Abstract
The transient receptor potential vanilloid-1 (TRPV1) is a non-specific cation channel known for its sensitivity to pungent vanilloid compound (i.e. capsaicin) and noxious stimuli, including heat, low pH or inflammatory mediators. TRPV1 is found in the somatosensory system, particularly primary afferent neurons that respond to damaging or potentially damaging stimuli (nociceptors). Stimulation of TRPV1 evokes a burning sensation, reflecting a central role of the channel in pain. Pharmacological and genetic studies have validated TRPV1 as a therapeutic target in several preclinical models of chronic pain, including cancer, neuropathic, postoperative and musculoskeletal pain. While antagonists of TRPV1 were found to be a valuable addition to the pain therapeutic toolbox, their clinical use has been limited by detrimental side effects, such as hyperthermia. In contrast, capsaicin induces a prolonged defunctionalisation of nociceptors and thus opened the door to the development of a new class of therapeutics with long-lasting pain-relieving effects. Here we review the list of TRPV1 agonists undergoing clinical trials for chronic pain management, and discuss new indications, formulations or combination therapies being explored for capsaicin. While the analgesic pharmacopeia for chronic pain patients is ancient and poorly effective, modern TRPV1-targeted drugs could rapidly become available as the next generation of analgesics for a broad spectrum of pain conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pain is the most frequent reason for patients to seek medical care. Efforts have been made to design new non-opioid analgesics. |
Targeting the capsaicin receptor (TRPV1 channel) using ligands that cause defunctionalisation of “pain-sensing” nerves has proved to be a promising therapeutic strategy in patients with herpetic neuropathic pain or osteoarthritic pain. |
Ongoing clinical trials are investigating the long-term efficacy of new capsaicin formulations and alternative modalities for administering TRPV1 agonists and antagonists to treat intractable pain. |
Side effects, sometime severe, that accompany capsaicin administration warrant the development of new capsaicin-derived agonists, or other modality-specific antagonists. |
Future clinical studies will address the long-term patient responses and the efficacy of these compounds, thus providing new pain therapeutics for healthcare practitioners. |
1 Introduction
Acute pain is a protective physiological response against harmful stimuli, such as extreme temperature, chemical irritants or tissue damage caused by injury or chronic inflammatory diseases [1]. Noxious or potentially noxious stimuli are transduced into nerve impulses by primary afferent neurons (nociceptors) and carried along the spinothalamic tract (Fig. 1) to reach the brainstem, thalamus and cerebral cortex, where nociceptive signals are encoded and perceived as painful.
Pain that persists beyond the time of usual tissue healing (~ 3 months) is considered chronic [2] and is the most frequent reason for seeking consultations at primary care units. In the USA alone, estimated costs of chronic pain exceed US$600 billion annually [3]. Chronic pain can stem from nerve damage (neuropathic pain) often associated with plastic changes in the peripheral and central nervous systems leading to altered detection, transmission, processing and regulation of pain [4, 5]. Abnormal hyperexcitable states that result in persistent and intensified pain sensations appear in conditions that produce continuous stimulation of the pain pathway such as chronic inflammation and cancer-associated pain [1] as well as dysfunctional pain disorders, which include bladder pain syndrome (previously interstitial cystitis), irritable bowel syndrome (IBS) and fibromyalgia [5, 6]. Current treatments for acute and chronic pain are lacking. Most of the drugs used, including opioids, non-steroidal anti-inflammatory drugs (NSAIDs), gabapentinoids, antidepressants, paracetamol or anticonvulsants achieve transient and partial pain relief and their use is often hampered by severe side effects [3, 7]. Insights into the understanding of the mechanisms of nociception at the periphery has been tremendous over the last 30 years and the discovery of the capsaicin receptor TRPV1 channel in the early 1990s has been a real breakthrough to provide new “druggable” targets [8]. TRPV1 is expressed by primary afferent nerve fibres, in which it functions as a sensor for noxious heat and diverse chemical irritants, or toxins produced by plants or pathogens. TRPV1 is a ligand-gated ion channel directly activated by arachidonic acid metabolites produced downstream of the 5-lipooxygenase pathway (5-LOX) and endocannabinoids (N-arachidonoyl dopamine (NADA), or N-oleoyl dopamine (OLDA)). At peripheral nerve endings, TRPV1 acts as a “receptor-operated” channel whose activation downstream of metabotropic receptors elicits inflammatory pain. Accordingly, prostaglandin, bradykinin or serotonin produced in inflamed tissues, through activation of their respective receptors, potentiate the TRPV1 channel response to endogenous agonists, and shift its threshold of activation by heat. Pharmacological and genetic studies have confirmed the contribution of TRPV1 in several models of pathological pain. TRPV1 null mice show virtually no thermal hyperalgesia during inflammation. Rapid desensitization of TRPV1-expressing fibers by administration of capsaicin or the potent agonist resiniferatoxin (RTX), attenuates experimental inflammatory hyperalgesia and neurogenic inflammation as well as naturally occurring cancer pain or debilitating arthritic pain. Overall, decades of fundamental research made the TRPV1 an attractive target for novel analgesic therapies.
With regard to drug development, the long-term analgesic effect of TRPV1 agonists has fostered many clinical trials, and all the research and development efforts have culminated with the introduction of the 8% capsaicin dermal patch for long lasting pain relief in some severe neuropathic pain conditions [9, 10]. In parallel, pharmaceutical companies have designed low molecular weight TRPV1 antagonists to avoid the sometimes severe side effects of TRPV1 agonist therapy. Despite several failed trials in this field, many achievements and new developments have occurred in the last couple of years. This review aimed to present the latest developments on TRPV1 agonists and antagonists undergoing clinical trials that were completed or started in the last 5 years. Perspectives and new achievements in the field of TRPV1 agonists are outlined with a special emphasis on capsaicin and its derivatives. Novel findings regarding the TRPV1 antagonists are presented and summarized and some promising recent findings are outlined.
2 The Vanilloid Receptor TRPV1
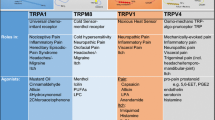
Although the first transient receptor potential channel was described in 1969 [11], it was not until the end of the 1990s that TRPV1 was identified and cloned [8]. The capsaicin receptor TRPV1 is the prototypical nociceptive channel widely expressed in primary afferent neurons of the dorsal root, trigeminal and nodose ganglia (Fig. 1). These are bipolar neurons that are the initial station in the pathway conveying sensory information from the periphery to the somatosensory cortex via the spinal cord (Fig. 1). TRPV1 is a non-selective cation channel with polymodal mechanisms of activation: temperature > 42 °C, low pH < 6.5, osmolarity changes, arachidonic acid metabolites: 5(S) and 12(s) arachidonic acid 5-hydroperoxide (HPETE); endocannabinoids: NADA and OLDA; and voltage sensitivity [1, 8, 12, 13]. The TRPV1 channel is mainly known for its ability of sensing a variety of pungent plant products, the most well-known being capsaicin, the bioactive compound in chilli peppers. Other compounds from this category include: RTX (from the latex of Eucalyptus resinifera), piperine (the pungent ingredient in black pepper), gingerol and zingerone (from ginger), camphor (from the wood of camphor) and eugenol (an essential oil found in cloves). Furthermore, the TRPV1 channel is activated by a variety of painful venoms from jellyfish, spiders, centipedes and scorpions [14,15,16,17].
Following TRPV1 activation, the nociceptors are known to release a variety of neuropeptides [among them substance P and calcitonin gene-related peptide (CGRP)], which activate secondary order neurons in the dorsal horn of the spinal cord, and elicit biochemical cascades at the periphery, known as neurogenic inflammation [1, 4, 18,19,20,21,22,23,24,25,26]. In the context of inflammation, it was also found that TRPV1 channels are activated by various pro-inflammatory agents such as prostaglandins, serotonin (5-HT), bradykinin, activators of the protease-activated receptors (PAR), ATP, nerve growth factor (NGF), histamine, calcitonin-gene-related peptide α (CGRP α), tumor necrosis factor α (TNFα) and granulocyte-colony stimulating factor (G-CSF) [27,28,29,30,31,32,33,34,35,36,37,38,39,40]. These algogenic mediators induce, through their cognate G protein-coupled receptors (GPCR), conformational changes in the TRPV1 channel protein, which elicits an increase in the probability of channel opening by heat, protons and capsaicin [1, 19, 41, 42]. This process of sensitization is an important mechanism leading to nociceptor hyperexcitability that underlies hyperalgesia and allodynia during tissue inflammation [2]. Hence, disrupting the activity or assembly of TRPV1 channels is able to attenuate the development of hyperalgesia [41,42,43]. Nevertheless, various studies, using genetic manipulation of TRPV1, showed only modest changes in pain sensitivity and acute noxious heat sensitivity in healthy conditions, yet highlighted the critical role of TRPV1 in mediating thermal hyperalgesia under inflammatory pain conditions, thus supporting the notion that targeting TRPV1 channels would be an adequate approach for individual living with pathological pain [44,45,46,47]. The use of pharmacological blockers of TRPV1 led to mixed results, depending on the preclinical model used, compared to knockout animal studies. TRPV1 antagonists were able to induce pain relief in various models of nerve injury [4, 48,49,50]. In models of painful diabetic neuropathy (PDN), animals present a biphasic pain behaviour characterized by hyperalgesia in the early phase of the disease followed by hypoalgesia in the later phase [4, 24, 51]. TRPV1-deficient mice show no signs of diabetes-induced heat hypersensitivity [51], while pharmacological inhibition of the channel reduces mechanical allodynia [52]. The underlying mechanism for these findings could be due to TRPV1 sensitization in conditions of hypoxia and hyperglycemia in the early phase [53, 54] or the direct TRPV1 modulation by insulin [55]. Furthermore, the decreased TRPV1 expression observed in the late phase of the disease in both animal models and patients [51, 56,57,58] could be the result of a decrease in insulin production. Finally in cancer- and chemotherapy-induced pain, either pharmacological blockade or genetic manipulation of the channel has proved to be effective [59,60,61,62,63,64].
Altogether, these findings confirmed the central role of TRPV1 as a molecular integrator of noxious stimuli and as an initiator of the neurogenic response. Further insights into the 3D structure of the channel, obtained by cryogenic electron microscopy (CryoEM), and identification of the binding sites for vanilloid compounds, using mutagenesis analysis and computational biology, has shed light on the mechanism of action of TRPV1, and provided key information for designing and developing TRPV1 activators, inhibitors and allosteric modulators for pain management [65,66,67]. Nevertheless, in recent years it became apparent that TRPV1 is expressed in non-nociceptive neurons and other tissues [41, 68], and thus TRPV1 is implicated, besides pain, in multiple physiological and pathophysiological processes such as: thermosensation [17, 69, 70], energy homeostasis [71, 72], cancer [73,74,75,76,77], regulation of diet-induced obesity [78], insulin and leptin resistance [71, 78,79,80,81], development of severe bronchial asthma [82,83,84,85], neuroimmunity [86] and itch [35, 87]. As such, tremendous interest has been shown by researchers and pharmaceutical companies to develop TRPV1 channel modulators not only for pain but also other clinical conditions such as stroke, cancer, dysphagia, diabetes and obesity.
3 Capsaicin: The Versatile Bioactive Compound of Chili Peppers
One of the striking, and perhaps counterintuitive, characteristics of capsaicin is its capacity to be used as a local analgesic. For millennia, this phytochemical has been used for a multitude of purposes, including medicinal [88,89,90]. Currently, topical treatment with low-dose (0.075%) capsaicin creams or high-dose (8%) patches is in use for the treatment of arthritis and skin conditions, muscle pain, neuropathic pain and migraine [9, 10, 91,92,93,94]. A number of pharmaceutical companies market topical capsaicin under trade names such as Menthacin, Zostrix, Zoderm and Capzasin-P [9, 88,89,90]. Furthermore, since 2009 the European Union and the US Food and Drug Administration (FDA) approved the use of the capsaicin 8% patch (Qutenza, NGX-4010, Transacin) for the treatment of postherpetic neuralgia, peripheral neuropathic pain and HIV-associated distal sensory polyneuropathy [88, 89, 95]. Although the mechanism by which capsaicin induces analgesia is not completely understood [88,89,90], it was determined that prolonged (acute or short-term desensitization) or repeated applications of capsaicin, a process named tachyphylaxis, induces a calcium-dependent desensitization of TRPV1, thus rendering the channel insensitive to capsaicin as well as other noxious stimuli [96,97,98,99]. This can be viewed as a neuronal protection mechanism preventing calcium overload during repetitive TRPV1 stimulation [98]. Some insight into understanding the process of desensitization came by establishing that capsaicin binds into a hydrophobic cavity constituted by residues Y512, S513, T551 and E571 in the human TRPV1 sequence [100]. While the first two residues are conserved between species, T551 is different in the rabbit and chicken [101]. This observation was an interesting finding since capsaicin detection is abrogated in birds and the avian channel is resistant to the typical desensitization produced by repeated application of protons [102]. Furthermore, it was shown that an important structural determinant for capsaicin-induced channel desensitization was mediated through an interaction with the calcium-binding protein calmodulin (CaM), at specific sites located at the N- and C-terminal regions of the channel [103,104,105]. In fact, differences in the binding site found in the C-terminal region between human and avian TRPV1 confer resistance to calcium-induced desensitization [102]. This is in concert with the previous findings of there being several possible mechanisms for desensitization, including the one that involved the binding of CaM to TRPV1 [103,104,105,106,107]. Other proposed mechanisms include: (1) dephosphorylation of TRPV1 by calcineurin [108, 109], which correlates with the fact that phosphorylation of TRPV1 by protein kinase C (PKC) and protein kinase A (PKA) reduces calcium-mediated desensitization [109,110,111,112] and (2) calcium-dependent stimulation of phospholipase C (PLC), leading to hydrolysis of PI(4,5)P2, which prevents channel function and thus promotes desensitization [113,114,115,116,117]. Therefore, desensitization of TRPV1 is a very useful feature from a pharmaceutical and clinical point of view. It begins a few hours after the treatment and may last up to several months. This process, named “defunctionalisation” of sensory neurons, includes a loss of membrane potential, membrane transport capabilities and a retraction of epidermal and dermal nerve fiber endings [9]. The downstream effect of the desensitization process is a reprogramming of the gene expression profile leading to the depletion of the pro-inflammatory, pro-algesic neuropeptides, such as substance P, and increased expression of the endogenous analgesic peptides, galanin and somatostatin [118,119,120,121,122].
Due to its multiple beneficial functions [90], new ways of using capsaicin in clinical practice have been explored. However, due to poor water solubility, the bioavailability and efficacy of capsaicin in vivo is limited [90]. Furthermore, systemic administration of capsaicin is not currently used to treat pain in a clinical setting partly because direct oral administration induces irritation of the oral cavity and the stomach leading to stomatitis, orofacial pain and gastric ulcers [123] as well as dangerous body temperature elevation [9, 78, 88, 89, 124]. Accordingly, treating neonatal rats systemically with capsaicin leads to not only lifelong TRPV1 desensitization and reduction in pain perception but also to abnormal body temperature and irregular circadian core body temperature rhythm [125]. To overcome the poor bioavailability and side effects of oral capsaicin delivery, different formulations have been designed, including micelles, lipid vesicles, micro/nano emulsions and nanoparticles [90, 126,127,128,129]. However, at this time most efforts have been concentrated on the development of capsaicin derivatives with no effect on body temperature, along with topical application, which is not hampered by severe systemic effects.
Today, many capsaicin topical compounds are used in clinical practice [9, 10, 88,89,90,91,92] and the interest for the use of capsaicin for the treatment of pain of various etiology, alone or in combination with other medications, has not faded. Besides the few that were completed in the last years, numerous clinical trials are still ongoing or will start in the near future (summarized in Table 1).
3.1 Capsaicin and Neuropathic Pain
In a randomized, double-blind, Phase 3 study (Boehringer Ingelheim) conducted in two countries on 746 patients with back and neck pain that appeared between 1 and 21 days before capsaicin application, Predel et al. reported that using capsaicin gel (0.075%) alone was sufficient to treat acute back and neck pain [130, 131]. Capsaicin alone was shown to significantly improve the pain. The incidence of adverse effects (skin burning sensation, nasopharyngitis, headache and application site pain) was relatively low (26.5%) and the addition of the diclofenac gel increased the adverse effects with no clinical benefit [130, 131]. In a Phase 4 multicenter study (Astellas Pharma), Galvez et al. reported a good efficacy of the capsaicin patch in the long-term treatment of peripheral neuropathic pain [132, 133]. The study tested the capsaicin patch, over a period of 52 weeks, on 305 patients with postherpetic neuralgia, painful HIV-associated neuropathy, post-traumatic or post-surgery nerve injury, and other peripheral neuropathic pain. A sustained reduction in the average daily pain was observed in these patients, which was associated with a minimal occurrence of sensory loss. In fact, the adverse effects were mild to moderate and only 3.6% of patients were unable to continue the treatment. Another ongoing Phase 4 multicenter randomized clinical trial is comparing the efficacy of the capsaicin (8%) and lidocaine patch (5%) in the treatment of localized peripheral pain secondary to herpes, surgery, amputation, radiation therapy and complex regional pain syndrome type 1 [134]. It would be interesting to determine if local capsaicin patches are as efficient as a local anesthetic, while preserving sensory function.
There are currently a number of clinical trials, either ongoing or recruiting, that suggest a real therapeutic interest for topical capsaicin. The vast majority of these trials are assessing the effect of the capsaicin patch (NGX-4010 or Qutenza) or gel in the treatment of neuropathic pain of various etiologies: following anticancer treatments [135, 136], following spinal cord injury in patients who failed other therapeutical approaches [137], and in the treatment of diabetic neuropathy [138]. Finally, another single-blind trial is testing intranasal capsaicin for the treatment of rhinogenic headache [139].
3.2 Capsaicin and Osteoarthritic Pain
Due to the therapeutic benefits of capsaicin formulations for musculoskeletal pain, the American College of Rheumatology/Arthritis recently included topical capsaicin as a therapeutic option in the treatment of knee osteoarthritis [92]. This sparked even more interest in the development of new approaches for capsaicin administration since high concentrations of capsaicin creams caused significant problems both for patients and healthcare providers. A Phase 2 clinical trial comparing the efficacy of 1% (CGS-200-1) versus 5% liquid capsaicin (CGS-200-5) in the treatment of osteoarthritis was started by Vizuri Health Sciences LLC [140, 141]. This was a multicenter, randomized, double-blind, parallel group, vehicle-controlled trial comparing topical CGS-200-1 (1%) or CGS-200-5 (5%) versus CGS-200-0 (no capsaicin) in 122 randomized subjects who had osteoarthritis of at least one knee, according to 1986 ACR criteria, and a Western Ontario and McMaster Osteoarthritis Index (WOMAC) pain score of ≥ 250. Capsaicin treatment was applied for 1 h to both knees on four consecutive days. The study showed that CGS-200-5, but not CGS-200-1, was well tolerated and had a durable effect (more than 60 days). The side effects noted, including application site pain, were only mild to moderate. The same company recently completed a Phase 4 clinical trial validating the efficacy and safety of liquid capsaicin (0.25%) administered once or twice daily in the treatment of knee osteoarthritis [142]. The drug is marketed as an over-the-counter topical analgesic under the name PainBloc24. However, there are unsuccessful clinical studies that were terminated prematurely such as a Phase 3 clinical trial assessing Qutenza in knee osteoarthritis of patients with obesity [143].
4 Synthetic Isomers and Precursors of Capsaicin
To avoid the, sometimes severe, side effects of capsaicin, synthetic isomers of capsaicin, with lower pungency, have also been produced. Cis-capsaicin (cis-8-methyl-N-vanillyl-6-nonenamide) (Zucapsaicin, Civanex, Civamide) was tested for neuropathic pain associated with herpes simplex infections, cluster headaches, migraine and also knee osteoarthritis [144,145,146]. In 2010, under the names Zuacta (Sanofi-Aventis) or Civanex (Winston Labs), cis-capsaicin was approved by Health Canada as a topical cream (0.075%) for the treatment of osteoarthritis. The drug is not FDA approved and it is still undergoing clinical trials. Civamide, in the form of an intranasal spray (0.01%), has been clinically studied for the treatment of postherpetic neuralgia [147]. Despite being a multicenter trial, the study had only 11 subjects and showed moderate to severe side effects that led to early termination of the trial. This was followed by another Phase 2 clinical trial designed to evaluate the safety and efficacy of a topically administered Civamide patch (Winston Laboratories) for the treatment of moderate to severe daily pain associated with (1) postherpetic neuralgia localized to the trunk, and (2) post-incisional neuropathic pain syndromes of the trunk [148]. Although this study has been completed, the results were not available. Intranasal Civamide for the treatment of cluster headaches has also been evaluated, with modest results [146]. Currently, the same approach is undergoing a Phase 3 double-blind, multicenter study employing 180 patients [149]. Another highly purified capsaicin isomer, trans-capsaicin, has been developed by Centrexion Therapeutics, this time in injectable form. This isomer, named CNTX-4975, was recently tested in the treatment of moderate to severe pain associated with osteoarthritis of the knee, in a Phase 1 followed by a Phase 3 clinical trial [150,151,152,153,154]. CNTX-4975 was administered by single intra-articular injection and because of its short half-life (less than 4 h), was expected to achieve a long-term analgesic effect with few side effects. This randomized, multicenter, double-blind study employed 172 patients with stable knee osteoarthritis and showed that a single intra-articular injection decreased pain scores at 12 and 24 weeks versus placebo [153]. This effect was dose dependent since injection of 1.0 mg of CNTX-4975 produced a decrease in osteoarthritis knee pain over 24 weeks while 0.5 mg improved pain, up to 12 weeks only. In addition, nearly one in four patients achieved complete pain relief. The administration of the drug was accompanied by cooling of the knee joint before and after. Only mild to moderate adverse effects were reported by 30%, 47% and 30% of patients in the placebo, CNTX-4975 0.5 mg and CNTX-4975 1.0 mg groups, respectively. The adverse effects included erythema, peripheral edema, nausea, oral hypoesthesia, hypotension and increased hepatic enzymes, but none of the patients had to drop out of the study. Although the results are promising, as the authors and others pointed out [153, 155], this was a small randomized study in a specific population of patients with moderate to severe osteoarthritis knee pain. Therefore, the findings cannot be generalized to the large population of individuals with knee osteoarthritis, and the data regarding the safety profile are limited.
Another approach has been to develop capsaicin precursors that would provide therapeutic benefits without the severe side effects of capsaicin. Such a compound has been developed by Concentric Analgesics and dubbed CA-008 [156]. This is a water-soluble prodrug that rapidly converts to capsaicin. After going through a series of clinical trials, CA-008 gained Breakthrough Therapy designation in 2018 from the FDA for the treatment of post-surgical pain [157]. The company announced good results in one of their Phase 2 study to control postsurgical pain in bunionectomy patients [157, 158]. The study included 144 patients who underwent bunionectomy at three sites across the USA. The patients were randomized to receive one of the three CA-008 doses or placebo. CA-008 (0.7, 2.1 or 4.2 mg) was infiltrated/instilled in the wound prior to closing the suture and the patients were followed for 29 days. The CA-008 4.2 mg dose group showed significant pain reduction (33%) versus placebo. Another important element was the reduction in opioid consumption by 50% versus placebo, while 26% of subjects required no opioid administration in the first 96 h post-surgery. Additionally, 32% of subjects in the (CA-008) 4.2 mg group compared to 5% in the placebo group were able to discontinue all opioids after 4 h. In terms of safety, 72.1% of subjects reported mild to moderate side effects. The most frequent were burning sensation (2), headache (4), polyuria (3), increased alanine transaminase (ALT) and aspartate transaminase (AST) (2), hypoesthesia and nausea, yet no major side effects caused early termination and no deaths resulted from the study. Of note, there were no relevant differences across CA-008 doses or placebo for wound healing, X-ray exams or neurosensory assessments. Additional studies testing CA-008 for postsurgical pain management are ongoing, including total knee arthroplasty, correction of hallux valgus deformity or complete abdominoplasty [159,160,161,162,163,164].
5 Resiniferatoxin and “Molecular Neurosurgery”
Resiniferatoxin (RTX) is a chemical found in the Euphorbia resinifera plant and is the most potent agonist of TRPV1. RTX binds with nanomolar affinity to TRPV1, causing prolonged channel opening and massive increase in intracellular calcium. This calcium increase has cytotoxic effect on the nociceptors, thus leading to rapid retraction and defunctionalisation of the pain fibers [165,166,167] (Fig. 1). An important observation regarding the RTX mode of action was the fact that RTX-induced defunctionalisation was not observed in the absence of TRPV1 [30], even at concentrations 1,000× higher than normally used. Additionally, cells that do not express TRPV1 remain intact even when adjacent to apoptotic TRPV1+ neurons exposed to RTX [168]. Preclinical data obtained in rodents showed a long-lasting, fully reversible desensitization of the neurogenic inflammatory pathway following one subcutaneous injection of RTX [169]. Both intrathecal and intraganglionic RTX administration leads to the selective targeting and permanent deletion of the TRPV1-expressing Aδ and C-fiber cell bodies in the DRG and trigeminal ganglia [166, 170,171,172,173]. As neurons in sensory ganglia collect noxious information from precisely delimited anatomical areas, called dermatomes, the idea to use RTX as a “molecular scalpel” for the treatment of highly localized pain became very attractive [174, 175]. However, the main caveat was that in order to avoid any undesirable effects, RTX had to be applied close to the site of action, on the cell bodies of sensory neurons or their axons (i.e. intrathecally or intraganglionically), or at the peripheral site of injury where pain is generated [174, 175].
Several animal models of cancer and inflammatory pain have been used to show the long-lasting analgesic efficacy of intrathecal or intraganglionic infusion of RTX [171, 172, 175,176,177,178,179], without affecting motor activity, coordination or mechanosensitivity. However, most studies currently agree on a localized route of injection of RTX since systemic administration can generate irreversible changes in the peripheral nervous system [174, 175]. Overall, complete removal of TRPV1 nociceptors using RTX is considered a treatment option for chronic, incurable conditions such as cancer pain, chronic phantom pain or diabetic neuropathy [175, 180].
Recently, intravesical RTX administration was also found to decrease the incidence and severity of catheter-related bladder discomfort in patients undergoing transurethral resection of the prostate, without any significant side effects [181]. In addition, the RTX efficacy in ablating pain secondary to canine osteosarcoma [176] raised the hopes of this treatment in human patients since human bone cancer is pathologically similar to canine osteosarcoma. These results led to a Phase 1 clinical trial with periganglionic RTX administration in human cancer patients [182] (Table 2). The first two human patients with intractable cervical cancer pain were enrolled into this trial at the National Cancer Institute in October 2009 [183]. Both patients reported sustained improvement in the original pain symptoms after RTX administration. So far, a total of ten patients have received intrathecal RTX in this ongoing study, and all have showed long-lasting analgesia with no significant adverse effects [184].
In pain syndromes affecting multiple dermatomes, RTX may be administered intrathecally [175, 177]. Currently, two clinical trials for cancer pain management using the intrathecal route of administration are ongoing. The first is a Phase 1, single-site, open-label, dose-escalation study to determine the safety and efficacy of intrathecal administration of RTX in patients with severe refractory pain due to advanced malignancy [185]. The study continues to recruit patients and the goal is to reach 45 participants. Another Phase 1b, multicenter study that explores the epidural route of administration of RTX for intractable cancer-induced pain is sponsored by Sorento Therapeutics [186]. In February 2020, Sorrento Therapeutics announced that an interim analysis of this study has generated positive results [187]. Data were available from 14 patients and showed that the lower doses used (0.4, 1, 2 and 4 µg) were ineffective and only three patients who received 8 and 15 µg of RTX showed significant decrease of pain 24 h after administration. The effect lasted for the entire period the patients were observed (weeks) and was accompanied by improvement in physical strength, mood and appetite. There were no dose-limiting toxicities, but one patient had increased blood pressure. The most common treatment-related adverse effect was transient and moderate post-procedural pain (50%). All these effects resolved in less than 2 days following drug administration. Further to this study, patients will be tested for doses up to 25 µg and Sorrento Therapeutics will initiate a Phase 3 study for epidural RTX administration in the treatment of intractable pain in advanced diseases. The results were presented by Nedeljkovic et al. at the American Academy of Pain Medicine Annual Meeting on 27 February 2020 [188]. Knowing the analgesic effects of capsaicin in osteoarthritic pain, it was expected that RTX, injected into the knee joint, would have similar beneficial effects at much lower concentrations than capsaicin. Indeed, preclinical data showed a long-lasting reduction in pain and inflammation, following intra-articular RTX administration in animal models [189,190,191]. The first clinical study of intra-articular RTX for osteoarthritic pain was initiated by Mestex AG and the first patient was treated in 2016 [192]. This was an open-label, dose-escalating Phase 1/2a study to determine the safety and clinical effects of a single or repeated intra-articular injections of Lopain (MTX-071) in patients with chronic osteoarthritic knee joint pain. No results have been published so far but, according to Mestex AG, as of October 2019 more than 60 patients with marked chronic knee pain have received Lopain in GCP-compliant clinical trials. After a single intra-articular administration, the joint pain was abolished for at least 6 months in most patients and, in some cases, for more than 12 months. Lopain was well tolerated and without any safety concerns. The company has initiated a randomized, double-blind, placebo-controlled Phase 2a [193] followed by a Phase 2b trial in 2019 [194]. Sorrento Therapeutics has also several Phase 1 and a Phase 3 clinical trials to study RTX for knee pain [195,196,197]. In 2019 the company announced good results for its Phase 1 trial [198]. Post administration of RTX, the patients showed fast relief (in less than a week), which lasted for the duration of the trial (84 days). There were no dose-limiting toxicities (doses between 5 and 30 µg) or any adverse events of interest noted. Five patients agreed to be followed further and showed no or very low levels of pain at day 168, supporting the long-term effect of RTX. These results will be expanded by two multicentre, double-blind Phase 3 trials, each planning to use 400 patients.
Other clinical applications for RTX treatment could be found in the future, including pain associated with thermal injuries [199] or migraine [88].
6 TRPV1 Antagonists
The findings that TRPV1 null mice showed attenuated thermal hyperalgesia after inflammation have triggered tremendous interest in developing TRPV1 antagonists with analgesic properties [45, 200]. In less than 20 years, a large array of potent and selective small molecules were identified and expected to provide a new generation of non-opioid analgesics [201,202,203]. Capsazepine was the first competitive TRPV1 antagonist described [88, 204]. Although a capsaicin derivative, capsazepine had poor selectivity for TRPV1, and was found to block nicotinic receptors, voltage-gated calcium channels and TRPM8, thus making it a poor clinical candidate [204, 205]. This first effort was followed by the development of other, more potent and selective TRPV1 antagonists. Many of these compounds showed promise in preclinical pain models; for example, BCTC (Pardue Pharma), A-784168 and A-425619 (Abbott) and GRC-6211 (Glenmark) were able to reverse pain in animal models of inflammatory and neuropathic pain; ABT-102 and ABT-116 (Abbott) and JNJ-39729209 (Janssen) proved to be efficacious in reversing bone cancer pain, postoperative and chronic inflammatory pain; A-995662 exhibited anti-pain effects in a model of rat knee joint pain; and AS1928370 and AS1725195 (Astellas) potently improved mechanical allodynia in neuropathic pain [201,202,203, 206,207,208,209,210,211].
As aforementioned, systemic administration of TRPV1 agonists was found to induce transient hypothermia [41, 89, 212]. Reciprocally, it became clear that numerous preclinical studies and, later on, clinical trials noted the appearance of hyperthermia (febrile reaction) with TRPV1 antagonist [124, 212]. The reported hyperthermic effect was variable depending on the compound. For instance, AZD-1386 (AstraZeneca) induced only a modest increase in body temperature and the effect disappeared upon repeated administration [124, 213], whereas AMG517 (Amgen) induced a marked (up to 40.2 °C) and long-lasting (1–4 days) effect, which ended in terminating a Phase 1b dental pain study [124]. The mechanism by which TRPV1 channel antagonists elevate the body temperature is still not clear, particularly because some antagonists can induce hypothermia [214]. This phenomenon raises an interesting question. Since all antagonists act on a single target, TRPV1, how do they induce opposite effects? From a pharmacological point of view, it would suggest that antagonists have multiple targets. The most simple model envisioned is that the predominant function of TRPV1 is body temperature regulation [215, 216]. It was postulated that TRPV1 has an endogenous tone that is important for the maintenance of normal body temperature [124, 212, 217]. If this tone is increased (e.g. by administering a TRPV1 agonist such as capsaicin), the core temperature starts dropping, whereas decreasing the tone (i.e. by the use of antagonists) leads to hyperthermia. This model cannot explain the wide range of hyperthermic and hypothermic effects of the various antagonists, and even is inconsistent with some experimental findings. It is interesting that although the target that mediates the effect of capsaicin on body temperature regulation was localized to the brain by microinjection studies [89, 218], both the blood-brain barrier crossing and not crossing TRPV1 channel antagonists caused a hyperthermic response [212, 219]. This led to the alternate explanation that TRPV1 channels involved in body temperature regulation are present in the viscera, probably in the gastrointestinal tract [124, 220]. This theory was, however, short lived since neither intra-abdominal administration of RTX nor vagotomy or transection of the greater splanchnic nerves were able to abolish the hyperthermia induced by TRPV1 channel antagonists [212]. Furthermore, the TRPV1 null mice have normal body temperature, although their circadian rhythm is disrupted [221] and the rats whose TRPV1-expressing neurons have been ablated by a high dose of neonatal capsaicin administration show abnormal body temperature [125]. Another phenomenon that argues against this hypothesis is the fact that administration of intra-abdominal RTX in adult mice is unable to prevent the antagonist-induced hyperthermia [212, 218].
Another deleterious adverse effect of TRPV1 channel antagonists is a long-lasting alteration of the noxious heat sensation that could lead to burn injuries. This could have been expected since the TRPV1 null mice are deficient in acute heat pain sensation, and RTX desensitization markedly increases the temperature required to evoke a nocifensive response [88, 89, 177, 212]. This effect also led to many clinical trials being discontinued. For instance, AZD1386 (AstraZeneca) was dropped after observation of elevated temperature and loss of heat pain perception during the Phase 2 trial [222]. MK-2295 (Merck/Neurogen) markedly increased the noxious heat pain threshold, and put the study participants at risk of scalding injury [223]. These results triggered the search for second-generation, “cleaner” antagonists that are not hampered by such deleterious side effects [88, 89]. Currently, efforts are made to separate the analgesic and hyperthermic effects of the TRPV1 blockers. One approach could be the development of soft short-lived capsaicin-derived agonists and antagonists [224]. Application of such antagonists in a mouse model of CFA-induced inflammation induced pain and itch relief but no changes in thermal nociception or hyperthermia [224]. Another way could be to specifically target one of the three modalities of channel activation (capsaicin, low pH and heat). Second-generation TRPV1 channel antagonists that do not interfere with proton activation of the receptor (modality-specific antagonists) do not induce hyperthermia in rats while preserving the analgesic activity [211, 225]. However, their action seems to be species specific. For instance JYL1421 had a good analgesic effect, without being accompanied by hyperthermia, in the rat [226], but increased the temperature when used in dogs and monkeys [217]. This raises the question if all the compounds developed using animal models will be able to translate to clinical trials.
SB-705489 (GlaxoSmithKline) was the first selective TRPV1 antagonist to enter clinical studies [227] (Table 3). In single-dose placebo-controlled Phase 1 studies, SB-705489 at 400 mg elevated heat pain thresholds in normal skin (NCT01673529) and reduced capsaicin-evoked rhinitis symptoms (NCT00731250). No hyperthermia or hypothermia adverse events were reported. A Phase 2 dental pain trial was completed [228] followed by a Phase 2 rectal pain trial [229]. The results have been published on the trial web page and show a similar trend like the Phase 1 study: pain reduction with mild and moderate side effects, and no hyperthermia.
Another such compound is NEO06860 developed by Neomed for osteoarthritic pain [230,231,232,233,234] (Table 3). While retaining a good analgesic action, this compound had only a small effect on the body core temperature and heat pain thresholds in the study subjects [230,231,232]. Several Phase 1 clinical studies with Mavatrep (JNJ-39439335) (Janssen Research & Development) have been completed both in healthy volunteers (NCT01454245, NCT01631487) and patients with osteoarthritis (NCT01343303, NCT00933582). Unfortunately, because of the observed safety issues, the efficacy of only a few TRPV1 antagonists has been reported. Mavatrep was no exception and although most of these trials ended almost a decade ago [235,236,237,238,239] the results have been only recently published [240,241,242,243]. The drug was well tolerated in healthy volunteers and none showed an increase in temperature above 38.5 °C following administration of a single 500 mg dose [240, 242, 243]. Mild oral and skin burn injuries were treated in 9% of the osteoarthritis patients taking mavatrep (JNJ-39439335) and there were no discontinuations. The primary goal of the study, to decrease pain, has been met [241]. Another oral TRPV1 antagonist that has been tested is DWP05195 (Daewoong Pharmaceutical). It underwent single- and multiple-dose Phase 1 trials [244,245,246] that showed a dose-dependent skin temperature increase. The drug showed a potent and dose-dependent decrease in pain, which allowed Daewoong Pharmaceutical to proceed to a, still ongoing, Phase 2 double-blind placebo-control clinical trial to evaluate the efficacy and safety of DWP05195 in the treatment of postherpetic neuralgia [247].
Another TRPV1 antagonist developed by Purdue Pharma was already tested on healthy volunteers [248,249,250]. V116517 compound could efficiently block capsaicin-induced irritation and inflammation-mediated thermal hyperalgesia, without affecting body temperature. One issue with this compound was the heat pain threshold of the subjects that increased to 50 °C on average and the tolerance to noxious temperature of 52 °C, which is potentially harmful. Nevertheless, two Phase 2 clinical trials have been started to explore the efficacy and safety of V116517 in patients with severe chronic pain associated with postherpetic neuralgia [251] or knee osteoarthritis [252].
A more recent Novartis compound was used in a Phase 2 clinical trial. The SAF312 compound had been tested for managing postoperative dental pain [253] as well as ocular pain associated with corneal epithelial defects such as photorefractive keratectomy surgery [254]. SAF312 showed a good dose effect for reducing dental pain, although a third of the patients developed hyperthermia above 38.5 °C at concentrations above 25 mg [255]. There were no serious side effects or discontinued treatment resulting from the SAF312 clinical trial. In human subjects with ocular pain, SAF312 showed efficacy in decreasing the severity of pain immediately after surgery [256], although one patient developed transient pyrexia.
With the discovery of microRNAs in 1993 [257] and the first gene silencing by RNAi in 1998 [258], a new era of genome editing or silencing emerged. In preclinical studies, mice treated with TRPV1-targeted siRNA had a phenotype similar to the one observed in the TRPV1 knockout mice [46, 47]. One such compound, Tivanisiran (SYL1001), has been developed by Sylentis and underwent both Phase 1 and Phase 2 clinical trials [259,260,261,262] followed by a Phase 3 HELIX trial for dry eye disease [263, 264]. The results of the Phase 1 and 2 trials indicated that Tivanisiran at 1.125% was well tolerated and able to reduce hyperemia and ocular pain/discomfort (visual analogue scale (VAS)) after 10 days of treatment. The Phase 3 study was a randomized, multicenter (six countries) study that enrolled 330 patients who received Tivanisiran or artificial tears over 4 weeks. The data, however, indicated that Tivanisiran was not better than artificial tears at controlling ocular pain. However, Sylentis disclosed other, more positive aspects of the data, starting with a statistically significant improvement in central corneal staining, a measure of cell damage and improving ocular pain symptoms, which were considered secondary endpoints. If the performance against the secondary endpoints is reproducible, it would suggest that Tivanisiran is effective at improving one part but not all of the cornea. The drug’s inability to perform better than artificial tears in those areas overshadows its performance against baseline, though, and the company announced it would be discussing the finding and other data points with the FDA before deciding on a path forward. Nevertheless, these findings open the possibility of using a similar approach for treating pain of other etiologies.
7 Conclusions
Pain represents a major clinical challenge due to the limited efficacy and side effects of available treatments. Drug combinations and surgical therapies are often required to provide pain relief in affected individuals. The discovery of the nociceptive TRPV1 receptor in primary afferent neurons that carry painful signals was a major breakthrough. Identification of several endogenous and exogenous ligands of the channel has offered novel therapeutic options for pain management. This was first validated by the use of capsaicin and other TRPV1 agonists in several neuropathic and musculoskeletal pain conditions. Topical capsaicin creams or high-dose capsaicin-containing patches, illustrated by the capsaicin 8% transdermal patch, have been approved as effective adjuvant therapy for the insistent pain of post-herpetic neuralgia and in the treatment of osteoarthritis. Topical capsaicin utilized as a single therapy or in conjunction with other analgesics offers a low-risk choice for patients who do not achieve pain control using other regimens and significantly improve their quality of life. Capsaicin injectable or in suspension formulations have been introduced in clinical trials with the hope of improving delivery and thus decreasing side effects. So far systemic administration was not an option given the adverse effects of capsaicin on blood pressure, breathing and other reflex pathways, but new ways of improving oral capsaicin delivery and decreasing its pungency are being tested [90]. The design and development of capsaicin analogues obtained naturally or synthetically is moving forward [78, 265, 266], and it is likely that, in the near future, we will see a wide range of injectable and topical TRPV1 agonist-based agents that will provide long-lasting analgesia with rapid onset after a single administration. The use of RTX as a “molecular scalpel” and therapies based on capsaicin and other capsaicin-derived TRPV1 agonists with non-pungent properties [265] may also soon be available in diseases associated with acute and/or chronic pain, such as cancer, thermal injuries or migraines. The future challenges to be addressed will be to determine the effective dose levels of capsaicin analogs and developing more efficient modes of administration.
On the other hand, the development of TRPV1 antagonists was marred by numerous setbacks related to hyperthermia or a decrease in noxious heat detection, which led to many roadblocks in the development of these compounds. The second generation of TRPV1 antagonists seem to show less propensity to cause these side effects.
Finally, alternative approaches could emerge in the future: (1) Targeting the expression of the TRPV1 channel using genome-editing tools. This approach could block certain populations of neurons and thus be much better tolerated. (2) Developing drugs that prevent inflammation-driven post-translational modification of the TRPV1 (i.e. phosphorylation, protein interactions, ubiquitination, etc.). In fact, blocking PKC-induced TRPV1 phosphorylation by site-directed mutagenesis has been shown to be effective in preclinical models [267]. This could be a means to prevent pathological hypersensitivity while preserving the thermosensitive function of the channel. (3) Modality-specific antagonists that cause less side effects must be further developed. In summary, a better comprehension of the biological role of the channel, the molecular chaperones that regulate its function, or the signaling effectors engaged upon activation will help to design and develop TRPV1-targeted drugs with a better therapeutic window.
References
Bourinet E, Altier C, Hildebrand ME, Trang T, Salter MW, Zamponi GW. Calcium-permeable ion channels in pain signaling. Physiol Rev. 2014;94(1):81–140 (2014/01/03).
Lapointe TK, Basso L, Iftinca MC, Flynn R, Chapman K, Dietrich G, et al. TRPV1 sensitization mediates postinflammatory visceral pain following acute colitis. Am J Physiol Gastrointest Liver Physiol. 2015;309(2):G87-99 (2015/05/30).
Domenichiello AF, Ramsden CE. The silent epidemic of chronic pain in older adults. Prog Neuro-psychopharmacol Biol Psychiatry. 2019;93:284–90 (6538291Epub 2019/04/21).
Basso L, Altier C. Transient receptor potential channels in neuropathic pain. Curr Opin Pharmacol. 2017;32:9–15 (2016/11/12).
Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32 (2768555Epub 2009/04/30).
Offiah I, McMahon SB, O’Reilly BA. Interstitial cystitis/bladder pain syndrome: diagnosis and management. Int Urogynecol J. 2013;24(8):1243–56 (2013/02/23).
Harbaugh CM, Suwanabol PA. Optimizing pain control during the opioid epidemic. Surg Clin N Am. 2019;99(5):867–83 (2019/08/27).
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–24 (1997/12/31 23:16).
Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth. 2011;107(4):490–502 (3169333Epub 2011/08/20).
Baranidharan G, Das S, Bhaskar A. A review of the high-concentration capsaicin patch and experience in its use in the management of neuropathic pain. Ther Adv Neurol Disord. 2013;6(5):287–97 (3755533Epub 2013/09/03).
Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224(5216):285–7 (1969/10/18).
Chavez AE, Chiu CQ, Castillo PE. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat Neurosci. 2010;13(12):1511–8 (3058928Epub 2010/11/16).
Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21(3):531–43 (1998/10/13).
Cuypers E, Yanagihara A, Karlsson E, Tytgat J. Jellyfish and other cnidarian envenomations cause pain by affecting TRPV1 channels. FEBS Lett. 2006;580(24):5728–32 (1800888Epub 2006/10/03).
Hakim MA, Jiang W, Luo L, Li B, Yang S, Song Y, et al. Scorpion toxin, BmP01, induces pain by targeting TRPV1 channel. Toxins. 2015;7(9):3671–87 (4591660Epub 2015/09/22).
Siemens J, Zhou S, Piskorowski R, Nikai T, Lumpkin EA, Basbaum AI, et al. Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature. 2006;444(7116):208–12 (2006/11/10).
Yang S, Yang F, Wei N, Hong J, Li B, Luo L, et al. A pain-inducing centipede toxin targets the heat activation machinery of nociceptor TRPV1. Nat Commun. 2015;6:8297 (4589873Epub 2015/10/01).
Boillat A, Alijevic O, Kellenberger S. Calcium entry via TRPV1 but not ASICs induces neuropeptide release from sensory neurons. Mol Cell Neurosci. 2014;61:13–22 (2014/05/06).
Gouin O, L’Herondelle K, Lebonvallet N, Le Gall-Ianotto C, Sakka M, Buhe V, et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: pro-inflammatory response induced by their activation and their sensitization. Protein Cell. 2017;8(9):644–61 (5563280Epub 2017/04/02).
Barnes PJ. Neurogenic inflammation and asthma. J Asthma. 1992;29(3):165–80 (1992/01/01).
Butler CA, Heaney LG. Neurogenic inflammation and asthma. Inflamm Allergy Drug Targets. 2007;6(2):127–32 (2007/08/19).
Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci. 2012;15(8):1063–7 (3520068Epub 2012/07/28).
Levine JD, Moskowitz MA, Basbaum AI. The contribution of neurogenic inflammation in experimental arthritis. J Immunol. 1985;135(2 Suppl):843s-s847 (1985/08/01).
Pabbidi MR, Premkumar LS. Role of transient receptor potential channels Trpv1 and Trpm8 in diabetic peripheral neuropathy. J Diabetes Treat. 2017;2017(4):29 (6317870Epub 2017/01/01).
Ramachandran R. Neurogenic inflammation and its role in migraine. Semin Immunopathol. 2018;40(3):301–14 (2018/03/24).
Schaper NC, Huijberts M, Pickwell K. Neurovascular control and neurogenic inflammation in diabetes. Diabetes Metab Res Rev. 2008;24(Suppl 1):S40–4 (2008/04/30).
Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, et al. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice. J Physiol. 2006;575(Pt 2):555–71 (1819458Epub 2006/06/24).
Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, et al. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci. 2004;24(18):4300–12 (6729438Epub 2004/05/07).
Asfaha S, Cenac N, Houle S, Altier C, Papez MD, Nguyen C, et al. Protease-activated receptor-4: a novel mechanism of inflammatory pain modulation. Br J Pharmacol. 2007;150(2):176–85 (2042908Epub 2006/12/21).
Cernit V, Senecal J, Othman R, Couture R. Reciprocal regulatory interaction between TRPV1 and Kinin B1 receptor in a rat neuropathic pain model. Int J Mol Sci. 2020;21(3):821 (7037982Epub 2020/02/06).
Devesa I, Ferrandiz-Huertas C, Mathivanan S, Wolf C, Lujan R, Changeux JP, et al. AlphaCGRP is essential for algesic exocytotic mobilization of TRPV1 channels in peptidergic nociceptors. Proc Natl Acad Sci USA. 2014;111(51):18345–50 (4280602Epub 2014/12/10).
Efendiev R, Bavencoffe A, Hu H, Zhu MX, Dessauer CW. Scaffolding by A-kinase anchoring protein enhances functional coupling between adenylyl cyclase and TRPV1 channel. J Biol Chem. 2013;288(6):3929–37 (3567646Epub 2012/12/25).
Eskander MA, Ruparel S, Green DP, Chen PB, Por ED, Jeske NA, et al. Persistent nociception triggered by nerve growth factor (NGF) is mediated by TRPV1 and oxidative mechanisms. J Neurosci. 2015;35(22):8593–603 (4452557Epub 2015/06/05).
Fu Q, Cheng J, Gao Y, Zhang Y, Chen X, Xie J. Protease-activated receptor 4: a critical participator in inflammatory response. Inflammation. 2015;38(2):886–95 (2014/08/15).
Shim WS, Tak MH, Lee MH, Kim M, Koo JY, Lee CH, et al. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27(9):2331–7 (6673467Epub 2007/03/03).
Sugiura T, Tominaga M, Katsuya H, Mizumura K. Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J Neurophysiol. 2002;88(1):544–8 (2002/07/02).
Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA. 2001;98(12):6951–6 (34459Epub 2001/05/24).
Wang Y, Feng C, He H, He J, Wang J, Li X, et al. Sensitization of TRPV1 receptors by TNF-alpha orchestrates the development of vincristine-induced pain. Oncol Lett. 2018;15(4):5013–9 (5840530Epub 2018/03/20).
Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24(24):4211–23 (1356334Epub 2005/12/02).
Basso L, Lapointe TK, Iftinca M, Marsters C, Hollenberg MD, Kurrasch DM, et al. Granulocyte-colony-stimulating factor (G-CSF) signaling in spinal microglia drives visceral sensitization following colitis. Proc Natl Acad Sci USA. 2017;114(42):11235–40 (5651747Epub 2017/10/05).
Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6(5):357–72 (2007/04/28).
Vay L, Gu C, McNaughton PA. The thermo-TRP ion channel family: properties and therapeutic implications. Br J Pharmacol. 2012;165(4):787–801 (3312478Epub 2011/07/30).
Flynn R, Chapman K, Iftinca M, Aboushousha R, Varela D, Altier C. Targeting the transient receptor potential vanilloid type 1 (TRPV1) assembly domain attenuates inflammation-induced hypersensitivity. J Biol Chem. 2014;289(24):16675–87 (4059113Epub 2014/05/09).
Bolcskei K, Helyes Z, Szabo A, Sandor K, Elekes K, Nemeth J, et al. Investigation of the role of TRPV1 receptors in acute and chronic nociceptive processes using gene-deficient mice. Pain. 2005;117(3):368–76 (2005/09/10).
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–13 (2000/04/15).
Christoph T, Bahrenberg G, De Vry J, Englberger W, Erdmann VA, Frech M, et al. Investigation of TRPV1 loss-of-function phenotypes in transgenic shRNA expressing and knockout mice. Mol Cell Neurosci. 2008;37(3):579–89 (2008/02/06).
Kasama S, Kawakubo M, Suzuki T, Nishizawa T, Ishida A, Nakayama J. RNA interference-mediated knock-down of transient receptor potential vanilloid 1 prevents forepaw inflammatory hyperalgesia in rat. Eur J Neurosci. 2007;25(10):2956–63 (2007/05/19).
Lee JY, Shin TJ, Choi JM, Seo KS, Kim HJ, Yoon TG, et al. Antinociceptive curcuminoid, KMS4034, effects on inflammatory and neuropathic pain likely via modulating TRPV1 in mice. Br J Anaesth. 2013;111(4):667–72 (2013/05/31).
Salat K, Filipek B. Antinociceptive activity of transient receptor potential channel TRPV1, TRPA1, and TRPM8 antagonists in neurogenic and neuropathic pain models in mice. J Zhejiang Univ Sci B. 2015;16(3):167–78 (4357366Epub 2015/03/07).
Yamamoto W, Sugiura A, Nakazato-Imasato E, Kita Y. Characterization of primary sensory neurons mediating static and dynamic allodynia in rat chronic constriction injury model. J Pharm Pharmacol. 2008;60(6):717–22 (2008/05/24).
Pabbidi RM, Yu SQ, Peng S, Khardori R, Pauza ME, Premkumar LS. Influence of TRPV1 on diabetes-induced alterations in thermal pain sensitivity. Mol Pain. 2008;4:9 (2275252Epub 2008/03/04).
Cui YY, Xu H, Wu HH, Qi J, Shi J, Li YQ. Spatio-temporal expression and functional involvement of transient receptor potential vanilloid 1 in diabetic mechanical allodynia in rats. PLoS ONE. 2014;9(7):e102052 (4096595Epub 2014/07/16).
Ristoiu V, Shibasaki K, Uchida K, Zhou Y, Ton BH, Flonta ML, et al. Hypoxia-induced sensitization of transient receptor potential vanilloid 1 involves activation of hypoxia-inducible factor-1 alpha and PKC. Pain. 2011;152(4):936–45 (2011/03/08).
Kahya MC, Naziroglu M, Ovey IS. Modulation of diabetes-induced oxidative stress, apoptosis, and Ca(2+) entry through TRPM2 and TRPV1 channels in dorsal root ganglion and hippocampus of diabetic rats by melatonin and selenium. Mol Neurobiol. 2017;54(3):2345–60 (2016/03/10).
Van Buren JJ, Bhat S, Rotello R, Pauza ME, Premkumar LS. Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol Pain. 2005;1:17 (1142339Epub 2005/04/29).
Facer P, Casula MA, Smith GD, Benham CD, Chessell IP, Bountra C, et al. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 2007;7:11 (1892784Epub 2007/05/25).
Khomula EV, Viatchenko-Karpinski VY, Borisyuk AL, Duzhyy DE, Belan PV, Voitenko NV. Specific functioning of Cav3.2 T-type calcium and TRPV1 channels under different types of STZ-diabetic neuropathy. Biochim Biophys Acta. 2013;1832(5):636–49 (2013/02/05).
Narayanaswamy H, Facer P, Misra VP, Timmers M, Byttebier G, Meert T, et al. A longitudinal study of sensory biomarkers of progression in patients with diabetic peripheral neuropathy using skin biopsies. J Clin Neurosci. 2012;19(11):1490–6 (2012/06/19).
Chen Y, Yang C, Wang ZJ. Proteinase-activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxel-induced neuropathic pain. Neuroscience. 2011;193:440–51 (2011/07/19).
Ghilardi JR, Rohrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci. 2005;25(12):3126–31 (6725088Epub 2005/03/25).
Menendez L, Juarez L, Garcia E, Garcia-Suarez O, Hidalgo A, Baamonde A. Analgesic effects of capsazepine and resiniferatoxin on bone cancer pain in mice. Neurosci Lett. 2006;393(1):70–3 (2005/10/26).
Niiyama Y, Kawamata T, Yamamoto J, Furuse S, Namiki A. SB366791, a TRPV1 antagonist, potentiates analgesic effects of systemic morphine in a murine model of bone cancer pain. Br J Anaesth. 2009;102(2):251–8 (2008/11/29).
Shinoda M, Ogino A, Ozaki N, Urano H, Hironaka K, Yasui M, et al. Involvement of TRPV1 in nociceptive behavior in a rat model of cancer pain. J Pain. 2008;9(8):687–99 (2008/05/06).
Ta LE, Bieber AJ, Carlton SM, Loprinzi CL, Low PA, Windebank AJ. Transient receptor potential vanilloid 1 is essential for cisplatin-induced heat hyperalgesia in mice. Mol Pain. 2010;6:15 (2848188Epub 2010/03/09).
Gao Y, Cao E, Julius D, Cheng Y. TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature. 2016;534(7607):347–51 (4911334Epub 2016/06/10).
Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504(7478):107–12 (4078027Epub 2013/12/07).
Moiseenkova-Bell VY, Stanciu LA, Serysheva II, Tobe BJ, Wensel TG. Structure of TRPV1 channel revealed by electron cryomicroscopy. Proc Natl Acad Sci USA. 2008;105(21):7451–5 (2396679Epub 2008/05/21).
Roberts JC, Davis JB, Benham CD. [3H]Resiniferatoxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res. 2004;995(2):176–83 (2003/12/16).
Palkar R, Lippoldt EK, McKemy DD. The molecular and cellular basis of thermosensation in mammals. Curr Opin Neurobiol. 2015;34:14–9 (4512934Epub 2015/01/27).
Yarmolinsky DA, Peng Y, Pogorzala LA, Rutlin M, Hoon MA, Zuker CS. Coding and plasticity in the mammalian thermosensory system. Neuron. 2016;92(5):1079–92 (5145739Epub 2016/11/15).
Christie S, Wittert GA, Li H, Page AJ. Involvement of TRPV1 channels in energy homeostasis. Front Endocrinol. 2018;9:420 (6079260Epub 2018/08/16).
Senaris R, Ordas P, Reimundez A, Viana F. Mammalian cold TRP channels: impact on thermoregulation and energy homeostasis. Pflugers Arch Eur J Physiol. 2018;470(5):761–77 (2018/04/28).
Amantini C, Ballarini P, Caprodossi S, Nabissi M, Morelli MB, Lucciarini R, et al. Triggering of transient receptor potential vanilloid type 1 (TRPV1) by capsaicin induces Fas/CD95-mediated apoptosis of urothelial cancer cells in an ATM-dependent manner. Carcinogenesis. 2009;30(8):1320–9 (2009/06/09).
Friedman JR, Richbart SD, Merritt JC, Brown KC, Denning KL, Tirona MT, et al. Capsaicinoids: multiple effects on angiogenesis, invasion and metastasis in human cancers. Biomed Pharmacother. 2019;118:109317 (6759410Epub 2019/08/14).
Sterle I, Zupancic D, Romih R. Correlation between urothelial differentiation and sensory proteins P2X3, P2X5, TRPV1, and TRPV4 in normal urothelium and papillary carcinoma of human bladder. BioMed Res Int. 2014;2014:805236 (4020497Epub 2014/05/29).
Zhang S, Wang D, Huang J, Hu Y, Xu Y. Application of capsaicin as a potential new therapeutic drug in human cancers. J Clin Pharm Ther. 2020;45(1):16–28 (2019/09/24).
Mistretta F, Buffi NM, Lughezzani G, Lista G, Larcher A, Fossati N, et al. Bladder cancer and urothelial impairment: the role of TRPV1 as potential drug target. BioMed Res Int. 2014;2014:987149 (4034493Epub 2014/06/06).
Baskaran P, Markert L, Bennis J, Zimmerman L, Fox J, Thyagarajan B. Assessment of pharmacology, safety, and metabolic activity of capsaicin feeding in mice. Sci Rep. 2019;9(1):8588 (6565628Epub 2019/06/15).
Baskaran P, Krishnan V, Fettel K, Gao P, Zhu Z, Ren J, et al. TRPV1 activation counters diet-induced obesity through sirtuin-1 activation and PRDM-16 deacetylation in brown adipose tissue. Int J Obes (Lond). 2017;41(5):739–49 (5413365Epub 2017/01/21).
Lee E, Jung DY, Kim JH, Patel PR, Hu X, Lee Y, et al. Transient receptor potential vanilloid type-1 channel regulates diet-induced obesity, insulin resistance, and leptin resistance. FASEB J. 2015;29(8):3182–92 (4511197Epub 2015/04/19).
Choowanthanapakorn M, Lu KW, Yang J, Hsieh CL, Lin YW. Targeting TRPV1 for body weight control using TRPV1(−/−) mice and electroacupuncture. Sci Rep. 2015;5:17366 (4664894Epub 2015/12/02).
Baker K, Raemdonck K, Dekkak B, Snelgrove RJ, Ford J, Shala F, et al. Role of the ion channel, transient receptor potential cation channel subfamily V member 1 (TRPV1), in allergic asthma. Respir Res. 2016;17(1):67 (4890475Epub 2016/06/04).
Cantero-Recasens G, Gonzalez JR, Fandos C, Duran-Tauleria E, Smit LA, Kauffmann F, et al. Loss of function of transient receptor potential vanilloid 1 (TRPV1) genetic variant is associated with lower risk of active childhood asthma. J Biol Chem. 2010;285(36):27532–5 (2934619Epub 2010/07/20).
Choi JY, Lee HY, Hur J, Kim KH, Kang JY, Rhee CK, et al. TRPV1 blocking alleviates airway inflammation and remodeling in a chronic asthma murine model. Allergy Asthma Immunol Res. 2018;10(3):216–24 (5911440Epub 2018/04/21).
McGarvey LP, Butler CA, Stokesberry S, Polley L, McQuaid S, Abdullah H, et al. Increased expression of bronchial epithelial transient receptor potential vanilloid 1 channels in patients with severe asthma. J Allergy Clin Immunol. 2014;133(3):704 e4-712 e4 (2013/11/12).
Li YR, Gupta P. Immune aspects of the bi-directional neuroimmune facilitator TRPV1. Mol Biol Rep. 2019;46(1):1499–510 (2018/12/17).
Alenmyr L, Hogestatt ED, Zygmunt PM, Greiff L. TRPV1-mediated itch in seasonal allergic rhinitis. Allergy. 2009;64(5):807–10 (2009/02/18).
Basith S, Cui M, Hong S, Choi S. Harnessing the therapeutic potential of capsaicin and its analogues in pain and other diseases. Molecules. 2016;21(8):966 (6272969Epub 2016/07/28).
Fattori V, Hohmann MS, Rossaneis AC, Pinho-Ribeiro FA, Verri WA. Capsaicin: current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules. 2016;21(7):844 (6273101Epub 2016/07/02).
Lu M, Chen C, Lan Y, Xiao J, Li R, Huang J, et al. Capsaicin-the major bioactive ingredient of chili peppers: bio-efficacy and delivery systems. Food Funct. 2020;11(4):2848–60 (2020/04/05).
Brederson JD, Kym PR, Szallasi A. Targeting TRP channels for pain relief. Eur J Pharmacol. 2013;716(1–3):61–76 (2013/03/19).
Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2020;72(2):149–62 (2020/01/08).
Bonezzi C, Costantini A, Cruccu G, Fornasari DMM, Guardamagna V, Palmieri V, et al. Capsaicin 8% dermal patch in clinical practice: an expert opinion. Expert Opin Pharmacother 2020:1-11 (2020/06/09).
Mayo Clinic—Drugs and Supplements. https://www.mayoclinic.org/drugs-supplements/capsaicin-topical-route/description/drg-20062561(2020/06/10).
Services USDoHaH. Pain management best practices inter-agency task force report: updates, gaps, inconsistencies, and recommendations. 2019. https://www.hhs.gov/ash/advisory-committees/pain/reports/index.html(2020/06/10).
Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci. 1997;17(10):3525–37.
Wang S, Asgar J, Joseph J, Ro JY, Wei F, Campbell JN, et al. Ca(2+) and calpain mediate capsaicin-induced ablation of axonal terminals expressing transient receptor potential vanilloid 1. J Biol Chem. 2017;292(20):8291–303 (5437236Epub 2017/04/01).
Touska F, Marsakova L, Teisinger J, Vlachova V. A “cute” desensitization of TRPV1. Curr Pharm Biotechnol. 2011;12(1):122–9 (2010/10/12).
Joseph J, Wang S, Lee J, Ro JY, Chung MK. Carboxyl-terminal domain of transient receptor potential vanilloid 1 contains distinct segments differentially involved in capsaicin- and heat-induced desensitization. J Biol Chem. 2013;288(50):35690–702 (3861621Epub 2013/11/01).
Yang F, Xiao X, Cheng W, Yang W, Yu P, Song Z, et al. Structural mechanism underlying capsaicin binding and activation of the TRPV1 ion channel. Nat Chem Biol. 2015;11(7):518–24 (4472570Epub 2015/06/09).
Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 2002;108(3):421–30 (2002/02/21).
Yuan P. Structural biology of thermoTRPV channels. Cell Calcium. 2019;84:102106 (6893863Epub 2019/11/15).
Lau SY, Procko E, Gaudet R. Distinct properties of Ca2+-calmodulin binding to N- and C-terminal regulatory regions of the TRPV1 channel. J Gen Physiol. 2012;140(5):541–55 (3483115Epub 2012/10/31).
Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci USA. 2003;100(13):8002–6 (164702Epub 2003/06/17).
Rosenbaum T, Gordon-Shaag A, Munari M, Gordon SE. Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J Gen Physiol. 2004;123(1):53–62 (2217413Epub 2003/12/31).
Grycova L, Lansky Z, Friedlova E, Obsilova V, Janouskova H, Obsil T, et al. Ionic interactions are essential for TRPV1 C-terminus binding to calmodulin. Biochem Biophys Res Commun. 2008;375(4):680–3 (2008/08/30).
Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007;54(6):905–18 (2007/06/22).
Docherty RJ, Yeats JC, Bevan S, Boddeke HW. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflugers Arch Eur J Physiol. 1996;431(6):828–37 (1996/04/01).
Mohapatra DP, Nau C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem. 2005;280(14):13424–32 (2005/02/05).
Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWT. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35(4):721–31 (2002/08/27).
Mandadi S, Numazaki M, Tominaga M, Bhat MB, Armati PJ, Roufogalis BD. Activation of protein kinase C reverses capsaicin-induced calcium-dependent desensitization of TRPV1 ion channels. Cell Calcium. 2004;35(5):471–8 (2004/03/09).
Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem. 2003;278(50):50080–90 (2003/09/25).
Brauchi S, Orta G, Mascayano C, Salazar M, Raddatz N, Urbina H, et al. Dissection of the components for PIP2 activation and thermosensation in TRP channels. Proc Natl Acad Sci USA. 2007;104(24):10246–51 (1891241Epub 2007/06/06).
Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. Dual regulation of TRPV1 by phosphoinositides. J Neurosci. 2007;27(26):7070–80 (6672228Epub 2007/06/29).
Lukacs V, Yudin Y, Hammond GR, Sharma E, Fukami K, Rohacs T. Distinctive changes in plasma membrane phosphoinositides underlie differential regulation of TRPV1 in nociceptive neurons. J Neurosci. 2013;33(28):11451–63 (3724548Epub 2013/07/12).
Senning EN, Collins MD, Stratiievska A, Ufret-Vincenty CA, Gordon SE. Regulation of TRPV1 ion channel by phosphoinositide (4,5)-bisphosphate: the role of membrane asymmetry. J Biol Chem. 2014;289(16):10999–1006 (4036241Epub 2014/03/07).
Ufret-Vincenty CA, Klein RM, Hua L, Angueyra J, Gordon SE. Localization of the PIP2 sensor of TRPV1 ion channels. J Biol Chem. 2011;286(11):9688–98 (3058964Epub 2011/01/13).
Gamse R, Lackner D, Gamse G, Leeman SE. Effect of capsaicin pretreatment on capsaicin-evoked release of immunoreactive somatostatin and substance P from primary sensory neurons. Naunyn–Schmiedeberg's Arch Pharmacol. 1981;316(1):38–41
Gamse R, Petsche U, Lembeck F, Jancso G. Capsaicin applied to peripheral nerve inhibits axoplasmic transport of substance P and somatostatin. Brain Res. 1982;239(2):447–-62 (1982/05/13).
Jessell TM, Iversen LL, Cuello AC. Capsaicin-induced depletion of substance P from primary sensory neurones. Brain Res. 1978;152(1):183–8 (1978/08/18).
Liu Z, Li Z. Regulation of galanin and galanin receptor 2 expression by capsaicin in primary cultured dorsal root ganglion neurons. In vitro Cell Dev Biol Anim. 2008;44(8-9):379-84 (2008/06/17).
Yaksh TL, Farb DH, Leeman SE, Jessell TM. Intrathecal capsaicin depletes substance P in the rat spinal cord and produces prolonged thermal analgesia. Science. 1979;206(4417):481–3 (1979/10/26).
Smutzer G, Jacob JC, Tran JT, Shah DI, Gambhir S, Devassy RK, et al. Detection and modulation of capsaicin perception in the human oral cavity. Physiol Behav. 2018;194:120-31 (2018/05/12).
Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008;136(1–2):202–10 (2008/03/14).
Jeong KY. Changes in TRPV1-Mediated physiological function in rats systemically treated with capsaicin on the neonate. Int J Mol Sci. 2020;21(9) (7247669Epub 2020/05/06).
Huang YB, Lin YH, Lu TM, Wang RJ, Tsai YH, Wu PC. Transdermal delivery of capsaicin derivative-sodium nonivamide acetate using microemulsions as vehicles. Int J Pharm. 2008;349(1–2):206–11 (2007/09/04).
Muzzalupo R, Tavano L, Cassano R, Trombino S, Ferrarelli T, Picci N. A new approach for the evaluation of niosomes as effective transdermal drug delivery systems. Eur J Pharm Biopharm. 2011;79(1):28–35 (2011/02/10).
Tavano L, Alfano P, Muzzalupo R, de Cindio B. Niosomes vs microemulsions: new carriers for topical delivery of Capsaicin. Colloids Surf B, Biointerfaces. 2011;87(2):333–9 (2011/06/21).
Teixeira MJ, Menezes LM, Silva V, Galhardoni R, Sasson J, Okada M, et al. Liposomal topical capsaicin in post-herpetic neuralgia: a safety pilot study. Arq Neuropsiquiatr. 2015;73(3):237–40 (2015/03/26).
Predel HG, Ebel-Bitoun C, Peil B, Weiser TW, Lange R. Efficacy and Safety of diclofenac + capsaicin gel in patients with acute back/neck pain: a multicenter randomized controlled study. Pain Ther. 2020;9(1):279–96 (7203310Epub 2020/03/30).
Capsaicin + diclofenac gel in acute back pain or neck pain. 2016. https://www.clinicaltrials.gov/ct2/show/results/NCT02700815(2020/06/20).
Galvez R, Navez ML, Moyle G, Maihofner C, Stoker M, Ernault E, et al. Capsaicin 8% patch repeat treatment in nondiabetic peripheral neuropathic pain: a 52-week, open-label, single-arm, safety study. Clin J Pain. 2017;33(10):921–31 (2017/09/06).
Safety and effectiveness of repeated administration of QUTENZA patches for treatment of pain caused by nerve damage (STRIDE). 2010. https://www.clinicaltrials.gov/ct2/show/NCT01252160(2020/06/23).
Localized neuropathic pain: topical treatment versus systemic treatment (PELICAN). 2017. https://www.clinicaltrials.gov/ct2/show/study/NCT03348735(2020/05/08).
Clinical trial assessing the efficacy of capsaicin patch (Qutenza®) in cancer patients with neuropathic pain (CAPSONCO). 2017. https://www.clinicaltrials.gov/ct2/show/NCT03317613(2020/05/08).
Evaluation in the treatment of neuropathic pain post breast surgery (CAPTRANE). 2018. https://www.clinicaltrials.gov/ct2/show/NCT03794388(2020/05/08).
Capsaicin 8% patch for spinal cord injury neuropathic pain (capsaicin). 2015. https://www.clinicaltrials.gov/ct2/show/NCT02441660(2020/05/09).
0.075% capsaicin lotion for the treatment of painful diabetic neuropathy. 2017. https://www.clinicaltrials.gov/ct2/show/study/NCT03113448(2020/06/11).
Capsaicin in treatment of rhinogenic headache. 2020. https://www.clinicaltrials.gov/ct2/show/NCT03330639(2020/06/11).
Billard M TJ, Fleming M, Warneke T, Qiu Y, Ly N, Aronstein W, Moore W. A Phase 2 double-blind clinical trial to examine the comparative effects on osteoarthritic knee pain of CGS-200-1 (1% capsaicin topical liquid), CGS-200-5 (5% capsaicin topical liquid), and CGS-200-0 (vehicle, no capsaicin) Arthritis Rheumatol 2019;71(suppl 10); https://www.acrabstracts.org/abstract/a-phase-2-double-blind-clinical-trial-to-examine-the-comparative-effects-on-osteoarthritic-knee-pain-of-cgs-200-1-1-capsaicin-topical-liquid-cgs-200-5-5-capsaicin-topical-liquid-and-cgs-200-0-v/(2020/06/11).
A phase 2 clinical trial examining the effects on osteoarthritic knee pain of CGS-200-1, CGS-200-5 and vehicle control. 2019. https://www.clinicaltrials.gov/ct2/show/NCT03528369(2020/06/11).
Osteoarthritis knee pain relief study of 0.25% 920-CGS-200. 2020. https://www.clinicaltrials.gov/ct2/show/results/NCT03124407(2020/06/11).
Capsaicin patches in knee osteoarthritis in obese patients (CHILI-OB). 2017. https://www.clinicaltrials.gov/ct2/show/NCT03153813(2020/06/11).
Bourne N, Bernstein DI, Stanberry LR. Civamide (cis-capsaicin) for treatment of primary or recurrent experimental genital herpes. Antimicrob Agents Chemother. 1999;43(11):2685–8 (89543Epub 1999/10/30).
Salat K, Jakubowska A, Kulig K. Zucapsaicin for the treatment of neuropathic pain. Expert Opin Investig Drugs. 2014;23(10):1433–40 (2014/08/30).
Saper JR, Klapper J, Mathew NT, Rapoport A, Phillips SB, Bernstein JE. Intranasal civamide for the treatment of episodic cluster headaches. Arch Neurol. 2002;59(6):990–4 (2002/06/18).
Civamide nasal solution for postherpetic neuralgia of the trigeminal nerve. 2017. https://www.clinicaltrials.gov/ct2/show/NCT01886313(2020/06/15).
Evaluation of civamide patch in treatment of postherpetic neuralgia and post-incisional neuralgia. 2010. https://www.clinicaltrials.gov/ct2/show/NCT00845923(2020/06/15).
Civamide Nasal solution for cluster headache (ECH). 2011. https://www.clinicaltrials.gov/ct2/show/NCT01341548(2020/06/15).
The effect of injection site cooling on pain experienced after the administration of CNTX-4975-05 into the knee. 2018. https://www.clinicaltrials.gov/ct2/show/NCT03472677(2020/07/01).
A study to compare levels of capsaicin after intra-articular injection and topical application in patients with painful knee osteoarthritis. 2018. https://www.clinicaltrials.gov/ct2/show/NCT03576508(2020/06/25).
A clinical study to test efficacy and safety of CNTX-4975-05 in patients with osteoarthritis knee pain. 2018. https://www.clinicaltrials.gov/ct2/show/NCT03429049(2020/07/01).
Stevens RM, Ervin J, Nezzer J, Nieves Y, Guedes K, Burges R, et al. Randomized, double-blind, placebo-controlled trial of intraarticular trans-capsaicin for pain associated with osteoarthritis of the knee. Arthritis Rheumatol. 2019;71(9):1524–33 (6772016Epub 2019/03/20).
A clinical study to test efficacy and safety of repeat doses of CNTX-4975-05 in patients with osteoarthritis knee pain. 2018. https://www.clinicaltrials.gov/ct2/show/NCT03660943(2020/07/01).
Yu Y, Lu ST. Trans-capsaicin for pain reduction and improvement in function in patients with knee osteoarthritis: comment on the article by Stevens et al. Arthritis Rheumatol. 2020. https://www.ncbi.nlm.nih.gov/pubmed/32293119(2020/07/08).
Concentric Analgesics-Press releases. https://www.concentricanalgesics.com/press-releases(2020/07/08).
Concentric Analgesics—Press release. 2018. https://www.concentricanalgesics.com/fda-grants-breakthrough-therapy-designation-for-concentric-analgesics-ca-008-in-post-surgical-pain(2020/07/08).
Gottlieb IJB, A.; Solanki, D.; Singla, N.; Shafer, S.; Brennan, T.; Hodge, C.; Donovan, J.; Royal, M. A Randomized Placebo-Controlled Trial of Intraoperative Administration of CA-008 for Postoperative Analgesia after Bunionectomy 44th annual regional anesthesiology and acute pain medicine meeting. 2019. https://www.epostersonline.com/ASRASPRING19/node/1194(2020/07/15).
Pain study in total knee arthroplasty. 2018. https://www.clinicaltrials.gov/ct2/show/NCT03731364(2020/06/10).
Safety, tolerability, pharmacokinetics, and preliminary efficacy of intraoperative administration of CA-008 for the correction of hallux valgus deformity. 2017. https://www.clinicaltrials.gov/ct2/show/results/NCT03307837(2020/06/10).
Study Evaluating the Safety, Efficacy and Pharmacokinetics of CA-008. 2019. https://www.clinicaltrials.gov/ct2/show/NCT04203537(2020/06/10).
Study in Subjects Undergoing Complete Abdominoplasty. 2018. https://www.clinicaltrials.gov/ct2/show/NCT03789318(2020/06/10).
Open-Label CA-008 in Bunionectomy. 2019. https://www.clinicaltrials.gov/ct2/show/NCT03885596(2020/06/14).
Study of CA-008 in Bunionectomy Patients. 2018. https://www.clinicaltrials.gov/ct2/show/record/NCT03599089(2020/06/14).
Acs G, Biro T, Acs P, Modarres S, Blumberg PM. Differential activation and desensitization of sensory neurons by resiniferatoxin. J Neurosci. 1997;17(14):5622–8 (6793835Epub 1997/07/15).
Karai LJ, Russell JT, Iadarola MJ, Olah Z. Vanilloid receptor 1 regulates multiple calcium compartments and contributes to Ca2+-induced Ca2+ release in sensory neurons. J Biol Chem. 2004;279(16):16377–87 (2004/02/14).
Szallasi A, Szabo T, Biro T, Modarres S, Blumberg PM, Krause JE, et al. Resiniferatoxin-type phorboid vanilloids display capsaicin-like selectivity at native vanilloid receptors on rat DRG neurons and at the cloned vanilloid receptor VR1. Br J Pharmacol. 1999;128(2):428–34 (1571651Epub 1999/10/08).
Caudle RM, Karai L, Mena N, Cooper BY, Mannes AJ, Perez FM, et al. Resiniferatoxin-induced loss of plasma membrane in vanilloid receptor expressing cells. Neurotoxicology. 2003;24(6):895–908 (2003/11/26).
Neubert JK, Karai L, Jun JH, Kim HS, Olah Z, Iadarola MJ. Peripherally induced resiniferatoxin analgesia. Pain. 2003;104(1–2):219–28 (2003/07/12).
Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398(6726):436–41 (1999/04/14).
Brown DC, Iadarola MJ, Perkowski SZ, Erin H, Shofer F, Laszlo KJ, et al. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology. 2005;103(5):1052–9 (2005/10/27).
Brown JD, Saeed M, Do L, Braz J, Basbaum AI, Iadarola MJ, et al. CT-guided injection of a TRPV1 agonist around dorsal root ganglia decreases pain transmission in swine. Science Transl Med. 2015;7(305):305ra145 (4854290Epub 2015/09/18).
Neubert JK, Mannes AJ, Karai LJ, Jenkins AC, Zawatski L, Abu-Asab M, et al. Perineural resiniferatoxin selectively inhibits inflammatory hyperalgesia. Mol Pain. 2008;4:3 (2242785Epub 2008/01/18).
Pecze L, Viskolcz B, Olah Z. Molecular surgery concept from bench to bedside: a focus on TRPV1+ pain-sensing neurons. Front Physiol. 2017;8:378 (5455100Epub 2017/06/20).
Brown DC. Resiniferatoxin: the evolution of the “molecular scalpel” for chronic pain relief. Pharmaceuticals (Basel). 2016;9(3):47 (5039500Epub 2016/08/17).
Brown DC, Agnello K, Iadarola MJ. Intrathecal resiniferatoxin in a dog model: efficacy in bone cancer pain. Pain. 2015;156(6):1018–24 (4431903Epub 2015/02/07).
Iadarola MJ, Gonnella GL. Resiniferatoxin for pain treatment: an interventional approach to personalized pain medicine. Open Pain J. 2013;6:95–107 (4711370Epub 2013/01/01).
Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, et al. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Investig. 2004;113(9):1344–52 (398431Epub 2004/05/05).
Neubert JK, Mannes AJ, Keller J, Wexel M, Iadarola MJ, Caudle RM. Peripheral targeting of the trigeminal ganglion via the infraorbital foramen as a therapeutic strategy. Brain Res Protoc. 2005;15(3):119–26 (2005/07/19).
Goso C, Piovacari G, Szallasi A. Resiniferatoxin-induced loss of vanilloid receptors is reversible in the urinary bladder but not in the spinal cord of the rat. Neurosci Lett. 1993;162(1–2):197–200 (1993/11/12).
Zhang N, Zhang P, Zhang X, Yang Y. The efficacy of resiniferatoxin in prevention of catheter related bladder discomfort in patients after TURP— a pilot, randomized, open study. Transl Androl Urol. 2012;1(1):14–8 (4713214Epub 2012/03/01).
Periganglionic resiniferatoxin for the treatment of intractable pain due to cancer-induced bone pain. 2015. https://www.clinicaltrials.gov/ct2/show/NCT02522611(2020/05/17).
Mannes AH, Quezado Z, Berger A, Fojo T, Smith R, Butman J, Lonser R, Iadarola M. Resiniferatoxin, a potent TRPV1 agonist: intrathecal administration to treat severe pain associated with advanced cancer—case report. J Pain. 2010;11(4):S43.
Heiss JI, Cantor F, Oughourli A, Smith R, Mannes A. A phase I study of the intrathecal administration of resiniferatoxin for treating severe refractory pain associated with advanced cancer. J Pain. 2014;15(Suppl):S67.
Resiniferatoxin to treat severe pain associated with advanced cancer. 2008. https://www.clinicaltrials.gov/ct2/show/NCT00804154(2020/05/17).
Study to evaluate safety and MTD of epidural resiniferatoxin injection for treatment of intractable cancer pain. 2017. https://www.clinicaltrials.gov/ct2/show/NCT03226574(2020/05/17).
Sorrento Therapeutics— Press release. 2020. https://www.globenewswire.com/news-release/2020/02/27/1992194/0/en/Sorrento-Therapeutics-Presents-Interim-Positive-Results-of-Phase-1b-Resiniferatoxin-RTX-in-Cancer-Pain-Trial.html(2020/05/17).
Nedeljkovic SS NS, Rickerson E, Levitt RC, Horn DB, Patin DJ, Albores-Ibarra N, Nahama A, Zhao T, Bharathi P, Luchi M, Minkowitz H, Solanki D, Leiman D. A multicenter, open-label, Phase 1b study to assess the safety and define the maximally tolerated dose of epidural Resiniferatoxin (RTX) injection for treatment of intractable pain associated with cancer. 2020. https://www.sorrentotherapeutics.com/research/product-candidates/rtx-cancer-pain/(2020/05/17).
Bert J, Mahowald ML, Frizelle S, Dorman CW, Funkenbusch SC, Krug HE. The effect of treatment with resiniferatoxin and capsaicin on dynamic weight bearing measures and evoked pain responses in a chronic inflammatory arthritis murine model. Intern Med Rev (Wash D C). 2016;2016(6):89 (5070479Epub 2016/10/25).
Iadarola MJ, Sapio MR, Raithel SJ, Mannes AJ, Brown DC. Long-term pain relief in canine osteoarthritis by a single intra-articular injection of resiniferatoxin, a potent TRPV1 agonist. Pain. 2018;159(10):2105–14 (2018/07/18).
Kissin EY, Freitas CF, Kissin I. The effects of intra-articular resiniferatoxin in experimental knee-joint arthritis. Anesth Analg. 2005;101(5):1433–9 (1409708Epub 2005/10/26).
Intra-articular lopain (MTX-071) phase I/IIa study in chronic osteoarthritic knee joint pain. 2015. https://www.clinicaltrials.gov/ct2/show/NCT02566564(2020/05/21).
Lopain (MTX-071 / resiniferatoxin) An open label, single dose phase Ib/IIa study to determine the safety and clinical effects of intra-articular injections of low doses of Lopain (MTX-071). 2017. https://www.clinicaltrialsregister.eu/ctr-search/search?query=Lopain(2020/05/21).
A randomized, double-blind, placebo-controlled, single dose phase IIb exploratory study to document the clinical effects and safety of intra-articular injections of Lopain (MTX-071) in patients witth knee osteoarthritis. 2019. https://www.clinicaltrialsregister.eu/ctr-search/search?query=Lopain(2020/05/09).
Study of resiniferatoxin for knee pain in moderate to severe osteoarthritis. 2018. https://www.clinicaltrials.gov/ct2/show/NCT03542838(2020/05/09).
A phase 3 study to evaluate the efficacy and safety of resiniferatoxin for pain due to osteoarthritis of the knee. 2019. https://www.clinicaltrials.gov/ct2/show/NCT04044742(2020/05/10).
Study to evaluate resiniferatoxin in patients with knee osteoarthritis whose total knee replacement surgery is delayed. 2020. https://www.clinicaltrials.gov/ct2/show/NCT04386980(2020/06/28).
Sorrento Therapeutics—Press release. 2019. https://www.globenewswire.com/news-release/2019/06/19/1871054/0/en/Sorrento-Therapeutics-Updates-Positive-Results-of-Phase-1b-Resiniferatoxin-RTX-in-Knee-Osteoarthritis-Pain-Trial.html(2020/06/28).
Salas MM, Clifford JL, Hayden JR, Iadarola MJ, Averitt DL. Local resiniferatoxin induces long-lasting analgesia in a rat model of full thickness thermal injury. Pain Med. 2017;18(12):2453–65 (6279302Epub 2016/10/31).
Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405(6783):183–7 (2000/05/23).
Lee Y, Hong S, Cui M, Sharma PK, Lee J, Choi S. Transient receptor potential vanilloid type 1 antagonists: a patent review (2011–2014). Expert Opin Ther Patents. 2015;25(3):291–318 (2015/02/11).
Trevisani M, Gatti R. TRPV1 antagonists as analgesic agents. Open Pain J. 2013;6(Suppl 1: M11):108–18.
Voight EA, Kort ME. Transient receptor potential vanilloid-1 antagonists: a survey of recent patent literature. Expert Opin Ther Patents. 2010;20(9):1107–22 (2010/07/01).
Bevan S, Hothi S, Hughes G, James IF, Rang HP, Shah K, et al. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br J Pharmacol. 1992;107(2):544–52 (1907893Epub 1992/10/01).
Weil A, Moore SE, Waite NJ, Randall A, Gunthorpe MJ. Conservation of functional and pharmacological properties in the distantly related temperature sensors TRVP1 and TRPM8. Mol Pharmacol. 2005;68(2):518–27 (2005/05/25).
Brown BS, Keddy R, Perner RJ, DiDomenico S, Koenig JR, Jinkerson TK, et al. Discovery of TRPV1 antagonist ABT-116. Bioorg Med Chem Lett. 2010;20(11):3291–4 (2010/05/12).
Honore P, Wismer CT, Mikusa J, Zhu CZ, Zhong C, Gauvin DM, et al. A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel transient receptor potential type V1 receptor antagonist, relieves pathophysiological pain associated with inflammation and tissue injury in rats. J Pharmacol Exp Ther. 2005;314(1):410–21 (2005/04/20).
Maher MP, Bhattacharya A, Ao H, Swanson N, Wu NT, Freedman J, et al. Characterization of 2-(2,6-dichloro-benzyl)-thiazolo[5,4-d]pyrimidin-7-yl]-(4-trifluoromethyl-phenyl) -amine (JNJ-39729209) as a novel TRPV1 antagonist. Eur J Pharmacol. 2011;663(1–3):40–50 (2011/05/18).
Puttfarcken PS, Han P, Joshi SK, Neelands TR, Gauvin DM, Baker SJ, et al. A-995662 [(R)-8-(4-methyl-5-(4-(trifluoromethyl)phenyl)oxazol-2-ylamino)-1,2,3,4-tetrahydr onaphthalen-2-ol], a novel, selective TRPV1 receptor antagonist, reduces spinal release of glutamate and CGRP in a rat knee joint pain model. Pain. 2010;150(2):319–26 (2010/07/14).
Surowy CS, Neelands TR, Bianchi BR, McGaraughty S, El Kouhen R, Han P, et al. (R)-(5-tert-butyl-2,3-dihydro-1H-inden-1-yl)-3-(1H-indazol-4-yl)-urea (ABT-102) blocks polymodal activation of transient receptor potential vanilloid 1 receptors in vitro and heat-evoked firing of spinal dorsal horn neurons in vivo. J Pharmacol Exp Ther. 2008;326(3):879–88 (2008/06/03).
Watabiki T, Kiso T, Kuramochi T, Yonezawa K, Tsuji N, Kohara A, et al. Amelioration of neuropathic pain by novel transient receptor potential vanilloid 1 antagonist AS1928370 in rats without hyperthermic effect. J Pharmacol Exp Ther. 2011;336(3):743–50 (2010/11/26).
Garami A, Shimansky YP, Rumbus Z, Vizin RCL, Farkas N, Hegyi J, et al. Hyperthermia induced by transient receptor potential vanilloid-1 (TRPV1) antagonists in human clinical trials: Insights from mathematical modeling and meta-analysis. Pharmacol Ther. 2020;208:107474 (2020/01/14).
Krarup AL, Ny L, Astrand M, Bajor A, Hvid-Jensen F, Hansen MB, et al. Randomised clinical trial: the efficacy of a transient receptor potential vanilloid 1 antagonist AZD1386 in human oesophageal pain. Aliment Pharmacol Ther. 2011;33(10):1113–22 (2011/03/18).
Garami A, Pakai E, McDonald HA, Reilly RM, Gomtsyan A, Corrigan JJ, et al. TRPV1 antagonists that cause hypothermia, instead of hyperthermia, in rodents: compounds’ pharmacological profiles, in vivo targets, thermoeffectors recruited and implications for drug development. Acta Physiol (Oxf). 2018;223(3):e13038 (6032921Epub 2018/01/21).
Gavva NR. Body-temperature maintenance as the predominant function of the vanilloid receptor TRPV1. Trends Pharmacol Sci. 2008;29(11):550–7 (2008/09/23).
Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, et al. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev. 2009;61(3):228–61 (2763780Epub 2009/09/15).
Gavva NR, Bannon AW, Surapaneni S, Hovland DN Jr, Lehto SG, Gore A, et al. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci. 2007;27(13):3366–74 (6672109Epub 2007/03/30).
Szolcsanyi J. Effect of capsaicin on thermoregulation: an update with new aspects. Temperature (Austin). 2015;2(2):277–96 (4843897Epub 2016/05/27).
Cui M, Honore P, Zhong C, Gauvin D, Mikusa J, Hernandez G, et al. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J Neurosci. 2006;26(37):9385–93 (6674601Epub 2006/09/15).
Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, et al. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci. 2007;27(28):7459–68 (6672610Epub 2007/07/13).
Szelenyi Z, Hummel Z, Szolcsanyi J, Davis JB. Daily body temperature rhythm and heat tolerance in TRPV1 knockout and capsaicin pretreated mice. Eur J Neurosci. 2004;19(5):1421–4 (2004/03/16).
Miller F, Bjornsson M, Svensson O, Karlsten R. Experiences with an adaptive design for a dose-finding study in patients with osteoarthritis. Contemp Clin Trials. 2014;37(2):189–99 (2014/01/08).
A study to evaluate the safety and efficacy of an investigational drug in the treatment of postoperative dental pain (MK-2295-005). 2006. https://www.clinicaltrials.gov/ct2/show/NCT00387140(2020/07/12).
Serafini M, Griglio A, Aprile S, Seiti F, Travelli C, Pattarino F, et al. Targeting transient receptor potential vanilloid 1 (TRPV1) channel softly: the discovery of passerini adducts as a topical treatment for inflammatory skin disorders. J Med Chem. 2018;61(10):4436–55 (2018/05/04).
Lehto SG, Tamir R, Deng H, Klionsky L, Kuang R, Le A, et al. Antihyperalgesic effects of (R, E)-N-(2-hydroxy-2,3-dihydro-1H-inden-4-yl)-3-(2-(piperidin-1-yl)-4-(trifluorom ethyl)phenyl)-acrylamide (AMG8562), a novel transient receptor potential vanilloid type 1 modulator that does not cause hyperthermia in rats. J Pharmacol Exp Ther. 2008;326(1):218–29 (2008/04/19).
Jakab B, Helyes Z, Varga A, Bolcskei K, Szabo A, Sandor K, et al. Pharmacological characterization of the TRPV1 receptor antagonist JYL1421 (SC0030) in vitro and in vivo in the rat. Eur J Pharmacol. 2005;517(1–2):35–44 (2005/06/28).
Chizh BA, O’Donnell MB, Napolitano A, Wang J, Brooke AC, Aylott MC, et al. The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain. 2007;132(1–2):132–41 (2007/07/31).
SB-705498 dental pain study after tooth extraction. 2006. https://www.clinicaltrials.gov/ct2/show/NCT00281684(2020/07/15).
Sb-705498 rectal pain study. 2007. https://www.clinicaltrials.gov/ct2/show/NCT00461682(2020/07/15).
Arsenault P, Chiche D, Brown W, Miller J, Treister R, Leff R, et al. NEO6860, modality-selective TRPV1 antagonist: a randomized, controlled, proof-of-concept trial in patients with osteoarthritis knee pain. Pain Rep. 2018;3(6):e696 (6344137Epub 2019/02/02).
Brown W, Leff RL, Griffin A, Hossack S, Aubray R, Walker P, et al. Safety, pharmacokinetics, and pharmacodynamics study in healthy subjects of oral NEO6860, a modality selective transient receptor potential vanilloid subtype 1 antagonist. J Pain. 2017;18(6):726–38 (2017/02/12).
Chiche DB, Walker P. NEO6860, a novel modality selective TRPV1 antagonist: results from a phase I, double-blind, placebo-controlled study in healthy subjects. J Pain. 2016;17:S79.
NEO6860, a TRPV1 antagonist, first in human study. 2015. https://www.clinicaltrials.gov/ct2/show/NCT02337543(2020/07/16).
A proof-of-concept study assessing NEO6860 in osteoarthritis pain. 2016. https://www.clinicaltrials.gov/ct2/show/NCT02712957(2020/07/16).
A clinical study to investigate the effect on pain relief of a single dose of JNJ-39439335 in patients with chronic osteoarthritis pain of the knee. 2009. https://www.clinicaltrials.gov/ct2/show/NCT00933582(2020/05/17).
A study to evaluate the bioavailability and food effect of JNJ-39439335 in healthy adult male volunteers. 2011. https://www.clinicaltrials.gov/ct2/show/NCT01454245(2020/05/17).
A multiple dose study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of JNJ-39439335 in patients with osteoarthritis. 2011. https://www.clinicaltrials.gov/ct2/show/NCT01343303(2020/05/17).
A study to investigate the safety, tolerability, and pharmacokinetics of JNJ-39439335 in healthy Japanese and Caucasian adult male participants. 2012. https://www.clinicaltrials.gov/ct2/show/NCT01631487(2020/08/18).
An exploratory study to assess the effects of JNJ-39439335 on the relief of pain using a thermal-grill experimental model. 2009. https://www.clinicaltrials.gov/ct2/show/NCT01006304(2020/05/18).
Manitpisitkul P, Shalayda K, Russell L, Sanga P, Williams Y, Solanki B, et al. Bioavailability and pharmacokinetics of TRPV1 antagonist mavatrep (JNJ-39439335) tablet and capsule formulations in healthy men: two open-label, crossover, single-dose phase 1 studies. Clin Pharmacol Drug Dev. 2018;7(7):699–711 (2017/11/11).
Mayorga AJ, Flores CM, Trudeau JJ, Moyer JA, Shalayda K, Dale M, et al. A randomized study to evaluate the analgesic efficacy of a single dose of the TRPV1 antagonist mavatrep in patients with osteoarthritis. Scand J Pain. 2017;17:134–43 (2017/08/30).
Manitpisitkul P, Mayorga A, Shalayda K, De Meulder M, Romano G, Jun C, et al. Safety, tolerability and pharmacokinetic and pharmacodynamic learnings from a double-blind, randomized, placebo-controlled, sequential group first-in-human study of the TRPV1 antagonist, JNJ-38893777 in healthy men. Clin Drug Investig. 2015;35(6):353–63 (2015/04/22).
Manitpisitkul P, Shalayda K, Russell L, Sanga P, Solanki B, Caruso J, et al. Pharmacokinetics and safety of mavatrep (JNJ-39439335), a TRPV1 antagonist in healthy japanese and caucasian men: a double-blind, randomized, placebo-controlled, sequential-group phase 1 study. Clin Pharmacol Drug Dev. 2018;7(7):712–26 (2017/11/11).
DWP05195 in healthy adult male volunteers. 2009. https://www.clinicaltrials.gov/ct2/show/NCT00969787(2020/07/18).
A multiple dose study of DWP05195 in healthy adult subjects. 2010. https://www.clinicaltrials.gov/ct2/show/NCT01094834(2020/07/18).
Lee J, Kim BH, Yu KS, Kim HS, Kim JD, Cho JY, et al. A first-in-human, double-blind, placebo-controlled, randomized, dose escalation study of DWP05195, a novel TRPV1 antagonist, in healthy volunteers. Drug Des Dev Ther. 2017;11:1301–13 (5411174Epub 2017/05/10).
Evaluate the efficacy and safety of DWP05195 in subjects with post-herpetic neuralgia. 2012. https://www.clinicaltrials.gov/ct2/show/NCT01557010(2020/07/18).
Experimental biomarker study for pain thresholds. 2016. https://www.clinicaltrials.gov/ct2/show/NCT02695745(2020/07/18).
Arendt-Nielsen L, Harris S, Whiteside GT, Hummel M, Knappenberger T, O’Keefe S, et al. A randomized, double-blind, positive-controlled, 3-way cross-over human experimental pain study of a TRPV1 antagonist (V116517) in healthy volunteers and comparison with preclinical profile. Pain. 2016;157(9):2057–67 (2016/05/12).
Tafesse L, Kanemasa T, Kurose N, Yu J, Asaki T, Wu G, et al. Structure–activity relationship studies and discovery of a potent transient receptor potential vanilloid (TRPV1) antagonist 4-[3-chloro-5-[(1S)-1,2-dihydroxyethyl]-2-pyridyl]-N-[5-(trifluoromethyl)-2-pyrid yl]-3,6-dihydro-2H-pyridine-1-carboxamide (V116517) as a clinical candidate for pain management. J Med Chem. 2014;57(15):6781–94 (2014/07/25).
Analgesic efficacy and safety of V116517 in subjects with moderate to severe chronic pain due to postherpetic neuralgia (PHN). 2012. https://www.clinicaltrials.gov/ct2/show/NCT01688947(2020/07/18).
Analgesic efficacy and safety of V116517 in subjects with moderate to severe chronic pain due to osteoarthritis (OA) of the knee. 2012. https://www.clinicaltrials.gov/ct2/show/NCT01688934(2020/07/18).
To evaluate the safety, tolerability and analgesic efficacy of SAF312 in postoperative dental pain patients. 2009. https://www.clinicaltrials.gov/ct2/show/NCT00986882(2020/05/22).
Study of SAF312 as an eye drop for treatment of eye pain following photorefractive keratectomy (PRK) surgery. 2016. https://www.clinicaltrials.gov/ct2/show/NCT02961062(2020/05/22).
Novartis—trial results. 2019. https://www.novctrd.com/CtrdWeb/displaypdf.nov?trialresultid=6323(2020/05/22).
Novartis—trial results. 2019. https://www.novctrd.com/CtrdWeb/displaypdf.nov?trialresultid=17355(2020/05/22).
Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–54 (1993/12/03).
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–11 (1998/03/05).
Pilot study to evaluate SYL1001 safety and effect in patients with ocular Pain. 2013. https://www.clinicaltrials.gov/ct2/show/NCT01776658(2020/07/28).
Benitez-Del-Castillo JM, Moreno-Montanes J, Jimenez-Alfaro I, Munoz-Negrete FJ, Turman K, Palumaa K, et al. Safety and efficacy clinical trials for SYL1001, a novel short interfering RNA for the treatment of dry eye disease. Investig Ophthalmol Vis Sci. 2016;57(14):6447–54 (2016/11/29).
Moreno-Montanes J, Bleau AM, Jimenez AI. Tivanisiran, a novel siRNA for the treatment of dry eye disease. Expert Opin Investig Drugs. 2018;27(4):421–6 (2018/03/24).
Dose-finding study to assess the safety and effect of SYL1001 in patients with ocular pain. 2015. https://www.clinicaltrials.gov/ct2/show/NCT02455999(2020/07/28).
HELIX, a double-masked study of SYL1001 in patients with moderate to severe dry eye disease (DED). 2017. https://www.clinicaltrials.gov/ct2/show/NCT03108664(2020/07/28).
Sylentis—Press release. 2019. https://www.sylentis.com/index.php/en/news/general-news/145-sylentis-announces-results-of-phase-3-helix-trial-with-tivanisiran-for-the-treatment-of-dry-eye-disease(2020/07/28).
Ann J, Kim HS, Thorat SA, Kim H, Ha HJ, Choi K, et al. Discovery of nonpungent transient receptor potential vanilloid 1 (TRPV1) agonist as strong topical analgesic. J Med Chem. 2020;63(1):418–24 (2019/11/09).
Mostinski Y, Noy G, Kumar R, Tsvelikhovsky D, Priel A. Tricyclic spirolactones as modular TRPV1 synthetic agonists. ACS Chem Neurosci. 2017;8(8):1688–96 (2017/05/19).
Wang S, Joseph J, Ro JY, Chung MK. Modality-specific mechanisms of protein kinase C-induced hypersensitivity of TRPV1: S800 is a polymodal sensitization site. Pain. 2015;156(5):931–41 (4402251Epub 2015/03/04).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by operating grants from the Canadian Institutes of Health Research (CIHR) and Natural Sciences and Engineering Research Council of Canada (NSERC) [CA]. CA holds a Canada Research Chair in Inflammatory Pain. MD has an ACHRI (Alberta Children's Hospital Research Institute) and CSM (Cumming School of Medicine) postdoctoral fellowship. No sponsor funding was received for the open access fee.
Conflicts of interest
No conflicts of interest.
Ethics approval
Not applicable.
Consent
Not applicable.
Author contributions
MI and CA wrote the paper. MD designed the figure.
Data availability statement
Not applicable.
Rights and permissions
About this article
Cite this article
Iftinca, M., Defaye, M. & Altier, C. TRPV1-Targeted Drugs in Development for Human Pain Conditions. Drugs 81, 7–27 (2021). https://doi.org/10.1007/s40265-020-01429-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01429-2