Abstract
Remimazolam (Anerem® in Japan; ByFavo™ in the USA; Aptimyda™ in the EU) is an ultra-short-acting intravenous (IV) benzodiazepine sedative/anesthetic being developed by PAION AG in conjunction with a number of commercial partners for use in anesthesia and procedural sedation. Remimazolam was approved on 23 January 2020 in Japan for use in general anesthesia in adult patients. Remimazolam is also undergoing regulatory assessment in South Korea for this indication and for use in procedural sedation in the USA, the EU and China. This article summarises the major milestones in the development of remimazolam for this first approval for the induction and maintenance of general anaesthesia, and its potential upcoming approvals in general anaesthesia and procedural sedation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
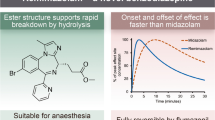
An ultra-short-acting IV benzodiazepine that is being developed by PAION AG in conjunction with Mundipharma, Cosmo Pharmaceuticals, Acacia Pharma, Hana Pharm, R-Pharm, Pharmascience and Yichang Humanwell Pharmaceutical Co. for use in anesthesia and sedation |
Received its first approval on 23 January 2020 in Japan |
Approved for use in the induction and maintenance of general anesthesia |
1 Introduction
Remimazolam (Anerem® in Japan [1]; ByFavo™ in the USA [2], Aptimyda™ in the EU) is an intravenous (IV) benzodiazepine sedative/anesthetic being developed by PAION AG in conjunction with a number of commercial partners for use in anesthesia and procedural sedation [3]. Remimazolam was approved on 23 January 2020 in Japan for use in general anesthesia [4]. Remimazolam is undergoing regulatory assessment in South Korea for use in general anesthesia [5], and for procedural sedation in the USA (PDUFA date of 5 July 2020) [3, 6, 7], the EU [8] and China [3].
Commonly used IV sedatives in anesthesia practice include the hypnotics propofol (an anesthetic alkylphenol) and midazolam (a benzodiazepine), both of which are often administered in combination with an IV opioid (e.g. fentanyl) for analgesia [9, 10]. Limitations of such regimens include the narrow therapeutic index of propofol (due to cardiorespiratory depression) and the onset and recovery time of midazolam. Consequently, there is a need for anesthetic agents that have a fast onset of action, good safety profile and short recovery time [9, 10]. Remimazolam is an ultra-short-acting GABAA receptor agonist and is thought to have a sedative/anesthetic effect by promoting GABA binding to the GABAA receptor via the receptor benzodiazepine binding site [11].

Key milestones in the development of remimazolam besylate leading to its first approval for general anesthesia. NDA new drug application, MAA marketing authorization application
In Japan, remimazolam is approved for the induction and maintenance of anesthesia [1]. In adults undergoing general anesthesia, remimazolam is administered at a rate of 12 mg/kg/h until the patient achieves the required level of unconsciousness (i.e. induction of anesthesia). For maintenance of anesthesia, an initial continuous infusion rate of 1 mg/kg/h is recommended, with the dose rate adjusted to maintain an appropriate depth of anesthesia, but not exceeding 2 mg/kg/h. The infusion rate should be reduced appropriately depending on the patient’s age and condition. If signs of arousal are evident, up to 0.2 mg/kg of remimazolam may be administered IV. Remimazolam should be used in combination with analgesics and muscle relaxants as part of an anesthetic regimen. If an overdose of remimazolam is suspected, flumazenil (a benzodiazepine receptor antagonist) should be administered. Remimazolam is contraindicated in patients with hypersensitivity to the drug, acute-angle glaucoma, myasthenia gravis, shock, coma, or acute alcoholism with depressed vital signs [1].
1.1 Company Agreements
In January 2020, Cosmo Pharmaceuticals N.V. (Cosmo) and Acacia Pharma Group plc (Acacia Pharma) entered into a licensing agreement for remimazolam. Under the agreement, Cosmo sublicensed the exclusive US rights to remimazolam to Acacia Pharma [2]. In June 2016, PAION had granted an exclusive license to Cosmo for the development and commercialisation of remimazolam in USA. PAION would be responsible for and would bear the cost associated with the completion of the ongoing US trials in procedural sedation. Cosmo would be responsible for further development and commercialisation of remimazolam in the USA, while bearing all future associated costs for market authorisation and distribution [12].
In January 2020, PAION extended its license agreement for remimazolam with Hana Pharm to include Southeast Asia (Indonesia, Philippines, Singapore, Thailand and Vietnam). According to the agreement Hana Pharm will manage the development and marketing approval process [13]. The development and commercialisation of remimazolam was licensed to Hana Pharm for Korea in 2013 [14].
In December 2017, PAION entered into a development and commercialisation agreement with Mundipharma Co., Ltd (Mundipharma), granting Mundipharma an exclusive license to develop and commercialise remimazolam, in Japan. Under the terms of the agreement, Mundipharma has the right and obligation to further develop remimazolam in all indications in Japan with PAION’s support. Mundipharma will bear all costs for market authorisation and distribution [15].
In July 2014, PAION entered into a license agreement with Pendopharm (through its European affiliate Pharmascience International Ltd) to develop and commercialise remimazolam in Canada [16]. In October 2013, PAION and R-Pharm entered into a license agreement for the development, manufacture and commercialisation of remimazolam in Russia and CIS. R-Pharm will manage the development and marketing approval process in Russia and CIS [17]. PAION and R-Pharm extended their license agreements (through the R-Pharm affiliate TRPharm) for remimazolam to include Turkey in November 2013 [18] and the MENA region (i.e. Middle East and North Africa) in June 2014 [19]. In July 2012, PAION entered into a license agreement with Yichang Humanwell for the development, manufacture and commercialisation of remimazolam in China [20].
In November 2014, Ono Pharmaceutical Co., Ltd (Ono), terminated the exclusive licensing agreement for Japanese rights to remimazolam and returned back the license to PAION [21, 22]; the knowledge and technology transfer from Ono was completed in July 2015 [23].
In June 2008, CeNeS Pharmaceuticals was acquired by PAION [24]. Previously, in November 2003, CeNeS Pharmaceuticals had acquired TheraSci Ltd and included in the assets of TheraSci was a simultaneous transaction under which GlaxoSmithKline assigned all rights to the pre-operative sedative programme with TheraSci to CeNeS. This transaction included remimazolam and several back-up compounds [25] In August 2007, CeNeS Pharmaceuticals had entered into a license agreement with Ono to develop and commercialize remimazolam in Japan [26].

Chemical structure of remimazolam besylate
1.2 Patent Information
PAION has been granted patents in numerous countries worldwide, including Japan and the USA, relating to the dosing regimen for sedation with remimazolam [27]. In September 2017, a formulation patent for remimazolam was granted to PAION in the EU by the European Patent Office. The patent, named “Compositions Comprising Short-Acting Benzodiazepines” (patent no. 2 852 389), relates to stable preparations of remimazolam and related compounds and will expire in May 2033 [28]. In February 2016, a patent related to crystalline forms of the besylate salt of remimazolam named “Short-Acting Benzodiazepine Salts and their Polymorphic Forms” was granted to PAION in the USA (patent no. US 919 373 0B2). The patent will expire in May 2031 [29]. PAION also holds a patent for remimazolam in Japan (patent no. JP 6199869 expiring in May 2033) [30]. CeNeS had announced in February 2006 that it had been granted a patent by the European Patent Office for a series of its short-acting sedatives in preclinical development, including remimazolam [31].
2 Scientific Summary
2.1 Pharmacodynamics
In vitro, remimazolam had a high affinity for the benzodiazepine binding site of the rat brain GABAA receptor (Ki 26.3 nmol/L) [1] and in tissue homogenates from human, rat and Yucatan micropig brain [11]. Remimazolam showed a 320- to 410-fold greater affinity for the GABAA benzodiazepine receptor than its carboxylic acid metabolite CNS 7054 in rat, micropig and human brain tissue homogenates; remimazolam and CNS 7054 (10 μM) showed no detectable affinity for other receptor sites [11]. Like midazolam, remimazolam enhanced GABA currents in Ltk cells stably transfected with GABAA receptor subtypes (α1, α2, α3, α5,) and did not show selectivity between receptor subtypes. IV remimazolam dose-dependently inhibited substantia nigra pars reticulata (SNpr) neuronal firing (to ≈ 20% of baseline levels; ED50 0.83 mg/kg) in a rat model. Recovery to baseline SNpr firing rates was achieved within ≈ 7 min of ceasing administration with the highest (8 mg/kg) dose of remimazolam. In rats, the loss of righting reflex after IV administration of remimazolam or midazolam was immediate; however, the duration of the drug-induced loss of righting reflex was shorter with remimazolam than with midazolam (9.6 vs 24.6 min). Pretreatment with the benzodiazepine receptor antagonist flumazenil significantly reduced the loss of righting reflex duration induced by IV remimazolam 30 mg/kg in mice (0.7 vs 8.4 min; p = 0.013) [11]. In animal models (mice, rats, minipigs and monkeys), remimazolam exerted a dose-dependent sedative effect [1]. Inhaled remimazolam was effective in producing sedation in rodent models and was not associated with pulmonary adverse events, including lung irritation or bronchospasm. Remimazolam potentiated the effects of remifentanil when both drugs were concurrently delivered via inhalation in a rat model [32].
Simulations based on population pharmacodynamic models showed that maximal sedation is reached within 3 min of IV remimazolam single-dose administration [33]. In healthy volunteers, rapid-onset, dose-dependent sedation [assessed using Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scores] was seen after IV administration of remimazolam ≥ 0.05 mg/kg in a single ascending-dose US study [34]. IV remimazolam 0.075–0.20 mg/kg induced peak sedation levels similar to or greater than those with midazolam 0.075 mg/kg, but with a more rapid median recovery time from sedation (5.5–20 vs 40 min) [34]. Peak sedation (assessed using MOAA/S and BiSpectral monitoring scores) occurred within 1–2 min after injection of IV remimazolam ≥ 0.075 mg/kg in a single ascending dose study in healthy volunteers conducted in China (ChiCTR180001518) and remimazolam achieved deeper sedation than midazolam during a 2 h continuous IV infusion of either drug (ChiCTR1800015186;) [induction over 1 min with remimazolam 0.2 mg/kg or midazolam 0.15 mg/kg over 1 min then maintenance with 1.0 mg/kg/h remimazolam or 0.05 mg/kg/h midazolam] [35].
A rapid decrease in the MOAA/S score from 5 (fully alert) to < 2 (loss of consciousness) was evident within 5 min of starting a 35-min IV infusion of remimazolam (5 mg/min for 5 min, then 3 mg/min for 15 min and 1 mg/min for 15 min) in a study in healthy volunteers (EudraCT201700045512). Full alertness (MOAA/S score of 5) was seen at 19 min after stopping the infusion [36]. EEG changes during the remimazolam infusion were characterized by an initial transient increase in the beta frequency band (shortly after the start of the infusion) and a late increase in the delta frequency band; no burst suppression patterns or isoelectric EEG were evident [37].
Time-to-event modelling using data from clinical trials of remimazolam found that the sedative effects of remimazolam are not cumulative when administered for up to ≈ 9 h during general anesthesia [38]. The sedative effect of remimazolam was rapidly reversed by flumazenil in vivo (in rats) and in humans (within a median 1–2 min of administration) in a phase 1b trial in healthy volunteers undergoing colonoscopy (multiple-dose procedural sedation) and a phase 2/3 general anesthesia trial (JapicCTI121973) [1, 39, 40]. Clinically relevant prolongation of the QTc interval was not evident in a thorough QT study of IV remimazolam [41].
2.2 Pharmacokinetics
The pharmacokinetic profile of remimazolam was linear when administered as a single IV 0.01–0.30 mg/kg dose over 1 min in a phase 1 dose-finding trial in healthy volunteers. The mean steady-state volume of distribution (Vss) of remimazolam was 34.8 L, while that of midazolam was 81.8 L [34]. The Vss of remimazolam administered as a 35-min IV infusion (5 mg/min for 5 min, then 3 mg/min for 15 min, then 1 mg/min for 15 min) in a phase 1 trial in healthy volunteers was 35.4 L [36]. In vitro, remimazolam was ≈ 92% bound to serum protein (predominantly albumin) and at a concentration of 1–10 μg/l showed a human blood cell translocation rate of 7.5–11.7% [1].
Remimazolam is rapidly hydrolyzed by tissue esterases (mainly liver carboxylesterase) to a pharmacologically inactive carboxylic acid metabolite (CNS7054) [1, 11, 42].
Systemic clearance (CL) after a single dose of IV remimazolam 0.01–0.35 mg/kg administered over 1 min was three times as rapid as that of a single dose of IV midazolam 0.075 mg/kg administered over 1 min (70.3 vs 23.0 L/h) in healthy volunteers [34]. CL was considered independent of bodyweight [33, 34]. The mean residence time of remimazolam was 0.51 h and the terminal half-life (t1/2) was 0.75 h (vs 3.62 and 4.29 h, respectively, with midazolam) [34]. In healthy volunteers who received remimazolam as a 35-min IV infusion (5 mg/min for 5 min, then 3 mg/min for 15 min, then 1 mg/min for 15 min), CL was 1.15 L/min and t1/2 was 70 min (i.e. 1.17 h). The simulated context-sensitive half-time after a 4 h infusion was predicted to be 6.8 min [36].
Remimazolam is predominantly excreted in urine; ≥ 80% of a dose was detected in urine as metabolites 24 h after administration of a single 0.2 or 0.3 mg/kg dose of IV remimazolam; the unchanged drug was not detected [1].
The pharmacokinetics of remimazolam did not differ between elderly (median age 66.0 years) and younger (median age 21.0 years) patients or between patients with normal renal function (eGFR ≥ 80 mL/min/1.73m2) and end-stage renal failure (eGFR < 15 mL/min/1.73m2). In patients with severe hepatic dysfunction (Child Pugh class C), AUC∞, Vss and t1/2 (171 ng·h/mL; 1.01 L/kg; 109 min) were increased compared to patients with normal hepatic function (132 ng·h/mL; 0.329 L/kg; 43.1 min). Careful titration to effect is recommended in patients with severe hepatic impairment [1].
Features and properties of remimazolam
Alternative names | Anerem®; ByFavo™; Aptimyda™; CNS 7056B, CNS 7056 besylate, ONO-2745BS, remimazolam besylate |
Class | Benzodiazepines; general anesthetics; hypnosedatives; small molecules |
Mechanism of action | GABAA receptor agonist |
Route of administration | IV |
Pharmacodynamics | |
Pharmacokinetics | Vss ≈ 35 L; ≈ 92% bound to serum protein; rapidly hydrolyzed by serum esterases; CL 70.3 L/h; t1/2 0.75 h |
Adverse events | |
Most frequent (general anesthesia) | Hypotension, vomiting, nausea |
ATC codes | |
WHO ATC code | N05C-D14 (Remimazolam) |
EphMRA ATC code | N5B (Hypnotics/Sedatives) |
Chemical name | Methyl 3-[(4S)-8-bromo-1-methyl-6-pyridin-2-yl-4H-imidazo[1,2-a][1,4]benzodiazepin-4-yl]propanoate |
2.3 Therapeutic Trials
2.3.1 General Anesthesia
IV remimazolam was as effective as propofol for the induction and maintenance of general anesthesia in adult patients undergoing surgery in a randomized Japanese phase 2/3 noninferiority trial (JapicCTI121973) [1, 39]. The primary efficacy endpoint of no intra-operative awakening or recall, no need for rescue sedative medication and no body movement was achieved in 100% of remimazolam or propofol recipients, thus achieving noninferiority. Patients were administered an IV infusion of remimazolam 6 or 12 mg/kg/h for induction and then a starting dose of remimazolam 1 mg/kg/h for maintenance, titrated up or down, as required (n = 150 and 150 evaluable) or a standard dose regimen of IV propofol (n = 75 evaluable). Anesthesia induction time was significantly shorter in the 12 mg/kg remimazolam group compared with the 6 mg/kg group (p < 0.001). Time to loss of consciousness was significantly longer with remimazolam 6 or 12 mg/kg/h than with propofol (102.0 and 88.7 vs 78.7 s; p < 0.001 and p = 0.0149) as was time to extubation (19.2 and 19.2 vs 13.1 min; p = 0.0007 and p = 0.0006), and the time to leaving the operating theatre (p = 0.007 for both remimazolam dosages vs propofol) [1, 39, 43].
Results from phase 3 trials conducted in Russia (in 150 patients; NCT03669484) [44] and South Korea (in 198 patients; REVOLUTION study) [45] that compared IV remimazolam with IV propofol in surgery requiring general anesthesia confirmed the efficacy of remimazolam for the induction and maintenance of general anesthesia. In REVOLUTION, remimazolam was considered to be as effective as propofol.
The efficacy of IV remimazolam in the induction and maintenance of general anesthesia in adult patients (n = 90) undergoing cardiac surgery was established in a randomized, single-blind phase 2 comparison (NCT01937767; EudraCT 2013-001113-32) with propofol and sevoflurane (the standard regimen for cardiac surgery general anesthesia) [46]. Success of anesthesia (primary endpoint; defined as no need for another sedative between the start of the study medication and the end of the surgical procedure) was achieved in 98% (61 of 62) of remimazolam recipients and 96% (27 of 28) of propofol recipients. The onset (time to loss of consciousness and time with Narcotrend Index ≤ 60) and offset (time to extubation in PACU/ICU post surgery) of action profile of remimazolam and propofol was comparable across all treatment arms [46]. Patients were administered an IV infusion of remimazolam 6 or 12 mg/kg/h for induction (n = 34 and 28) and then remimazolam 1–3 mg/kg/h for maintenance (n = 62) or IV propofol 2–2.5 mg/kg administered over ≈ 1 min for induction and inhaled sevoflurane 0.8–2.5 MAC for maintenance (until the start of extracorporeal circulation) then maintenance IV propofol 3–12 mg/kg/h as an infusion (n = 28). During recovery, the remimazolam or propofol dosage was downtitrated then stopped. The anesthetic regimen also included fentanyl and remifentanil as opioid analgesics and neuromuscular blockade with rocuronium. Patients were aged 18–84 years (mean 60–64 years) and surgery was assumed to require > 2 h of maintenance of general anesthesia and use of extracorporeal circulation [46].
2.3.2 Procedural Sedation
2.3.2.1 Bronchoscopy
IV remimazolam provided effective procedural sedation for patients undergoing bronchoscopy (446 evaluable) in a randomized, double-blind, placebo-controlled phase 3 trial that also included an open-label IV midazolam arm (NCT02296892) [47]. The success rate with remimazolam (n = 310) was significantly higher than with placebo [n = 63] (80.6% vs 4.8%; p < 0.0001; primary outcome); the success rate in the midazolam arm (n = 73) was 32.9%. The success rate was a composite measure of completion of the procedure; no need for rescue sedative; and ≤ 5 doses of remimazolam or placebo in any 15-min window or ≤ 3 doses of midazolam in any 12-min window. Bronchoscopy started sooner with remimazolam than with placebo (after a mean 6.4 vs 17.2 min; p < 0.0001) or midazolam (mean 16.3 min).Time to full alertness at the end of bronchoscopy was shorter with remimazolam than placebo (median 6 vs 13.6 min; p = 0.0001) or midazolam (median 12 min); placebo recipients received midazolam rescue treatment because of inadequate sedation, which accounts for the time required to regain of full alertness [47]. A post-hoc analysis found that success rates with remimazolam in older (aged > 65 years) patients were consistent with those in younger patients (84% vs 77%), as was the time to being fully alert (7 vs 6 min) [48]. In this trial, patients received an initial dose of IV fentanyl 25–75 μg, followed by a single IV dose of remimazolam 5.0 mg or an equal volume of placebo (double-blinded) or IV midazolam 1–1.75 mg. If sedation (assessed using MOAA/S scores) was insufficient after the first dose, patients could receive up to 5 doses of remimazolam 2.5 mg or placebo in any 15-min window or 3 doses of midazolam 0.5–1.0 mg in any 12-min window at intervals of ≥ 2 min; in all three treatment arms, top-up doses of fentanyl 25 μg were permitted every 5–10 min until adequate analgesia was achieved or 200 μg had been administered. If sedation was then still inadequate, treatment failure was declared and rescue midazolam (dose determined by examining physician) was permitted [47].
2.3.2.2 Gastrointestinal Endoscopy
IV remimazolam provided effective procedural sedation for patients undergoing colonoscopy in a randomized, double-blind, placebo-controlled phase 3 trial (n = 461) that also included an open-label IV midazolam arm (NCT02290873) [49]. The success rate (primary endpoint), was significantly higher in remimazolam (n = 298) than placebo (n = 60) recipients (91.3% vs 1.7%; p < 0.0001); the success rate was 25.2% in the midazolam (n = 103) arm. Success rate was a composite measure of completion of the colonoscopy; no need for rescue medication; and ≤ 5 doses of remimazolam or placebo in any 15-min window or ≤ 3 doses of midazolam in any 12-min window. The median time to the start of the procedure was significantly shorter in the remimazolam arm than in the placebo group (4.0 vs 19.5 min; p < 0.0001), and was 19.0 min in midazolam recipients. The mean total dose of fentanyl administered in the remimazolam, placebo and midazolam groups was 88.6, 121.3 and 106.9 μg. The mean time to full alertness from the end of the procedure was significantly shorter with remimazolam than placebo (7.35 vs 21.95 min; p < 0.0001; placebo recipients received midazolam rescue treatment because of inadequate sedation, which accounts for the time required to regain of full alertness) and was 15.84 min with midazolam [49]. In this trial, patients received an initial dose of IV fentanyl 50–75 μg, followed by a single IV dose of remimazolam 5.0 mg or an equal volume of placebo (double-blinded) or IV midazolam 1–1.75 mg. Sedation (assessed using MOAA/S scores) was maintained by top-up doses of remimazolam 2.5 mg or an equal volume of placebo not more than 2 min apart or midazolam 0.5–1 mg. Patients could receive up to 5 doses of remimazolam or placebo in any 15-min window or 3 doses of midazolam in any 12-min window at intervals of ≥ 2 min; in all three treatment arms, top-up doses of fentanyl 25 μg were permitted every 5–10 min until adequate analgesia was achieved or 200 μg had been administered. If sedation was then still inadequate, treatment failure was declared and rescue midazolam (dose determined by the endoscopist) was permitted [49].
In patients with severe or life-threatening systemic disease (i.e. American Society of Anesthesiologists Grade III or IV status) who required colonoscopy and were enrolled in a randomized, double-blind, placebo-controlled phase 3 trial (n = 79) that also included an open-label IV midazolam arm (NCT02532647), IV remimazolam provided effective procedural sedation [50]. The success rate (primary endpoint), was achieved in 84.4% of remimazolam recipients (n = 32), no placebo recipients (n = 16) and 12.9% of midazolam recipients (n = 31). Success rate was a composite measure of completion of the colonoscopy; no need for rescue medication; and ≤ 5 doses of remimazolam or placebo in any 15-min window or ≤ 3 doses of midazolam in any 12-min window. The median time to full alertness from the end of the procedure was 3.0 min with remimazolam, 5.3 min with placebo and 7.0 min with midazolam. In this trial, patients received an initial dose of IV fentanyl 25–50 μg, followed by a single IV dose of remimazolam 2.5–5.0 mg or an equal volume of placebo (double-blinded) or IV midazolam 1 mg. Sedation (assessed using MOAA/S scores) was maintained by top-up doses of remimazolam 1.25–2.5 mg or an equal volume of placebo or midazolam 0.5 mg. Patients could receive up to 5 doses of remimazolam or placebo in any 15-min window or 3 doses of midazolam up to a maximum dose of 200 μg in any 12-min window at intervals of ≥ 2 min; in all three treatment arms, top-up doses of fentanyl 25 μg (maximum dose not reported) were permitted [50].
IV remimazolam provided significantly better procedural sedation than IV midazolam in a randomized, double-blind, phase 2b trial in patients undergoing colonoscopy (NCT01145222) [51]. The success rate with remimazolam [loading//maintenance doses of 8.0/3.0 mg (n = 40), 7.0/2.0 mg (n = 40) or 5.0/3.0 mg (n = 40)] was significantly higher than with midazolam at a loading/maintenance dose of 2.5/1.0 mg (n = 40) [92.5–97.5% vs 75.0%; p ≤ 0.025]. Success rate was a composite measure of: adequate sedation (assessed using MOAA/S scores) on three consecutive measurements taken every minute; completion of the procedure; no need for alternative and/or rescue sedative; and no manual or mechanical ventilation [51]. An exploratory, dose-finding, phase 2a comparison of IV remimazolam with IV midazolam in patients (n = 100) undergoing upper gastrointestinal endoscopy (NCT00869440) found that a single dose of IV remimazolam (0.1, 0.15 or 0.2 mg/kg) or IV midazolam 0.075 mg/kg induced rapid sedation (assessed using MOAA/S scores), with a quick recovery profile. A successful procedure was achieved in 32%, 56% and 64% of remimazolam recipients and 44% of midazolam recipients and the mean time to being fully alert in each of the treatment arms was 6.8, 9.0, 9.9 and 11.5 min [52].
Key clinical trials of remimazolam besylate
Drug(s) | Indication | Phase | Status | Sponsor | Location(s) | Identifier |
|---|---|---|---|---|---|---|
Anesthesia | ||||||
Remimazolam, propofol | Intravenous anesthesia | III | Recruiting | PAION | Europe | NCT03661489; EudraCT2018-000174-29 |
Remimazolam, propofol, fentanyl, rocuronium | General anesthesia | III | Completed | R-Pharm | Russia | NCT03669484 |
Remimazolam, propofol, sevoflurane, remifentanil, fentanyl, rocuronium | General anesthesia (cardiac surgery + post-operative ICU sedation) | II | Completed | PAION | Germany | NCT01937767; EudraCT2013-001113-32 |
Remimazolam, propofol | General anesthesia | IIb/III | Completed | Ono | Japan | JapicCTI121973 |
Remimazolam, propofol | General anesthesia | III | Completed | Hana Pharm | Korea | REVOLUTION |
Procedural sedation | ||||||
Remimazolam, midazolam, placebo | Bronchoscopy | III | Completed | PAION | USA | NCT02296892 |
Remimazolam, midazolam, placebo | Colonoscopy | III | Completed | PAION | USA | NCT02290873 |
Remimazolam, midazolam, placebo | Colonoscopy | III | Completed | PAION | USA | NCT02532647 |
Remimazolam, midazolam | Upper GI endoscopy | II | Completed | PAION | USA | NCT00869440 |
Remimazolam, midazolam | Colonoscopy | II | Completed | PAION | USA | NCT01145222 |
2.4 Adverse Events
In patients undergoing general anesthesia in the Japanese phase 2/3 trial (JapicCTI121973), adverse reactions were reported in 39.3% (59/150 patients) remimazolam 6 mg/kg/h recipients, 42% (64 of 150) of remimazolam 12 mg/kg/h recipients and 61.3% (46 of 75) of propofol recipients [1, 43]. The most frequent adverse reaction (incidence ≥ 5% in any treatment group) in the remimazolam 6 or 12 mg/kg/h and propofol groups were hypotension (20.0%, 24.0% and 49.3%), injection-site pain (0%, 0% and 18.7%) vomiting (4.7%, 7.3% and 4.0%) and nausea (7.3%, 6.7% and 5.3%). Vasopressor treatment for hypotension was required in 41.3% of remimazolam (n = 300) and 64.0% of propofol (n = 75) recipients and treatment for bradycardia was required in 6.3% and 9.3% of patients in either treatment group [1, 39].
The most common treatment-emergent adverse events (> 2% in any arm) reported in patients undergoing procedural sedation in the remimazolam 5 mg initial dose (n = 303), placebo (n = 59) or midazolam 1–1.75 mg initial dose (n = 69) arms in the phase 3 bronchoscopy trial (NCT02296892) [47] were hypertension (61.4%, 52.5% and 59.4%), hypotension (41.9%, 62.7% and 49.3%), hypoxia (21.8%, 20.3% and 18.8%), bradycardia (4.3%, 6.8% and 7.2%), decreased respiratory rate (2.3%, 3.4% and 4.3%), nausea (4.0%, 3.4% and 2.9%) and vomiting (2.0%, 1.7% and 2.9%). 90.5% of placebo recipients received rescue midazolam during the procedure; no patients in any treatment arm required reversal agents.
The most common treatment-emergent adverse events (> 2% in any arm) reported in the remimazolam 5 mg initial dose (n = 296), placebo (n = 60) or midazolam 1–1.75 mg initial dose (n = 102) arms in patients undergoing procedural sedation in the phase 3 colonoscopy trial (NCT02290873) [49] were hypotension (38.9%, 41.7% and 61.8%), hypertension (19.9%, 28.3% and 17.6%), bradycardia (11.1%, 11.7% and 15.7%), tachycardia (7.8%, 11.7% and 12.7%), nausea (1.7%, 6.7% and 2.0%), bradypnea (1.4%, 3.3% and 2.9%), hypoxia (1.0%, 3.3% and 1.0%), vomiting (1.0%, 3.3% and 0.0%) and headache (1.7%, 0.0% and 2.9%).
2.5 Ongoing Clinical Trials
A phase 3 trial of IV remimazolam in general anesthesia (NCT03661489; EudraCT2018-000174-29) being conducted in Europe is ongoing.
3 Current Status
Remimazolam was approved on 23 January 2020 in Japan for use in general anesthesia [4]; approval for use as procedural sedation in the USA and the EU is pending.
References
Mundipharma Co. Ltd. Remimazolam besylate (Anerem): Japanese prescribing information. 2020. https://www.pmda.go.jp/. Accessed 2 Mar 2020.
Cosmo Pharmaceuticals. Cosmo sub-licenses its ByFavo™ (remimazolam) US rights to Acacia Pharma, takes an initial 14.1% stake and provides finance for Acacia Pharma’s US expansion [media release]. 10 Jan 2020. https://www.cosmopharma.com/.
PAION AG. About remimazolam. 2020. https://www.paion.com/remimazolam/indikationen/leitsubstanz-remimazolam/. Accessed 2 Mar 2020.
PAION AG. PAION AG: Mundipharma receives market approval for Anerem® (remimazolam) in general anesthesia in Japan [media release]. 23 Jan 2020. https://www.paion.com/.
PAION AG. PAION announces submission of new drug application for remimazolam by its licensee Hana Pharm in South Korea [media release]. 30 Dec 2019. https://www.paion.com/.
Cosmo Pharmaceuticals. FDA accepts filing of NDA for remimazolam [media release]. 10 Jun 2019. https://www.cosmopharma.com.
Acacia Pharma Group plc. Acacia Pharma announces brief extension of FDA review period for NDA for BYFAVO™ [media release]. 12 Mar 2020. https://www.acaciapharma.com/.
PAION AG. PAION announces submission of the marketing authorization application for remimazolam in procedural sedation to the European Medicines Agency [media release]. 20 Nov 2019. https://www.paion.com/.
Wesolowski AM, Zaccagnino MP, Malapero RJ, et al. Remimazolam: pharmacologic considerations and clinical role in anesthesiology. Pharmacotherapy. 2016;36(9):1021–7.
Pambianco DJ, Cash BD. New horizons for sedation: the ultrashort acting benzodiazepine remimazolam. Tech Gastrointest Endosc. 2016;18(1):22–8.
Kilpatrick GJ, McIntyre MS, Cox RF, et al. CNS 7056: a novel ultra-short-acting benzodiazepine. Anesthesiology. 2007;107(1):60–6.
PAION AG. PAION grants Cosmo Pharmaceuticals remimazolam license in the U.S. and Cosmo becomes largest shareholder of PAION AG [media release]. 24 June 2016. https://www.paion.com.
PAION AG. PAION grants remimazolam license for Southeast Asia to Hana Pharm [media release]. 8 Jan 2020. https://www.paion.com.
PAION AG. PAION announces clinical development progress with remimazolam by its partner Hana Pharm in South Korea [media release]. 22 Mar 2018. https://www.paion.com/.
PAION AG. Paion grants exclusive license to Mundipharma for development and commercialization of remimazolam in Japan [media release]. 18 Dec 2017. https://www.paion.com.
PAION AG. PAION grants licence to remimazolam in Canada to Pendopharm [media release]. 9 July 2015. https://www.paion.com/.
PAION AG. PAION AG grants license to R-Pharm for remimazolam [media release]. 30 Oct 2013. https://www.paion.com.
PAION AG. PAION AG grants license for Turkey to add to R-Pharm territory [media release]. 26 Nov 2013. https://www.paion.com/.
TRPharm. PAION AG (ISIN DE000A0B65S3; Frankfurt Stock Exchange Prime Standard: PA8), a specialty pharma company, and R-Pharm, Russia, today announced that they have extended their license agreements for remimazolam to include the "MENA Region" (Middle East andNorth Africa) [media release]. 17 June 2014. https://www.trpharm.com.
PAION AG. PAION AG grants license to Yichang Humanwell for remimazolam in China [media release]. 18 July 2012. https://www.paion.com/.
CeNeS Pharmaceuticals plc. CeNeS licenses new intravenous anaesthetic to Ono in Japan [media release]. 6 Aug 2007. https://www.cenes.com.
PAION AG. PAION's partner Ono decides not to file for regulatory approval of remimazolam in Japan and will return its remimazolam rights for Japan to Paion [media release]. 5 Nov 2014. https://www.paion.de.
PAION AG. PAION AG reports on financial year and financial results 2015 [media release]. 22 Mar 2016. https://www.paion.de.
PAION AG. PAION AG completes acquisition of CeNeS [media release]. 23 June 2008. https://www.paion.de.
CeNeS Pharmaceuticals plc. CeNeS announces preliminary results for the year ended 31 December 2003 [media release]. 20 Apr 2004. https://www.cenes.com.
Ono Pharmaceutical Co L. Ono enters into license agreement with CeNeS Limited [media release]. 6 Aug 2007. https://www.cenes.com.
Google Patents. Dosing regimen for sedation with CNS 7056 (remimazolam). 2020. https://patents.google.com/patent/US10195210B2/en. Accessed 17 Feb 2020.
PAION AG. European Patent Office grants formulation patent for remimazolam in the EU [media release]. 28 Sep 2017. https://www.paion.com.
PAION AG. US Patent & Trademark office grants substance patent for remimazolam besylate [media release]. 12 Feb 2016. https://www.paion.com/.
PAION AG. Japan Patent Office grants dosing patent for remimazolam in Japan [media release]. 13 Oct 2017. https://www.paion.com/.
CeNeS Pharmaceuticals plc. CeNeS is granted patent for short-acting sedatives, including CNS 7056X, by the European Patent Office [media release]. 8 Feb 2006. https://www.cenes.com.
Bevans T, Deering-Rice C, Stockmann C, et al. Inhaled remimazolam potentiates inhaled remifentanil in rodents. Anesth Analg. 2017;124(5):1484–90.
Wiltshire HR, Kilpatrick GJ, Tilbrook GS, et al. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part II—population pharmacokinetic and pharmacodynamic modeling and simulation. Anesth Analg. 2012;115(2):284–96.
Antonik LJ, Goldwater DR, Kilpatrick GJ, et al. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part I—safety, efficacy, and basic pharmacokinetics. Anesth Analg. 2012;115(2):274–83.
Sheng XY, Liang Y, Yang XY, et al. Safety, pharmacokinetic and pharmacodynamic properties of single ascending dose and continuous infusion of remimazolam besylate in healthy Chinese volunteers. Eur J Clin Pharmacol. 2020;76(3):383–91.
Schuttler J, Eisenried A, Lerch M, et al. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part I—pharmacokinetics and clinical pharmacodynamics. Anesthesiology. 2020;132(4):636–51.
Eisenried A, Schuttler J, Lerch M, et al. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part II—pharmacodynamics of electroencephalogram effects. Anesthesiology. 2020;132(4):652–66.
Lohmer LL, Schippers F, Petersen KU, et al. Time-to-event modeling for remimazolam for the indication of induction and maintenance of general anesthesia. J Clin Pharmacol. 2020. https://doi.org/10.1002/jcph.1552.
Sato S, Doi M, Morita K, et al. Remimazolam a new ultra-short acting anesthetic shows similar efficacy and superior hemodynamic stability vs. propofol in general surgery patients with TIVA: results of a randomised, non-inferiority, phase IIb/III trial [abstract no. A5018]. In: 2015 Annual Meeting of the American Society of Anesthesiologists. 2015.
Worthington MT, Antonik LJ, Goldwater DR, et al. A phase Ib, dose-finding study of multiple doses of remimazolam (CNS 7056) in volunteers undergoing colonoscopy. Anesth Analg. 2013;117(5):1093–100.
Kleiman RB, Darpo B, Thorn M, et al. Potential strategy for assessing QT/QTc interval for drugs that produce rapid changes in heart rate: electrocardiographic assessment of the effects of intravenous remimazolam on cardiac repolarization. Br J Clin Pharmacol. 2020.
Freyer N, Knospel F, Damm G, et al. Metabolism of remimazolam in primary human hepatocytes during continuous long-term infusion in a 3-D bioreactor system. Drug Des Dev Ther. 2019;13:1033–47.
Mundipharma Co. Ltd. Remimazolam pharmaceutical interview form. 2020 https://www.info.pmda.go.jp/go/interview/1/770098_11194A1F1029_1_1F.pdf. Accessed 12 Feb 2020.
R-Pharm Group, PAION AG. R-Pharm Group and PAION announces successful completion of remimazolam phase III clinical trial [media release]. 6 Nov 2018. http://www.r-pharm.com/en/.
Hana Pharma. Remizolam, “identical to propofol in general anesthesia, demonstrating reduced side effects” [media release]. 2019. https://www.hanaph.co.kr/.
EU Clinical Trials Register. A randomized, single-blind phase II study evaluating the efficacy, safety and pharmacokinetics of remimazolam in general anesthesia in adult patients undergoing cardiac surgery, including follow-up sedation in the post-anesthesia care unit/intensive care unit. 2016. https://www.clinicaltrialsregister.eu/ctr-search/trial/2013-001113-32/results. Accessed 2 Mar 2020.
Pastis NJ, Yarmus LB, Schippers F, et al. Safety and efficacy of remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest. 2019;155(1):137–46.
Hill N, Pastis NJ, Schippers F, et al. Comparing the efficacy and safety of remimazolam versus midazolam based on age and ASA class in sedation for bronchoscopy [abstract no. A1399]. Am J Respir Crit Care Med. 2019;199(9).
Rex DK, Bhandari R, Desta T, et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88(3):427–37.e6.
ClinicalTrials.gov. Safety and efficacy of remimazolam in ASA III and IV patients undergoing colonoscopy [NCT02532647]. 2015. https://clinicaltrials.gov/. Accessed 12 Feb 2020.
Pambianco DJ, Borkett KM, Riff DS, et al. A phase IIb study comparing the safety and efficacy of remimazolam and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2016;83(5):984–92.
Borkett KM, Riff DS, Schwartz HI, et al. A phase IIa, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth Analg. 2015;120(4):771–80.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Susan Keam is a salaried employee of Adis International Ltd/Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
Enhanced material for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.12017124.
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Keam, S.J. Remimazolam: First Approval. Drugs 80, 625–633 (2020). https://doi.org/10.1007/s40265-020-01299-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01299-8