Abstract
Voxelotor (Oxbryta™) is a haemoglobin S polymerization inhibitor that has been developed for the treatment of sickle cell disease. In November 2019, voxelotor received its first global approval in the USA for the treatment of sickle cell disease in adults and paediatric patients aged ≥ 12 years. The drug was granted accelerated approval based on the results of the phase III HOPE trial. Phase III clinical development of voxelotor for sickle cell disease is ongoing worldwide. Voxelotor also has Orphan Drug designation and Priority Medicine status in Europe for the treatment of sickle cell disease. This article summarizes the milestones in the development of voxelotor leading to this first approval as a disease-modifying agent for sickle cell disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A haemoglobin S polymerization inhibitor being developed by Global Blood Therapeutics for the treatment of sickle cell disease |
Received its first approval on 25 November 2019 in the USA |
Approved for the treatment of sickle cell disease in adults and paediatric patients aged ≥ 12 years |
1 Introduction
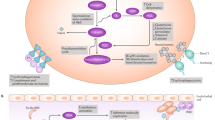
Sickle cell disease is one of the most common inherited diseases worldwide [1]. It is caused by a single amino acid substitution in the gene encoding the β-globin subunit of haemoglobin [1, 2]. Deoxygenated haemoglobin molecules that include two mutant sickle β-globin subunits (i.e. haemoglobin S) can polymerize within red blood cells (RBCs), causing them to take on a characteristic sickle-like shape [1, 2]. Sickle RBCs are prone to haemolysis, vaso-occlusion and inflammation, which lead to acute complications (e.g. vaso-occlusive pain crises, acute chest syndrome, stroke) as well as chronic complications (e.g. anaemia, chronic kidney disease) which can result in multiorgan damage and premature death [2].
Voxelotor (Oxbryta™) is a haemoglobin S polymerization inhibitor developed by Global Blood Therapeutics for the treatment of sickle cell disease. The US FDA granted voxelotor Fast Track [3], Orphan Drug [4], Rare Pediatric Disease [5] and Breakthrough Therapy [6] designations for the treatment of sickle cell disease. In September 2019, the US FDA accepted the New Drug Application and granted a priority review [7]. Voxelotor received its first global approval on 25 November 2019, in the USA, for the treatment of sickle cell disease in adults and paediatric patients aged ≥ 12 years [8]. Voxelotor was granted accelerated approval based on the results of the phase III HOPE trial (NCT03036813) [9]. As a condition of approval, Global Blood Therapeutics will conduct a post-approval confirmatory trial (HOPE-KIDS 2; EudraCT2017-000903-26) in paediatric patients (Sect. 2.5) [10]. The recommended dosage of voxelotor in adults and adolescents aged ≥ 12 years is 1500 mg once daily, with or without food [8]. The dosage of voxelotor should be reduced to 1000 mg in patients with severe hepatic impairment (Child–Pugh class C) and in patients receiving concomitant strong CYP3A4 inhibitors or fluconazole, and increased to 2500 mg in patients receiving concomitant strong or moderate CYP3A4 inducers (Sect. 2.2). Voxelotor may be given with or without hydroxyurea [8].
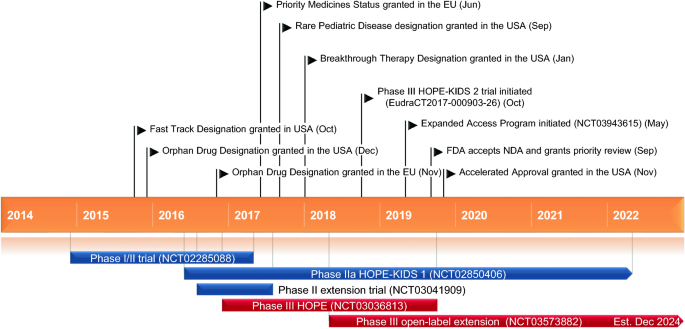
Key milestones in the development of voxelotor for the treatment of sickle cell disease
Voxelotor is in phase III development for the treatment of sickle cell disease worldwide. It was granted Orphan Drug designation in Europe in December 2016 [11] and was given Priority Medicines status by the European Medicines Agency in June 2017 [12]. Development of voxelotor for the treatment of idiopathic pulmonary fibrosis and hypoxia has been discontinued.
1.1 Patent Information
Global Blood Therapeutics holds US patents covering voxelotor which will expire between 2032 and 2035. The company also owns or co-owns additional pending patent applications in multiple counties worldwide relating to voxelotor.
2 Scientific Summary
2.1 Pharmacodynamics
The haemoglobin S polymerization inhibitor voxelotor binds reversibly to the N-terminal α chain of haemoglobin S in a 1:1 stoichiometry [13,14,15], partitioning highly and favourably into blood with a RBC/plasma ratio of ≈ 150 [13]. Voxelotor inhibits the polymerization of haemoglobin S by increasing haemoglobin’s affinity for oxygen [8].

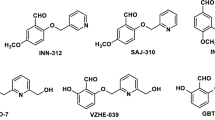
Chemical structure of voxelotor
In preclinical studies, voxelotor dose-dependently increased the affinity of haemoglobin S for oxygen, as determined by the change in partial pressure of oxygen at which haemoglobin is 50% saturated with oxygen (p50) [14]. Voxelotor inhibited RBC sickling in vitro and in a murine model of sickle cell disease [14]. Moreover, voxelotor reversed in vitro sickling of already sickled RBCs under hypoxic conditions [16]. Voxelotor also maintained the deformability of sickle RBCs and reduced the hyperviscosity of sickle cell blood under deoxygenated conditions [17].
In a phase I/II trial in patients with sickle cell disease (NCT02285088), voxelotor (500–1000 mg daily) demonstrated rapid and durable haematological improvements [18]. Treatment with voxelotor was associated with dose-dependent reductions in p20 and p50, indicating increased haemoglobin-oxygen affinity. Voxelotor increased haemoglobin levels and reduced other measures of haemolysis, including unconjugated bilirubin, reticulocyte count, dense RBCs and percentage of sickled red cells, with these improvements maintained over the longer term (≥ 90 days) [18]. Voxelotor also improved RBC physiology in children aged 4–11 years with sickle cell disease participating in the phase IIa HOPE-KIDS 1 trial (abstract; NCT02850406; Sect. 2.3.2) [19].
Administration of voxelotor at plasma concentrations ≈ 2-fold above therapeutic concentrations was not associated with clinically relevant QT interval prolongation [8].
2.2 Pharmacokinetics
After being absorbed into the blood, voxelotor is primarily distributed into RBCs due to its preferential binding to haemoglobin [8]. Voxelotor exhibits a linear pharmacokinetic profile [8, 15]. Dose-proportional increases in maximum concentration (Cmax) and area under the concentration–time curve (AUC) in whole blood, plasma and RBCs are seen with single or multiple doses of voxelotor 1500 mg [8]. Steady state concentrations are reached within 8 days of repeated administration. The median Cmax in plasma and whole blood is observed after 2 h, while the mean time to Cmax in whole blood and RBCs is between 6 and 18 h. Voxelotor can be administered with or without food (Sect. 1). Administration of a high-fat, high-caloric meal increased the voxelotor AUC by 42% in both whole blood and plasma, and increased the voxelotor Cmax by 45% in whole blood and by 95% in plasma [8].
The apparent volume of distribution of voxelotor in plasma in the central and peripheral compartment is 338 L and 72 L [8]. The drug is highly protein-bound (99.8%) and the blood-to-plasma ratio in patients with sickle cell disease is ≈ 15:1. Metabolism of voxelotor involves oxidation, reduction and glucuronidation. Oxidation of voxelotor is catalyzed primarily by CYP3A4 and, to a lesser extent, by CYP2B6, CYP2C9 and CYP2C19. Following administration of radiolabeled voxelotor, ≈ 63% of the radioactivity is excreted in the faeces (33% as unchanged drug) and 36% is excreted in the urine (0.1% as unchanged drug). The estimated apparent clearance after oral administration of voxelotor in patients with sickle cell disease is 6.7 L/h [8]. Following single doses of voxelotor (100–2800 mg) in a phase I/II trial (NCT02285088), the terminal half-life of voxelotor was shorter in patients with sickle cell disease (50 h) than in healthy volunteers (61–85 h) [15].
The phase IIa HOPE-KIDS 1 trial (NCT02850406; Sect. 2.3.2) demonstrated that the pharmacokinetics of voxelotor in adolescents aged 12 to < 18 years were similar to those observed in adults with sickle cell disease [20]. Voxelotor exposure was higher in children aged 6 to < 12 years compared with adolescents and adults; therefore, lower dosages of voxelotor are recommended in children aged < 12 years [20]. Age (12–59 years), sex, bodyweight (28–135 kg) and mild to severe renal impairment (creatinine clearance of 15–89 mL/min) had no clinically meaningful effect on the pharmacokinetics of voxelotor [8]. Following a single dose of voxelotor 900 mg, whole blood exposure was 25% lower in subjects with severe renal impairment (estimated glomerular filtration rate of < 30 mL/min/1.73 m2) than in subjects with normal renal function. Whole blood exposure to voxelotor was 14%, 15% and 90% higher in subjects with mild, moderate and severe hepatic impairment (Child–Pugh classes A, B and C) than in subjects with normal hepatic function. Whole blood exposure to voxelotor was also higher in patients with haemoglobin SC genotype than in patients with haemoglobin SS genotype [8].
2.2.1 Drug Interactions
Voxelotor inhibits CYP3A4, but not CYP1A2, CYP2C8, CYP2C9, CYP2C19 or CYP2D6 [8]. Voxelotor is a reversible and time-dependent inhibitor and inducer of CYP2B6. In vitro, voxelotor is not an inhibitor of P-glycoprotein, BRCP, OATP1B1, OATP1B3, OCT2, OAT1, OAT3, MATE1, MATE2-K or BSEP, and is not a substrate of P-glycoprotein, BRCP, OATP1A2, OATP1B1, OATP1B3 or BSEP. Coadministration of voxelotor with strong CYP3A4 inhibitors (e.g. ketoconazole) or fluconazole should be avoided since coadministration may increase voxelotor plasma concentrations and increase the risk of toxicity. If concomitant use is unavoidable, the dosage of voxelotor should be reduced to 1000 mg once daily (Sect. 1). Coadministration of voxelotor with fluconazole (a moderate CYP3A4 inhibitor, a moderate CYP2C9 inhibitor and a strong CYP2C19 inhibitor) is predicted to increase the voxelotor AUC by 40–116%. Coadministration of voxelotor with strong (e.g. rifampin) or moderate (e.g. efavirenz) CYP3A4 inducers should be avoided since coadministration may decrease voxelotor plasma concentrations and lead to reduced efficacy. If concomitant use is unavoidable, the dosage of voxelotor should be increased to 2500 mg once daily (Sect. 1). Coadministration of voxelotor with sensitive CYP3A4 substrates with a narrow therapeutic index (e.g. midazolam) should be avoided since coadministration may increase the exposure of the sensitive CYP3A4 substrate. If concomitant use is unavoidable, dose reduction of the sensitive CYP3A4 substrate should be considered [8].
Features and properties of voxelotor
Alternative names | GBT-440; GTX-011; Oxbryta |
Class | Antianaemics; benzaldehydes; ethers; pyrazoles; pyridines; small molecules |
Mechanism of action | Sickle haemoglobin modulator |
Route of administration | Oral (tablets) |
Pharmacodynamics | Binds to haemoglobin S with a 1:1 stoichiometry; dose-dependently inhibits haemoglobin S polymerization by increasing the affinity of haemoglobin for oxygen; inhibits RBC sickling, improves RBC deformability and reduces whole blood viscosity |
Pharmacokinetics | Linear, dose-dependent pharmacokinetics; apparent volume of distribution 338 L and 72 L in central and peripheral compartments; highly protein-bound (99.8%); estimated apparent oral clearance 6.7 L/h; terminal half-life 50 h in patients with sickle cell disease and 61–85 h in healthy volunteers |
Most frequent adverse events | Abdominal pain, diarrhoea, headache, nausea, rash |
ATC codes | |
WHO ATC code | B03X (other antianemic preparations); R07A (other respiratory system products) |
EphMRA ATC code | B3X (other anti-anaemic products, including folic acid, folinic acid); R7X (all other respiratory system products) |
Chemical name | Benzaldehyde,2-hydroxy-6-((2-(1-(1-methylethyl)-1H-pyrazol-5-yl)-3-pyridinyl)methoxy)- |
2.3 Therapeutic Trials
2.3.1 Phase III
Voxelotor significantly increased haemoglobin levels and reduced markers of haemolysis in patients with sickle cell disease participating in the multicentre, double-blind, randomized, phase III HOPE trial (NCT03036813) [21]. The trial enrolled 274 patients aged 12–65 years with confirmed sickle cell disease, a haemoglobin level of 5.5–10.5 g/L and 1–10 vaso-occlusive crises (VOCs) in the previous 12 months. Patients were randomized to receive oral voxelotor 900 mg (n = 92), voxelotor 1500 mg (n = 90) or placebo (n = 92) once daily for up to 72 weeks [21]. Patients who completed 72 weeks of treatment with voxelotor were eligible to enrol in the open-label extension of the HOPE trial (Sect. 2.5).
The proportion of patients with a haemoglobin response (defined as an increase from baseline of > 1.0 g/dL at week 24; primary endpoint) in the intention-to-treat (ITT) population was 33 and 51% in the voxelotor 900 and 1500 mg once daily groups, respectively, compared with 7% in the placebo group; the difference between voxelotor 1500 mg once daily and placebo was statistically significant (p < 0.001) [21]. Similar results were seen in the per-protocol (PP) population (38 and 59% vs 9%). Higher rates of haemoglobin response rates were observed with voxelotor 1500 mg once daily than with placebo regardless of concomitant hydroxyurea use or baseline anaemia severity [21].
The annualized incidence rates of acute anaemia (defined as a decrease from baseline in the haemoglobin level of > 2.0 g/dL at any time during the trial) were lower with voxelotor 900 mg once daily (0.04 episodes per person-year) and voxelotor 1500 mg once daily (0.06 episodes per person-year) than with placebo (0.18 episodes per person-year) [21]. The adjusted mean change in haemoglobin level from baseline to week 24 in the ITT population was 0.6 g/dL with voxelotor 900 mg once daily, 1.1 g/dL with voxelotor 1500 mg once daily and –0.1 g/dL with placebo. Similar results were seen in the PP population (0.7, 1.3 and 0 g/dL, respectively). The proportion of patients with a haemoglobin level of ≥ 10 g/dL at week 24 was 20% with voxelotor 900 mg once daily, 41% with voxelotor 1500 mg once daily and 9% with placebo. Haemoglobin occupancy (geometric mean percentage), defined as the percentage of haemoglobin bound by voxelotor, was 17.1% in the voxelotor 900 mg once daily group and 26.5% in the voxelotor 1500 mg once daily group [21].
At week 24, voxelotor 1500 mg was associated with significantly (p < 0.001) greater reductions from baseline in indirect bilirubin level and percentage of reticulocytes than placebo [21]. Among patients in the PP population receiving voxelotor 1500 mg once daily (n = 74), those with haemoglobin changes of > 1.0 g/dL had the greatest reductions in markers of haemolysis (indirect bilirubin level, percentage of reticulocytes, absolute reticulocyte count and lactate dehydrogenase level) compared with those with haemoglobin changes of ≤ 1.0 g/dL [22].
The proportion of patients who underwent red-cell transfusions during the trial was 32% in the voxelotor 900 mg once daily group, 33% in the voxelotor 1500 mg once daily group and 25% in the placebo group [21]. Most transfusions were performed because of acute VOCs. The annualized incidence rate of VOC were lower was 2.76 crises per person-year with voxelotor 900 mg once daily, 2.77 crises per person-year with voxelotor 1500 mg once daily and 3.19 crises per person-year with placebo. The proportion of patients with ≥ 1 VOC was 66% with voxelotor 900 mg once daily, 67% with voxelotor 1500 mg once daily and 69% with placebo. A total of 183 VOCs occurred in the voxelotor 900 mg once daily group, 179 occurred in the voxelotor 1500 mg once daily group and 219 occurred in the placebo group [21].
2.3.2 Phase IIa
Voxelotor was associated with a robust improvement in haemoglobin and reduced haemolysis in adolescents with sickle cell disease in the HOPE-KIDS 1 trial (NCT02850406) [23]. The trial enrolled 15 patients aged 12–17 years, all of whom received voxelotor 1500 mg/day for up to 24 weeks. At week 16, 55% of patients achieved an increase from baseline in the haemoglobin level of > 1.0 g/dL (median increase 1.1 g/dL). Median reductions from baseline in indirect bilirubin, percentage of reticulocytes and lactate dehydrogenase were 36.9, 5.8 and 23.1%, respectively [23].
Voxelotor did not increase cerebral blood flow (CBF) in an ancillary study of three patients enrolled in HOPE-KIDS 1 [24]. One patient experienced no change in CBF after 56 days of treatment with voxelotor 1500 mg/day. In the other two patients, treatment with voxelotor 900 mg/day for 36–80 days reduced global CBF and increased haemoglobin levels, suggesting improved oxygen delivery to the brain. No new or evolving areas of ischaemia were detected on MRI with angiography [24].
2.3.3 Case Series
The efficacy of voxelotor for the treatment of sickle cell disease was confirmed in a case series of seven patients aged 22–67 years who received voxelotor via a compassionate access scheme [25]. All patients had severe, treatment-refractory sickle cell disease with a haemoglobin level of < 6.0 g/dL or extreme/life-threatening complications. The dosage of voxelotor was 900 mg once daily, which could be increased to 1500 mg once daily. Haemoglobin levels increased rapidly (as early as 2 weeks after starting voxelotor), with five patients experiencing an increase of ≥ 1 g/dL. The number of transfusions decreased by 60% during 24 weeks of voxelotor treatment, compared with the 24-week period before treatment initiation (13 vs 33). Improvements in blood oxygenation were observed in all four patients who had low room air oxygen saturation at baseline. The number of hospitalizations for VOC pain decreased by 67% during 24 weeks of voxelotor treatment, compared with the 24-week period prior to treatment initiation (9 vs 28). All patients experienced improved well-being, most often within 2–3 weeks of starting treatment [25].
Key clinical trials of voxelotor in sickle cell disease (Global Blood Therapeutics)
Drug(s) | Phase | Status | Location(s) | Identifier |
|---|---|---|---|---|
Voxelotor, placebo | III | Ongoing | USA | HOPE-KIDS 2; EudraCT2017-000903-26; GBT440-032 |
Voxelotor, placebo | III | Completed | Multinational | HOPE; NCT03036813; EudraCT2016-003370-40; GBT440-031 |
Voxelotor | III | Ongoing | Multinational | NCT03573882; EudraCT2017-004045-25; GBT440-034 |
Voxelotor | IIa | Ongoing | Multinational | HOPE-KIDS 1; NCT02850406; GBT440-007 |
Voxelotor | II | Completed | United Kingdom | NCT03041909; GBT440-024 |
Voxelotor, placebo | I/II | Completed | United Kingdom | NCT02285088; UKCRN18490; GBT440-001 |
Voxelotor | I | Completed | USA | NCT02497924; GBT440-002 |
Voxelotor | I | Completed | USA | NCT02567695; GBT440-004 |
Voxelotor, caffeine, warfarin, omeprazole, midazolam | I | Completed | USA | NCT02567682; GBT440-003 |
Voxelotor | I | Completed | USA | NCT03114540; GBT440-0112 |
Voxelotor | I | Completed | USA | NCT03151015; GBT440-0110 |
2.4 Adverse Events
Voxelotor was generally well tolerated in patients with sickle cell disease in the phase III HOPE trial (NCT03036813) [21]. Most adverse events (AEs) were grade 1 or 2 in severity and were considered to be unrelated to study treatment. Treatment-related AEs (TRAEs) occurred in 32, 39 and 25% of patients in the voxelotor 900 mg once daily, voxelotor 1500 mg once daily and placebo groups, respectively. Corresponding rates of serious TRAEs were 3, 3 and 1%, respectively. The most common TRAEs occurring in ≥ 5% of patients and with a numerically higher incidence in the voxelotor 900 or 1500 mg once daily groups than the placebo group were diarrhoea (9 and 13 vs 3%), nausea (7 and 7 vs 6%), abdominal pain (7 and 7 vs 1%), headache (3 and 6 vs 3%) and rash (2 and 8 vs 4%). The proportions of patients who discontinued treatment due to AEs were 5% in the voxelotor 900 mg once daily group, 9% in the voxelotor 1500 mg group and 4% in the placebo group.
Voxelotor was also generally well tolerated in adolescents with sickle cell disease in the phase IIa HOPE-KIDS 1 trial (NCT02850406) [23]. Most TRAEs were grade 1 or 2 in severity, with the exception of one patient with grade 3 rash. No patients discontinued treatment due to AEs [23].
2.5 Ongoing Clinical Trials
An open-label extension of the HOPE trial is currently underway to assess the effect of long-term treatment with voxelotor in patients with sickle cell disease (NCT03573882). A total of 176 patients from ≈ 100 sites worldwide who participated in the HOPE trial have been enrolled. The estimated study completion date is December 2024.
The phase III HOPE-KIDS 2 trial (EudraCT2017-000903-26) is using a novel approach to determine a weight band-based voxelotor dosing regimen for infants and children with sickle cell disease to help simplify dosing in the paediatric population [26]. The primary objective of part A of the trial is to assess the efficacy of long-term voxelotor in paediatric patients aged 2 to < 12 years with sickle cell disease, as measured by improvement in anaemia. The primary objective of part B of the trial is to evaluate the safety and tolerability of long-term voxelotor in infants aged 9 months to < 2 years with sickle cell disease. Following completion of the trial, patients may be eligible for additional treatment with voxelotor in a separate, open-label study.
3 Current Status
Voxelotor received its first global approval on 25 November 2019 in the USA for the treatment of sickle cell disease in adults and paediatric patients aged ≥ 12 years [8].
References
Gardner RV. Sickle cell disease: advances in treatment. Ochsner J. 2018;18(4):377–89.
Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers. 2018. https://doi.org/10.1038/nrdp.2018.10.
Global Blood Therapeutics. Global Blood Therapeutics announces GBT440 receives FDA fast track designation for sickle cell disease [media release]. 12 Oct 2015. http://www.globalbloodtx.com.
Global Blood Therapeutics. Global Blood Therapeutics receives FDA orphan drug designation for GBT440 in sickle cell disease [media release]. 31 Dec 2015. http://www.globalbloodtx.com.
Global Blood Therapeutics. Global Blood Therapeutics receives rare pediatric disease designation from FDA for GBT440 for treatment of sickle cell disease (SCD) [media release]. 5 Sep 2017. http://www.globalbloodtx.com.
Global Blood Therapeutics. GBT receives FDA breakthrough therapy designation for voxelotor for treatment of sickle cell disease (SCD) [media release]. 9 Jan 2018. http://www.gbt.com.
Global Blood Therapeutics. GBT announces U.S. Food and Drug Administration acceptance of new drug application and priority review for voxelotor for the treatment of sickle cell disease [media release]. 5 Sep 2019. http://www.gbt.com.
Global Blood Therapeutics. Oxbryta™ (voxelotor) tablets, for oral use: US prescribing information. 2019. http://www.accessdata.fda.gov. Accessed 6 Jan 2020.
US Food & Drug Administration. FDA approves novel treatment to target abnormality in sickle cell disease [media release]. 25 Nov 2019. http://www.fda.gov.
Global Blood Therapeutics. FDA approves Oxbryta(TM) (voxelotor), the first medicine specifically targeting the root cause of sickle cell disease [media release]. 25 Nov 2019. http://www.gbt.com.
Global Blood Therapeutics. Global Blood Therapeutics announces GBT440 granted orphan drug designation in Europe for treatment of sickle cell disease [media release]. 30 Nov 2016. http://www.globalbloodtx.com.
Global Blood Therapeutics. Global Blood Therapeutics receives EMA PRIME designation for GBT440 for the treatment of sickle cell disease (SCD) [media release]. 28 Jun 2017. http://www.globalbloodtx.com.
Metcalf B, Chuang C, Dufu K, et al. Discovery of GBT440, an orally bioavailable R-state stabilizer of sickle cell hemoglobin. ACS Med Chem Lett. 2017;8(3):321–6.
Oksenberg D, Dufu K, Patel MP, et al. GBT440 increases haemoglobin oxygen affinity, reduces sickling and prolongs RBC half-life in a murine model of sickle cell disease. Br J Haematol. 2016;175(1):141–53.
Hutchaleelaha A, Patel M, Washington C, et al. Pharmacokinetics and pharmacodynamics of voxelotor (GBT440) in healthy adults and patients with sickle cell disease. Br J Clin Pharmacol. 2019;85(6):1290–302.
Dufu K, Oksenberg D. GBT440 reverses sickling of sickled red blood cells under hypoxic conditions in vitro. Hematol Rep. 2018;10(2):7419.
Dufu K, Patel M, Oksenberg D, et al. GBT440 improves red blood cell deformability and reduces viscosity of sickle cell blood under deoxygenated conditions. Clin Hemorheol Microcirc. 2018;70(1):95–105.
Howard J, Hemmaway CJ, Telfer P, et al. A phase 1/2 ascending dose study and open-label extension study of voxelotor in patients with sickle cell disease. Blood. 2019;133(17):1865–75.
Chonat S, Fields E, Baratz H, et al. Improvement in red blood cell physiology in children with sickle cell anemia receiving voxelotor [abstract]. Blood. 2019;134(Suppl 1):2281.
Washington CB, Green M, Inati AC, et al. Pharmacokinetics (PK) of voxelotor (GBT440) using population pharmacokinetic (PPK) and physiologically based pharmacokinetic (PBPK) modeling in pediatric subjects with sickle cell disease (SCD) [abstract no. PF713]. HemaSphere. 2018;2(Suppl 2):306.
Vichinsky E, Hoppe CC, Ataga KI, et al. A phase 3 randomized trial of voxelotor in sickle cell disease. N Engl J Med. 2019;381(6):509–19.
Howard J, vichinsky E, Knight-Madden J, et al. Correlation of voxelotor exposure with hemoglobin response and measures of hemolysis in patients from the HOPE study [abstract no. 712]. Blood. 2019;134(Suppl 1):1020.
Brown C, Hoppe C, Inati A, et al. Phase 2a study (GBT440-007) of voxelotor in adolescents with sickle cell disease [abstract no. 711]. Pediatr Blood Cancer. 2019;66(Suppl 2):S241–S2.
Estepp JH, Wang W, Hwang S, et al. Cerebral blood flow in adolescents with sickle cell anemia receiving voxelotor [abstract no. PF740 plus poster]. HemaSphere. 2019;3(Suppl 1):324.
Blyden G, Bridges KR, Bronte L. Case series of patients with severe sickle cell disease treated with voxelotor (GBT440) by compassionate access. Am J Hematol. 2018;93(8):E188–90.
Washington CB, Goldstein B, Dixon S, et al. Voxelotor dose extrapolation in a phase 3, randomized, double-blind, placebo-controlled study in pediatric patients with sickle cell disease (GBT440-032, HOPE KIDS 2) [abstract no. PS1453]. HemaSphere. 2018;2(Suppl 2):666.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Hannah A. Blair is a salaried employee of Adis International Ltd/Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
Enhanced material for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.11658999.
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Blair, H.A. Voxelotor: First Approval. Drugs 80, 209–215 (2020). https://doi.org/10.1007/s40265-020-01262-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01262-7