-small cell lung cancer, PDUFA Prescript ion Drug User Fee Act
On 18 March 2022, nivolumab plus relatlimab as a fixed-dose combination therapy received its first approval in the USA for the treatment of unresectable or metastatic melanoma in adult patients and paediatric patients aged ≥ 12 years who weigh ≥ 40 kg [6]. The recommended dosage of nivolumab plus relatlimab is nivolumab 480 mg and relatlimab 160 mg administered intravenously (over a maximum infusion volume of 160 mL) once every 4 weeks until disease progression or unacceptable toxicity occurs [1]. The maximum infusion volume in adults weighing < 40 kg is 4 mL/kg. The recommended dosage in paediatric patients aged ≥ 12 years and weighing < 40 kg has not been established. No dose reductions are recommended [1]. Clinical studies investigating the use of nivolumab plus relatlimab in various cancer types are currently underway in multiple countries worldwide.
1.1 Company Agreements
In July 2014, Bristol Myers Squibb and Ono Pharmaceutical entered into a licensing agreement to develop and commercialise multiple immunotherapies for patients with cancer in Japan, Taiwan and South Korea [7]. The agreement covers relatlimab, ipilimumab, nivolumab, lirilumab, and urelumab. Under the terms of this agreement, Bristol Myers Squibb and Ono will jointly develop monotherapy and combination regimens, using nivolumab as the foundational therapy, in the above countries. The companies will also leverage their global clinical studies by including patients from these countries. Ono will be primarily in charge of monotherapy and Bristol Myers Squibb will be primarily in charge of combination therapies, and development costs and profits will be shared accordingly [7].
In September 2017, Bristol Myers Squibb and Halozyme entered into a global collaboration and license agreement to develop subcutaneously administered Bristol Myers Squibb immuno-oncology medicines using Halozyme’s ENHANZE® drug delivery technology [8]. Bristol Myers Squibb has designated multiple immuno-oncology targets including PD-1 and has an option to select additional targets within five years from the effective date; the collaboration may extend to a maximum of 11 targets [8].
In June 2020, Bristol Myers Squibb selected three targets on an exclusive basis and exercised their option to convert a co-exclusive license to an exclusive license [9]. Bristol Myers Squibb has selected eight targets on an exclusive basis as of December 2020 [9].
2 Scientific Summary
2.1 Pharmacodynamics
Relatlimab is a human immunoglobulin G4 (IgG4) monoclonal blocking antibody which reduces LAG-3 pathway-mediated inhibition of the immune response by binding to the LAG-3 receptor, blocking interaction with its ligands (e.g. MHC II) and therefore promoting T-cell proliferation and cytokine secretion [1]. In vitro, treating peripheral blood mononuclear cells from patients with chronic lymphocytic leukaemia with relatlimab depleted leukemic cells and restored T-cell and NK cell-mediated immune responses [10].
Nivolumab is a human IgG4 monoclonal blocking antibody that reduces the inhibition of the PD-1 pathway-mediated immune response (including the anti-tumour immune response) by binding to the PD-1 receptor which is found on T cells, blocking its interaction with programmed death ligands 1 and 2 (PD-L1 and PD-L2); the interaction between these ligands (which are upregulated in some tumour types) and the PD-1 receptor inhibits T-cell proliferation and cytokine production [1].
Nivolumab combined with relatlimab increases T-cell activation to a greater degree than either drug alone [1]. In mouse tumour models, the anti-tumour effect of the PD-1 receptor inhibition is increased by the inhibition of the LAG-3 receptor, both inhibiting tumour growth and promoting tumour regression [11].
2.2 Pharmacokinetics
Data from two clinical trials in patients with varying types of cancer indicate that relatlimab demonstrates non-linear and time-varying pharmacokinetics when administered alone at a dosage of 160 mg once every 4 weeks [12]. When relatlimab was administered intravenously in cancer patients over dosages of 160–1440 mg once every 4 weeks as monotherapy or in combination with nivolumab (80 mg or 240 mg every 2 weeks, or 480 mg once every 4 weeks), steady-state concentrations of relatlimab were reached by 16 weeks, with a systemic accumulation that was 1.9-fold [1]. After the first-dose, the average plasma concentration of relatlimab increased dose-proportionally with doses ≥ 160 mg administered every 4 weeks. When nivolumab plus relatlimab was administered at the recommended dosage, the steady-state, geometric mean maximum and average plasma concentrations of nivolumab were 187 µg/mL and 94.4 µg/mL, and those of relatlimab were 62.2 µg/mL and 28.8 µg/mL [1].
Features and properties of nivolumab plus relatlimab
Alternative names | BMS-936558/BMS-986016; BMS-986213; Nivolumab and relatlimab-rmbw - Bristol Myers Squibb; Nivolumab/relatlimab-rmbw - Bristol Myers Squibb; Opdualag; Relatlimab-rmbw/nivolumab - Bristol Myers Squibb; Relatlimab/nivolumab - Bristol Myers Squibb |
Class | Antineoplastics; immunotherapies; monoclonal antibodies |
Mechanism of action | Antibody-dependent cell cytotoxicity; CD223 antigen inhibitors; programmed cell death-1 receptor antagonists; T lymphocyte stimulants |
Route of administration | Intravenous |
Pharmacodynamics | Nivolumab: Binds to the PD-1 receptor, reducing inhibition of the PD-1 pathway-mediated immune response and therefore promoting T-cell proliferation and cytokine production |
Relatlimab: Binds to the LAG-3 receptor, blocking interaction with its ligands (e.g. MHC II); promotes T-cell proliferation and cytokine secretion | |
Pharmacokinetics | Nivolumab: Comparable mean Cmin when administered with relatlimab vs administered alone; mean CL 7.6 mL/h at steady state; terminal t½ = 26.5 days when administered with relatlimab |
Relatlimab: Plasma concentration increases dose-proportionally with doses ≥ 160 mg every 4 weeks; mean CL 5.5 mL/h at steady state; effective t½ = 26.2 days when administered with nivolumab | |
Adverse events | |
Most frequent (treatment-related) | Pruritus, fatigue, rash, arthralgia, hypothyroidism, diarrhoea, vitiligo |
Grade 3–4 | Musculoskeletal pain, fatigue, rash, diarrhoea, increased AST and ALT levels, decreased sodium, decreased haemoglobin, decreased lymphocytes |
Serious | Adrenal insufficiency, anaemia, colitis, pneumonia, acute myocardial infarction, back pain, diarrhoea, myocarditis, pneumonitis |
ATC codes | |
WHO ATC code | L01X-C (Monoclonal antibodies); L01X-C17 (Nivolumab) |
EphMRA ATC code | L1G (Monoclonal Antibody Antineoplastics) |
In the phase II/III RELATIVITY-047 study, the steady-state, geometric mean minimum concentration of nivolumab in patients receiving nivolumab plus relatlimab was comparable with that in those receiving nivolumab alone [1].
Nivolumab and relatlimab each have a geometric mean volume of distribution of 6.6 L at steady state [1]. The geometric mean clearance (CL) of nivolumab is 9.6 mL/h after the first dose and 7.6 mL/h at steady state, while those of relatlimab are 6 mL/h after the first dose and 5.5 mL/h at steady state. Following the administration of nivolumab 480 mg and relatlimab 160 mg every 4 weeks, the geometric mean terminal half-life (t½) of nivolumab is 26.5 days, and the geometric mean effective t½ of relatlimab is 26.2 days [1].
2.3 Therapeutic Trial
2.3.1 Unresectable or Metastatic Melanoma
Relative to nivolumab alone, nivolumab in combination with relatlimab improved progression-free survival (PFS) in patients with previously untreated metastatic or unresectable melanoma in the ongoing, randomized, double-blind, phase II/III RELATIVITY-047 trial (NCT03470922) [13]. Eligible patients are aged ≥ 12 years and had histologically confirmed stage III or IV melanoma, measurable disease according to Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1, and expression of LAG-3 and PD-L1 assessable in tumour tissue. Those who had previously received adjuvant or neoadjuvant therapies with a PD-1, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), B-Raf (BRAF), or mitogen-activated protein kinase kinase (MEK) inhibitor were eligible if the treatment was completed ≥ 6 months before disease recurrence, and patients previously treated with interferon were eligible if their last dose was received ≥ 6 weeks prior to randomization. Patients with uveal melanoma and untreated, active brain or leptomeningeal metastases were not eligible for the study. Study participants (n = 714) were randomized 1:1 to receive nivolumab 480 mg plus relatlimab 160 mg in a fixed-dose combination or nivolumab 480 mg alone, both of which were administered intravenously once every 4 weeks [13].
Key clinical trials of nivolumab plus relatlimab
Drug(s) | Indication | Phase | Status | Location(s) | Identifier | Sponsor |
|---|---|---|---|---|---|---|
Nivolumab/relatlimab, nivolumab | Malignant melanoma | III | Recruiting | Multinational | NCT05002569, EudraCT2021-001641-13, RELATIVITY-098 | Bristol Myers Squibb |
Nivolumab/relatlimab, nivolumab | Malignant melanoma, ALM | II/III | Active, no longer recruiting | Multinational | NCT03470922, EudraCT2017-003583-12, RELATIVITY-047 | Bristol Myers Squibb |
Nivolumab/relatlimab/CTx, nivolumab/CTx | GC/GEJ | II | Active, no longer recruiting | Multinational | NCT03662659, EudraCT2018-001069-18 | Bristol Myers Squibb |
Nivolumab/relatlimab | Metastatic uveal melanoma | II | Recruiting | USA | NCT04552223 | Bristol Myers Squibb |
Nivolumab/relatlimab/CTx, nivolumab/PL/CTx | NSCLC | II | Recruiting | Multinational | NCT04623775; RELATIVITY-104 | Bristol Myers Squibb |
Nivolumab/relatlimab, nivolumab, relatlimab | Metastatic melanoma | II | Recruiting | USA | NCT03743766 | Bristol Myers Squibb |
Nivolumab/relatlimab, nivolumab | Advanced liver cancer | II | Recruiting | Multinational | NCT04567615, RELATIVITY-073 | Bristol Myers Squibb |
Nivolumab/relatlimab, relatlimab | Solid tumours | I/IIa | Active, no longer recruiting | Multinational | NCT01968109, EudraCT2014-002605-38, JapicCTI183890 | Bristol Myers Squibb, Ono Pharmaceuticals |
Nivolumab/relatlimab | Solid tumours | I/II | Recruiting | China | NCT05134948, RELATIVITY 059 | Bristol Myers Squibb |
Nivolumab/relatlimab/bevacizumab, nivolumab/PL/bevacizumab | Advanced liver cancer | I/II | Not yet recruiting | France, Taiwan | NCT05337137, RELATIVITY-106 | Bristol Myers Squibb |
Nivolumab/relatlimab (subcutaneous) | Multiple cancer types | I | Active, no longer recruiting | USA | NCT04112498 | Bristol Myers Squibb |
After a median follow-up duration of 13.2 months, the median PFS was significantly greater with nivolumab plus relatlimab compared with nivolumab alone [10.1 months vs 4.6 months; hazard ratio for progression or death (HR) 0.75, 95% CI 0.62–0.92; p = 0.006) [13]. At 12 months, 47.7% of nivolumab plus relatlimab recipients and 36.0% of nivolumab-only recipients achieved PFS. The median PFS in patients with a PD-L1 expression of ≥ 1% (n = 293) was 15.7 months in nivolumab plus relatlimab recipients and 14.7 months in nivolumab-only recipients (HR 0.95; 95% CI 0.68–1.33), and 6.4 months and 2.9 months in patients with a PD-L1 expression of ≤ 1% (n = 421) [HR 0.66, 95% CI 0.51–0.84]. With respect to LAG-3 expression, the median PFS in patients with LAG-3 expressions of ≥ 1% (n = 537) was 12.6 months and 4.8 months in the nivolumab plus relatlimab and nivolumab-only groups (HR 0.75; 95% CI 0.59–0.95), and 4.8 months and 2.8 months in patients with LAG-3 expressions of < 1% (HR 0.78; 95% CI 0.54–1.15) [13].
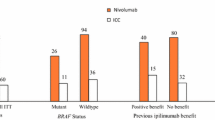
Nivolumab plus relatlimab was also efficacious regardless of BRAF mutation status (n = 275 and 439 in the BRAF mutation and wild type subgroups); in both BRAF mutation and wild type subgroups, the median PFS was 10.1 months with nivolumab plus relatlimab and 4.6 months with nivolumab alone (BRAF mutation subgroup: HR 0.74, 95% CI 0.54–1.03; wild-type BRAF subgroup: HR 0.76; 95% CI 0.59–0.98) [13]. PFS findings across a range of other patient subgroups, including those of other key prognostic indicators (e.g. metastatic stage of the tumour, lactate dehydrogenase levels) generally favoured nivolumab plus relatlimab therapy over nivolumab alone. There was no clinically meaningful change in health-related quality of life in either treatment group [13].
The median PFS remained consistent after a median follow-up of 19.3 months (10.2 months vs 4.6 months in the nivolumab plus relatlimab and nivolumab-only groups) [14]. The median overall survival (OS) was not reached with nivolumab plus relatlimab and 34.1 months with nivolumab alone; statistical significance was not reached (HR 0.80; 95% CI 0.6–1.0; p = 0.059); OS rates were 77.0% and 71.6% in the respective groups at 12 months, and 63.7% and 58.3% at 24 months. Objective response rates (ORR) were 43.1% and 32.6%, with complete responses seen in 16.3% and 14.2% of patients [14].
2.3.2 Solid Tumours
Interim findings from an ongoing, multinational phase I/IIa dose-escalation and -expansion study (NCT01968109) have indicated that nivolumab plus relatlimab is effective with respect to ORR, disease control rate (DCR) and duration of response (DOR) in patients with solid tumours previously treated with prior anti-PD-1 or PD-L1 therapy [15]. The interim assessment was performed in the melanoma cohort progressed disease despite prior anti-PD-1/PD-L1 therapy. The study participants received intravenous nivolumab 240 mg plus relatlimab 80 mg once every two weeks [15].
At the data cut-off date (15 June 2017), 68 patients were treated and 61 patients were efficacy-evaluable [15]. The ORR with nivolumab plus relatlimab therapy was 11.5% (1 complete responder and 6 partial responders; 1 unconfirmed responder), with ORR rates ≥ 3.5 times higher in patients with LAG-3 expression levels of ≥ 1% compared with < 1%. The DCR was 49%, and the median DOR was not reached [15].
2.4 Adverse Events
Nivolumab plus relatlimab had an acceptable tolerability profile in patients with previously untreated metastatic or unresectable melanoma, with no new safety signals observed [1, 13]. After a median of 5.6 months of treatment with nivolumab plus relatlimab (vs 4.9 months with nivolumab) in the RELATIVITY-047 study, 97.2% and 94.4% of patients in the respective groups experienced an adverse event (AE) [13]. The most common treatment-related AEs (TRAEs) of any grade with nivolumab plus relatlimab (incidence > 15%) were pruritus (23.4% vs 15.9% with nivolumab alone), fatigue (23.1% vs 12.8%) and rash (15.5% vs 12.0%). AEs related to infusion were reported in 5.9% of nivolumab plus relatlimab recipients and 3.6% of nivolumab-only recipients. The most common (incidence > 20%) AEs of any grade relating to laboratory abnormalities in the nivolumab plus relatlimab group include decreased haemoglobin (37%), lymphocytes (32%) and sodium (24%), and increased levels of aspartate aminotransferase (AST; 30%) and alanine aminotransferase (ALT; 26%) [1]. Grade 3 or 4 TRAEs were reported in 18.9% and 9.7% of the respective groups, with the most common in the nivolumab plus relatlimab group being increased levels of lipase (1.7%), ALT (1.4%), and AST (1.4%), and fatigue (1.1%) [13].
Serious AEs (SAEs) occurred in 36% of the nivolumab plus relatlimab group, with the most common (incidence > 1%) being adrenal insufficiency, anaemia, colitis, and pneumonia (incidence 1.4% for each), as well as acute myocardial infarction, back pain, diarrhoea, myocarditis, and pneumonitis (1.1% for each) [1]. AEs requiring dosage interruptions were reported in 43% of the nivolumab plus relatlimab group, with the most common of these (incidence > 2%) being diarrhoea (3.9%), increased levels of troponin (3.9%), troponin T (2.8%), AST (2.8%), and ALT (2.3%), and hyperthyroidism (2.3%) [1]. TRAEs leading to treatment discontinuation were reported in 14.6% of the nivolumab plus relatlimab group and 6.7% of the nivolumab monotherapy group [13]. Three deaths in the nivolumab plus relatlimab group were considered to be related to treatment (haemophagocytic lymphohistiocytosis, acute pulmonary oedema, and pneumonitis), as were two in the nivolumab only group (sepsis and myocarditis in one patient, pneumonia in the other) [13].
The most common immune-mediated AEs of any grade with nivolumab plus relatlimab (incidence ≥ 5%) were relating to hypothyroidism or thyroiditis (18% vs 13.9% with nivolumab alone), rash (9.3% vs 6.7%), diarrhoea or colitis (6.8% vs 3.1%), hyperthyroidism (6.2% vs 6.7%) and hepatitis (5.6% vs 2.5%) [13]. Myocarditis occurred in 1.7% of the nivolumab plus relatlimab group (vs 0.6% in the nivolumab only group), with 0.6% being grade 3 or 4 in severity (vs 0%); however, these events in this group resolved completely [13].
Interim findings from the previously discussed phase I/IIa study in patients with solid tumours (NCT01968109) indicate that nivolumab plus relatlimab is generally well tolerated [15]. TRAEs occurred in 51% of the total study population (n = 262), with 10% experiencing grade 3 or 4 TRAEs and 3.8% discontinuing treatment. In melanoma patients who had progressed disease despite prior anti-PD-1/PD-L1 therapy (n = 68), 41% experienced TRAEs, with 4.4% of patients reporting grade 3 or 4 TRAEs and 1.5% discontinuing treatment [15].
2.5 Ongoing Clinical Trials
In addition to the ongoing phase II/III RELATIVITY-047 trial, which has an estimated completion date of November 2023, the multinational phase III trial, RELATIVITY-098, is currently recruiting participants to further investigate the efficacy and tolerability of nivolumab plus relatlimab compared with nivolumab monotherapy in completely resected malignant melanoma. Another phase II trial (NCT03743766) is recruiting participants who have not previously received immunotherapy to assess the efficacy and tolerability of nivolumab/relatlimab compared with nivolumab monotherapy and relatlimab monotherapy in metastatic melanoma.
A phase II trial (NCT03662659) is currently underway to investigate the use of nivolumab plus relatlimab, or nivolumab only, in combination with chemotherapy in patients with gastric or gastroesophageal adenocarcinoma.
The phase I/IIa safety study (NCT01968109) assessing the use of nivolumab plus relatlimab in patients with solid tumours is active and ongoing. Recruitment is currently underway for phase I/II or II studies investigating the efficacy, tolerability, and/or pharmacological properties of nivolumab plus relatlimab in non-small cell lung cancer (NCT04623775; RELATIVITY-104), metastatic uveal melanoma (NCT04552223), advanced liver cancer (NCT04567615; RELATIVITY-073), and solid tumours (NCT05134948; RELATIVITY-059). A phase I/II study assessing the use of nivolumab plus relatlimab, or nivolumab plus placebo, in combination with bevacizumab in patients with advanced liver cancer (NCT05337137; RELATIVITY-106) has recently been initiated.
An ongoing phase I study (NCT04112498) in patients with a range of cancer types in the USA is investigating the bioavailability and safety of nivolumab plus relatlimab when formulated with recombinant human hyaluronidase for subcutaneous drug administration.
3 Current Status
Nivolumab plus relatlimab received its first approval on 18 March 2022 in the USA for unresectable or metastatic melanoma in adult and paediatric patients aged ≥ 12 years who weigh ≥ 40 kg [6].
References
Bristol-Myers Squibb. OPDUALAG™ (nivolumab and relatlimab-rmbw) injection, for intravenous use: US prescribing information. 2022. https://www.accessdata.fda.gov/. Accessed 26 Apr 2022.
Shi AP, Tang XY, Xiong YL, et al. Immune checkpoint LAG3 and its ligand FGL1 in cancer. Front Immunol. 2022;12(785091):1–11.
LaFleur MW, Muroyama Y, Drake CG, et al. Inhibitors of the PD-1 pathway in tumor therapy. J Immunol. 2018;200(2):375–83.
Barbari C, Fontaine T, Parajuli P, et al. Immunotherapies and combination strategies for immuno-oncology. Int J Mol Sci. 2020;21(14):5009.
Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–86.
US Food & Drug Administration. FDA approves Opdualag™ for unresectable or metastatic melanoma [media release]. 18 Mar 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-opdualag-unresectable-or-metastatic-melanoma.
Bristol-Myers Squibb. Bristol-Myers Squibb and Ono Pharmaceutical Co., Ltd. announce strategic immuno-oncology collaboration in Japan, South Korea and Taiwan [media release]. 23 Jul 2014. http://www.bms.com.
Halozyme Therapeutics, Bristol-Myers Squibb. Bristol-Myers Squibb and Halozyme enter global collaboration and license agreement for ENHANZE® technology [media release]. 14 Sep 2017. http://www.halozyme.com.
Halozyme Therapeutics Inc. Annual report on Form 10-K. 2020. https://www.sec.gov/. Accessed 26 Apr 2022.
Sordo-Bahamonde C, Lorenzo-Herrero S, González-Rodríguez AP, et al. LAG-3 blockade with relatlimab (BMS-986016) restores anti-leukemic responses in chronic lymphocytic leukemia. Cancers (Basel). 2021;13(9):2122.
Thudium K, Selby M, Zorn JA, et al. Preclinical characterization of relatlimab, a human LAG-3-blocking antibody, alone or in combination with nivolumab. bioRxiv. 2022. https://doi.org/10.1101/2022.01.24.477551.
Zhao Y, Hu Z, Suryawanshi R, et al. Model-informed clinical pharmacology (CP) profile of a novel fixed-dose combination (FDC) of relatlimab and nivolumab in patients with solid tumors. Clin Pharmacol Ther. 2022;111(Suppl. 1):S61.
Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386(1):24–34.
Long GV, Hodi FS, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in previously untreated metastatic or unresectable melanoma: overall survival and response rates from RELATIVITY-047 (CA224-047). J Clin Oncol. 2022;40(36 Suppl.): 360385.
Ascierto PA, Bono P, Bhatia S, et al. Efficacy of BMS-986016, a monoclonal antibody that targets lymphocyte activation gene-3 (LAG-3), in combination with nivolumab in pts with melanoma who progressed during prior anti-PD-1/PD-L1 therapy (mel prior IO) in all-comer and biomarkerenriched populations. Ann Oncol. 2017;28(Suppl 5):v611–2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Julia Paik is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paik, J. Nivolumab Plus Relatlimab: First Approval. Drugs 82, 925–931 (2022). https://doi.org/10.1007/s40265-022-01723-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01723-1