Abstract
Vosoritide (VOXZOGO®) is a modified recombinant human C-type natriuretic peptide (CNP) analogue, being developed by BioMarin Pharmaceutical for the treatment of achondroplasia. Achondroplasia is caused by a gain-of-function mutation in the fibroblast growth factor receptor 3 gene (FGFR3), which is a negative regulator of bone growth. Vosoritide acts to restore chondrogenesis through its binding to natriuretic peptide receptor B (NPR-B), resulting in the inhibition of downstream signalling pathways of the overactive FGFR3 gene. Vosoritide was approved in August 2021 in the EU for the treatment of achondroplasia in patients aged ≥ 2 years whose epiphyses are not closed; the diagnosis of achondroplasia should be confirmed by appropriate genetic testing. The drug is also under regulatory review in the USA for the treatment of achondroplasia and clinical development is underway in several countries. This article summarizes the milestones in the development of vosoritide leading to this first approval for achondroplasia in patients aged ≥ 2 years whose epiphyses are not closed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.16746472. |
A modified recombinant human C-type natriuretic peptide analogue is being developed by BioMarin Pharmaceutical for the treatment of achondroplasia |
Received its first approval on 27 August 2021 in the EU |
Approved for the treatment of achondroplasia in patients aged ≥ 2 years whose epiphyses are not closed; the diagnosis of achondroplasia should be confirmed by appropriate genetic testing |
1 Introduction
Achondroplasia is the most common form of skeletal dysplasia, carrying hallmark clinical features of a short stature with rhizomelic limb shortening, long trunk and macrocephaly [1, 2]. The condition is caused by a gain-of-function mutation in the fibroblast growth factor receptor 3 gene (FGFR3), a negative regulator of bone growth, resulting in sustained activation of the downstream signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) pathway in chondrocytes, which inhibits endochondral ossification [2,3,4]. Vosoritide (VOXZOGO®) is a modified recombinant human C-type natriuretic peptide (CNP) analogue, being developed by BioMarin Pharmaceutical for the treatment of achondroplasia and short stature. Vosoritide mimics the actions of natural CNP and binds to natriuretic peptide receptor B (NPR-B); this results in stimulation of intracellular cyclic guanosine monophosphate (cGMP) production and inhibition of the overactive FGFR3 pathway, thereby restoring chondrogenesis [2, 5]. The 39 amino acid peptide analogue of vosoritide includes the 37 C terminal amino acids of the human CNP53 sequence, with two additional amino acids (Pro Gly) [6]. These properties convey resistance to neutral endopeptidase (NEP) degradation, thereby prolonging the half-life of vosoritide compared with endogenous CNP (rapidly degraded by NEP), allowing for once-daily administration [6].

Key milestones in the development of vosoritide for the treatment of achondroplasia. CHMP Committee for Medicinal Products for Human Use, FDA Food and Drug Administration, NDA New Drug Application
Vosoritide received its first approval in August 2021 in the EU [7] for the treatment of achondroplasia in patients 2 years of age and older whose epiphyses are not closed; the diagnosis of achondroplasia should be confirmed by appropriate genetic testing [6]. The drug is also under regulatory review in the USA, for the treatment of achondroplasia [8]. Vosoritide is administered subcutaneously, with the volume of the drug to be administered at the recommended dose being based on the patient's weight and the vosoritide concentration; the usual dose is 15 μg/kg body weight. Treatment with vosoritide should be stopped upon confirmation of no further growth potential, indicated by a growth velocity of < 1.5 cm/year and closure of epiphyses [6].
1.1 Company Agreements
In March 2016, Chugai Pharmaceutical concluded an exclusive sublicense agreement with BioMarin Pharmaceutical on the patent of Chugai's CNP intended for treatment of achondroplasia in the regions of the US and Japan, in order to make vosoritide available for patients as a new treatment for achondroplasia as quickly as possible [9].
2 Scientific Summary
2.1 Pharmacodynamics
Endochondral bone growth is negatively regulated in patients with achondroplasia owing to a gain-of-function mutation in FGFR3, resulting in sustained activation of the downstream ERK/MAPK pathway and thereby leading to negative regulation of chondrocyte proliferation and differentiation [5, 6]. As seen with native CNP, following the binding of vosoritide to NPR-B, the downstream signalling pathway of FGFR3 is inhibited at the level of rapidly accelerating fibrosarcoma serine/threonine protein kinase (RAF-1), thereby inhibiting ERK1/2 in the MAPK pathway. This results in positive regulation of endochondral bone growth through stimulation of chondrocyte proliferation and differentiation [2, 4, 6, 10]. In an in vitro study in achondroplasia human growth plate chondrocytes, vosoritide was associated with decreased phosphorylation of ERK1 and ERK2, demonstrating that vosoritide inhibits FGFR3-mediated MAPK activation [10].
In an in vivo study in a transgenic mouse model for dwarfism, vosoritide administration resulted in a significant recovery of bone growth, with an increase in axial and appendicular skeleton lengths and improvements in dwarfism-related clinical features [10]. In normal juvenile cynomolgus monkeys treated with vosoritide for 6 months, growth plate expansion was observed, including significant expansions in total growth plate thickness, proliferating zone thickness and hypertrophic zone thickness [4].
Given the structural similarity between vosoritide and atrial natriuretic peptide, an in vivo study assessing the haemodynamic effects of vosoritide on juvenile cynomolgus monkey was conducted, with only transient, mild haemodynamic changes (a decrease in blood pressure and increase in heart rate) observed [4].
In an open-label phase II study of vosoritide in children aged 5–14 years with achondroplasia (NCT02055157), dose-dependent increases from baseline in urinary cyclic guanosine monophosphate (cGMP, a biomarker for NPR-B activity) and serum collagen type X marker (CXM, a biomarker for endochondral ossification) were observed with vosoritide treatment [1]. Increases in cGMP and CXM occurred within the first 4 h of receiving vosoritide, with levels remaining elevated beyond 24 months of treatment [1, 5, 6]. The activity of vosoritide 15 μg/kg administered subcutaneously once daily (as measured by urine cGMP) was near saturation and maximal increase in growth plate activity (as observed by CXM levels) was achieved, with no additional meaningful improvements in annualized growth velocity (AGV, efficacy measure) seen with vosoritide 30 μg/kg [5]. These data were confirmed in a phase III study in patients with achondroplasia (NCT03197766) [11]. Longer-term data from the phase 2 extension trial (NCT02724228) demonstrated that the increase in urine cGMP with vosoritide was sustained after 60 months of therapy [12].
2.2 Pharmacokinetics
The pharmacokinetics of once-daily subcutaneous vosoritide 15 μg/kg vosoritide have been evaluated in phase II and III trials in patients aged 5 to < 18 years with achondroplasia up to 52 weeks [5, 6]. Across a vosoritide dose range of 2.5–30.0 μg/kg/day, a greater than dose proportional increase in plasma exposure was observed overall.
Subcutaneous vosoritide 15 μg/kg is rapidly absorbed, reaching a median peak concentration (Tmax) within 15 min [5, 6]. After 52 weeks of once-daily subcutaneous vosoritide treatment, the mean Cmax and AUC0−t observed was 5.8 ng/mL and 290 ng ·min/mL, respectively; exposure was consistent across patient visits and no evidence of accumulation seen with once-daily dosing. The mean apparent volume of distribution was 2.91 L/kg and the mean apparent clearance was 79.4 mL/min/kg after 52 weeks. After reaching maximal plasma concentrations, vosoritide levels rapidly decline, with a t½ of 27.9 min. Vosoritide metabolism is expected to occur via catabolic pathways, with degradation into small peptide fragments and amino acids.
Increased body weight was associated with an increase in the apparent clearance and volume of distribution of vosoritide [5, 6]; therefore, the use of doses above (in patients between 10 and 16 kg body weight), or below (in those above a body weight of 44 kg) the 15 μg/kg “standard dose” are recommended, in order to enable a similar level of exposure across all weight-ranges [6] No clinically significant differences in the pharmacokinetics of vosoritide were seen based on age (0.9–16 years), sex, race or ethnicity [5, 6].
While no drug-drug interaction studies have been undertaken to date, results from in vitro studies suggest that vosoritide is unlikely to cause CYP- or transporter-mediated drug-drug interactions in humans when administered concomitantly with other medicinal products [6]. Vosoritide is an unlikely candidate for drug-drug interactions given it is a recombinant human protein.
Features and properties of vosoritide
Alternative names | BMN-111; ProCNP38; VOXZOGO |
Class | Cyclic peptides; natriuretic agents; natriuretic peptides; recombinant proteins |
Mechanism of action | Atrial natriuretic factor receptor B agonist |
Route of administration | Subcutaneous |
Pharmacodynamics | Binds to NPR-B, inhibiting the downstream signalling pathway of FGFR3 at the level of RAF-1 and thereby inhibiting ERK1/2 in the MAPK pathway. Dose-dependent increase in cGMP and CXM in patients with achondroplasia receiving vosoritide; increases occurred within 4 h, levels remained elevated with > 24 months’ treatment |
Pharmacokinetics | Mean Cmax and AUC0-t of 5.8 ng/mL and 290 ng · min/mL, respectively; rapidly absorbed with Tmax reached within 15 min, no evidence of accumulation; volume of distribution was 2.91 L/kg and the mean apparent clearance was 79.4 mL/min/kg after 52 weeks; t½ 27.9 min; increased body weight associated with increase in vosoritide clearance and volume of distribution |
Adverse events | |
Most frequent | Injection site reaction, injection site erythema, injection site swelling, vomiting, arthralgia, injection site urticaria, hypotension |
ATC codes | |
WHO ATC code | M05B-X07 (Vosoritide) |
EphMRA ATC code | M5B (Bone Calcium Regulators) |
Chemical name | L-Cysteine, L-prolylglycyl-L-glutaminyl-L-.alpha.-glutamyl-L-histidyl-L-prolyl-L-asparaginyl-L-alanyl-L-arginyl-L-lysyl-L-tyrosyl-L-lysylglycyl-L-alanyl-L-asparaginyl-L-lysyl-L-lysylglycyl-L-leucyl-L-seryl-L-lysylglycyl-L-cysteinyl-L-phenylalanylglycyl-L-leucyl-L-lysyl-L-leucyl-L-.alpha.-aspartyl-L-arginyl-L-isoleucylglycyl-L-seryl-L-methionyl-L-serylglycyl-L-leucylglycyl-, cyclic (23->39)-disulfide |
2.3 Therapeutic Trials
2.3.1 Phase III Trial
The efficacy of vosoritide in children aged 5 to <18 years with achondroplasia with confirmed FGFR3 mutation has been shown in a randomized, double-blind, placebo-controlled, phase III trial (NCT03197766) [11] and its open-label extension (NCT03424018) [13]. The study comprised a 52-week placebo-controlled treatment phase, followed by the open-label treatment extension study where all patients received vosoritide (i.e. placebo recipients were switched to vosoritide) for up to 2 years [11, 13]. Eligible patients had already completed at least 6 months of treatment in a lead-in, observational growth study (NCT01603095). In the placebo-controlled phase, patients were randomized to either once-daily subcutaneous vosoritide 15 μg/kg (n = 60) or placebo (n = 61) [11].
Vosoritide was effective in the treatment of children with achondroplasia, with improvements seen in growth velocity and height Z-scores over the 52 week trial period compared with placebo (NCT03197766) [11]. Vosoritide treatment was associated with significant (p < 0.0001) improvements in AGV, with a mean change from baseline of 1.71 cm/year in vosoritide 15 μg/kg/day recipients compared with 0.13 cm/year in placebo recipients, corresponding to an adjusted mean difference of 1.57 cm/year (95% CI 1.22–1.93) in favour of vosoritide at week 52. Height Z-scores were also significantly (p < 0.0001) higher in vosoritide recipients compared with placebo recipients (mean changes from baseline of 0.27 vs − 0.1, respectively), corresponding to a between group difference of 0.28 (95% CI 0.17–0.39) in favour of vosoritide (key secondary endpoint). There was no statistical difference in the change from baseline in upper to lower body segment ratio for vosoritide versus placebo recipients (mean between-group difference − 0.01 [95% CI − 0.05 to 0.02]). Results from a pre-specified subgroup analysis demonstrated that improvements in AGV seen with vosoritide versus placebo in the primary analysis were consistent across all subgroups analysed, namely sex, age group, Tanner stage, baseline height Z-score and baseline AGV; all estimates of the mean between group differences were in the favour of vosoritide (all 95% CIs were overlapping) [11].
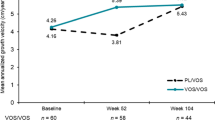
The therapeutic efficacy of daily subcutaneous administration of vosoritide seen in the randomized placebo-controlled study [11] was maintained for up to two years in children with achondroplasia aged 5 to <18 years in the open-label extension study (NCT03424018) [13]. In children originally randomized to vosoritide who continued treatment (n = 58), AGV increased from 4.26 cm/year at baseline to 5.52 cm/year at week 104. In placebo recipients who had crossed over to the vosoritide treatment group in the extension study (n = 61), AGV increased from 3.81 cm/year at week 52 to 5.43 cm/year at week 104 [13]. Comparative analyses (using ANCOVA model) of height gain, height Z-score and upper-to-lower body segment ratio were also conducted to assess improvements with vosoritide treatment (n = 45) versus those receiving placebo (i.e. 2 years of untreated follow up; n = 38); accounting for the placebo period and an additional year from the observational study prior to start of the randomized study. In patients receiving vosoritide for up to 2 years, improvements were seen in terms of mean between-group-differences in height gain (+3.34 cm; 95% CI 2.76–3.93), height Z-score (+ 0.44; 95% 0.25–0.63) and upper-to-lower body segment ratio (− 0.05; 95% CI − 0.09 to − 0.01) compared with untreated patients at week 104, according to the comparative analyses [13].
2.3.2 Phase II Trials
The efficacy of vosoritide was also evident in an open label, dose-escalation study (NCT02055157) and in its long-term extension study (NCT02724228) in children aged 5–14 years with achondroplasia (n = 35) who had already completed at least 6 months of treatment in a lead-in, observational growth study (NCT01603095) [1, 6, 12]. A dose-dependent increase in AGV was seen during the first 6 months of vosoritide treatment, with changes from baseline observed in patients receiving vosoritide 7.5, 15 and 30 μg/kg/day at 6 months (1.28, 2.01 and 2.08 cm/year, respectively); the change from baseline in AGV with vosoritide 2.5 μg/kg/day was − 0.37 cm/year at this time point [1]. Improvements in AGV were sustained over the extension period, with increases from baseline seen in all treatment groups at the interim time point up to 42 months; in vosoritide 15 μg/kg/day recipients, the mean AGV was 5.51 cm/year (as calculated between 30 and 42 months), representing an annual increase from baseline of 1.46 cm [1]. Growth velocity was sustained over the longer-term in an updated analysis in patients who completed treatment for up to 5 years (included vosoritide 2.5, 7.5, 15 μg/kg/day cohorts; n = 19), with a mean improvement from baseline seen in AGV of 1.35 cm/year after 60 months of treatment (NCT02724228) [12]. When compared with an age and sex matched historical control, a statistically significant (p = 0.0002) mean difference in height in favour of vosoritide was seen compared with untreated patients after 5 years (9.08 cm [95% CI 5.77–12.38]), according to an analysis adjusted for baseline height differences [6]. Vosoritide was also associated with a sustained increase in height Z-scores through both 42 (n = 22) and 60 (n = 19) month time points; mean overall increases in height Z-score of 0.87 and 0.78 across vosoritide treatment groups, respectively [1, 12].
Key clinical trials of Vosoritide (BioMarin Pharmaceutical)
Indication | Phase | Status | Location(s) | Identifier |
|---|---|---|---|---|
Achondroplasia | Phase 3 | Completed | Australia, England, France, Germany, Italy, Japan, Spain, Turkey, UK, USA | NCT03197766, EudraCT2015-003836-11, JapicCTI184167 |
Achondroplasia | Phase 3 | Ongoing | Africa, Australia, England, Germany, Japan, Spain, Turkey, UK, USA | NCT03424018, EudraCT2017-002404-28 |
Achondroplasia | Observational | Completed | Australia, England, France, Germany, Italy, Japan, Spain, Turkey, UK, USA | NCT01603095, EudraCT2017-000701-21 |
Achondroplasia | Phase 2 | Ongoing | Australia, England, Japan, UK, USA | NCT03583697, EudraCT2016-003826-18 |
Achondroplasia | Phase 2 | Ongoing | Australia, England, France, UK, USA | NCT02724228, EudraCT2015-004004-30 |
Achondroplasia | Phase 2 | Ongoing | Australia, England, Japan, UK, USA | NCT03989947, EudraCT2018-004364-66 |
Achondroplasia | Phase 2 | Completed | Australia, England, France, UK, USA | NCT02055157, EudraCT2013-004137-32 |
Achondroplasia | Phase 2 | Ongoing | Australia, England, UK, USA | NCT04554940, EudraCT2020-001055-40 |
Genetic short stature | Phase 2 | Ongoing | USA | NCT04219007 |
2.4 Adverse Events
Vosoritide was generally well tolerated in the placebo-controlled, phase III trial (NCT03197766). The most commonly reported (incidence >10%) adverse events seen with subcutaneous vosoritide 15 μg/kg/day occurring at a higher rate than with placebo were injection site reaction (73% vs 48% for placebo), injection site erythema (68% vs 66%), injection site swelling (38% vs 10%), vomiting (27% vs 20%), arthralgia (15% vs 7%), injection site urticaria (13% vs 3%) and hypotension (12% vs 5%) [11]. Decreases in blood pressure were transient and resolved without intervention; median time to onset from injection was 31 (range 18–120) min with resolution within 31 (5–90) min [6, 11].
All injection site reactions observed with vosoritide were mild (grade 1) in severity except for five injection site reactions observed in two patients that were considered moderate (grade 2); reported grade 2 events included two events of injection site urticarial (in two patients), and one event of injection site vesicles [6].
Vosoritide treatment was generally well tolerated over the longer term, with no new adverse events detected in the open-label phase III extension study (NCT03424018; up to 2 years treatment) [13] and in the ongoing open-label phase II study (NCT02724228; up to 5 years treatment) [12]. Consistent with shorter term findings, most adverse events were mild in nature and no serious adverse events were attributed to vosoritide; the most common adverse event were mild and transient injection site reactions [12, 13].
As with all therapeutic proteins, vosoritide has a potential for immunogenicity. In clinical trials, anti-drug antibodies (ADA) were detected in 35% of vosoritide recipients, with the earliest time to ADA development of 85 days; no anti-vosoritide neutralising antibodies were seen in ADA-positive patients. The presence of ADA was not associated with any impact on pharmacokinetic properties, efficacy and safety of vosoritide. No correlation was seen between ADA positivity or mean ADA titre and the number, duration, or severity of hypersensitivity adverse reactions or injection site reactions [6].
2.5 Ongoing Clinical Trials
In addition to ongoing phase 2 (NCT02724228) and phase 3 (NCT03424018) trials previously mentioned, the safety and efficacy of vosoritide is also being assessed in an ongoing study in patients aged between 0 and < 5 years that had enrolled 62 patients by a 30 June 2020 data cut-off (NCT03583697). Interim data from this trial showed a positive effect on growth in 4 patients aged ≥ 2 to < 5 years treated with vosoritide 15 μg/kg/day for 2 years; data are currently unavailable for children aged < 2 years [6]. An open label, long-term extension to this phase 2 trial is underway and aims to evaluate the safety and efficacy of vosoritide in children with achondroplasia until patients reach near-adult final height (NCT03989947). The safety and efficacy of vosoritide is also being evaluated in an ongoing randomized, controlled, open-label clinical trial in infants and young children (aged 0 to ≤ 12 months) with achondroplasia at risk of requiring cervicomedullary decompression surgery (NCT04554940) [14]. Vosoritide is also being evaluated in an interventional study in patients with selected genetic causes of short stature (NCT04219007).
References
Savarirayan R, Irving M, Bacino CA, et al. C-Type natriuretic peptide analogue therapy in children with achondroplasia. N Engl J Med. 2019;381(1):25–35.
Semler O, Rehberg M, Mehdiani N, et al. Current and emerging therapeutic options for the management of rare skeletal diseases. Paediatr Drugs. 2019;21(2):95–106.
Wrobel W, Pach E, Ben-Skowronek I. Advantages and disadvantages of different treatment methods in achondroplasia: a review. Int J Mol Sci. 2021;22(11):5573. https://doi.org/10.3390/ijms22115573.
Wendt DJ, Dvorak-Ewell M, Bullens S, et al. Neutral endopeptidase-resistant C-type natriuretic peptide variant represents a new therapeutic approach for treatment of fibroblast growth factor receptor 3-related dwarfism. J Pharmacol Exp Ther. 2015;353(1):132–49.
Chan ML, Qi Y, Larimore K, et al. Pharmacokinetics and exposure-response of vosoritide in children with achondroplasia. Clin Pharmacokinet. 2021. https://doi.org/10.1007/s40262-021-01059-1.
BioMarin International Limited. Voxzogo: EU summary of product characteristics. 2021. https://ec.europa.eu/health/documents/community-register/2021/20210826152503/anx_152503_en.pdf. Accessed 20 Sep 2021.
BioMarin Pharmaceutical. European Commission approves BioMarin's VOXZOGO® (vosoritide) for the treatment of children with achondroplasia from age 2 until growth plates close [media release]. 27 Aug 2021. https://investors.biomarin.com/.
BioMarin Pharmaceutical. BioMarin submits new drug application to U.S. Food and Drug Administration for vosoritide to treat children with achondroplasia [media release]. 21 Aug 2020. http://www.biomarin.com.
Chugai P. Chugai and BioMarin enter exclusive sublicense agreement on the patent of C- type natriuretic peptide intended for treatment of achondroplasia [media release]. 3 Mar 2016. http://www.chugai-pharm.co.jp.
Lorget F, Kaci N, Peng J, et al. Evaluation of the therapeutic potential of a CNP analog in a Fgfr3 mouse model recapitulating achondroplasia. Am J Hum Genet. 2012;91(6):1108–14.
Savarirayan R, Tofts L, Irving M, et al. Once-daily, subcutaneous vosoritide therapy in children with achondroplasia: a randomised, double-blind, phase 3, placebo-controlled, multicentre trial. Lancet. 2020;396(10252):684–92.
Hoover-Fong J, Irving M, Bacino C, et al. Vosoritide for children with achondroplasia: a 60-month update from an ongoing phase 2 clinical trial. Mol Genet Metab. 2021;132(Suppl 1):S101.
Savarirayan R, Tofts L, Irving M et al. Safe and persistent growth-promoting effects of vosoritide in children with achondroplasia: 2-year results from an open-label, phase 3 extension study. Genet Med. 2021. https://doi.org/10.1038/s41436-021-01287-7
Savarirayan R, Irving M, Maixner W, et al. Rationale, design, and methods of a randomized, controlled, open-label clinical trial with open-label extension to investigate the safety of vosoritide in infants, and young children with achondroplasia at risk of requiring cervicomedullary decompression surgery. Sci Prog. 2021;104(1):368504211003782.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sean Duggan is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duggan, S. Vosoritide: First Approval. Drugs 81, 2057–2062 (2021). https://doi.org/10.1007/s40265-021-01623-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01623-w