Abstract
Zanubrutinib (Brukinsa®), an orally-administered Bruton tyrosine kinase (BTK) inhibitor, is being developed by BeiGene for the treatment of B-cell malignancies. Zanubrutinib received accelerated approval in the USA on 14 November 2019 for the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy, based on overall response rate (ORR) seen in phase II and I/II clinical trials. This article summarizes the milestones in the development of zanubrutinib leading to this first approval for the treatment of MCL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A BTK inhibitor is being developed by BeiGene for B-cell malignancies |
Received its first approval on 14 Nov 2019 in USA |
Approved for use in adult patients with MCL who have received at least one prior therapy |
1 Introduction
Zanubrutinib (Brukinsa®) is a next-generation, highly potent, selective, irreversible Bruton tyrosine kinase (BTK) inhibitor developed by BeiGene for the treatment of B-cell malignancies [1]. BTK plays a key role in B-cell malignancies [2]. Zanubrutinib covalently binds to a cysteine residue in the BTK active site to inhibit BTK activity [3]. Designed to maximise target occupancy and minimize off-target binding, zanubrutinib may have potential pharmacodynamic and pharmacokinetic advantages over ibrutinib, a standard-of-care BTK inhibitor for B-cell malignancies [4].
Zanubrutinib received its first approval in the USA on 14 November 2019 for the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy [3]. This is an accelerated approval based on overall response rate (ORR) seen in two clinical studies (Sect. 2.3.1.1); continued approval may be contingent upon demonstration of clinical benefit in a confirmatory trial. Zanubrutinib is available as oral 80 mg capsules and the recommended dosage is 160 mg twice daily or 320 mg once daily [3].
Zanubrutinib is under regulatory review in China for the treatment of relapsed/refractory (RR) MCL and RR chronic lymphocytic leukaemia (CLL)/small lymphocytic lymphoma (SLL). The drug is undergoing clinical development worldwide for other B-cell malignancies, including CLL/SLL, Waldenstrom’s macroglobulinaemia (WM), follicular lymphoma (FL), diffuse large B cell lymphoma (DLBCL), marginal zone lymphoma (MZL) and Non-Hodgkin’s lymphoma (NHL). In July 2018, the US FDA granted fast track designation to zanubrutinib in WM [5]. Zanubrutinib has orphan drug designation in the USA for WM, MCL and CLL [6].
1.1 Company Agreements
In November 2019, Catalent entered into a long-term manufacturing and supply agreement with BeiGene for zanubrutinib [7].
1.2 Patent Information
As of February 2019, BeiGene owns three issued patents (two in the USA and one in China) related to zanubrutinib and zanubrutinib combination therapies for the treatment of haematological malignancies or autoimmune disease [6]. These patents expire in 2034. BeiGene also has pending patent applications in the USA and other jurisdictions [6].

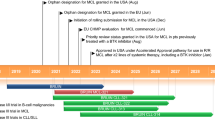
Key milestones in the development of zanubrutinib monotherapy for second-line or greater treatment of MCL. CTA clinical trial application, IND Investigational New Drug application, MCL mantle-cell lymphoma
2 Scientific Summary
2.1 Pharmacodynamics
Zanubrutinib inhibited MCL cell proliferation and induced apoptosis in vitro, and inhibited tumour growth in MCL patient-derived xenograft mouse models [8]. This activity is attributed to its pleiotropic effects (including suppression of the Akt/mTOR pathway) on several MCL survival mechanisms [8]. In vitro, zanubrutinib has similar potency to ibrutinib at inhibiting BTK but ≈ 20-times less potent than ibrutinib at inhibiting IL-2 inducible kinase (ITK) [9]. Thus, zanubrutinib is associated with lesser off-target kinase inhibition than ibrutinib. In patients with MCL, ibrutinib but not zanubrutinib significantly suppressed natural killer cell toxicity, likely because of ITK inhibition [9]. Zanubrutinib showed synergistic or additive antitumour activity when combined with birabresib, pimasertib, selinexor or venetoclax in several MCL and activated B-cell DLBCL cell lines [10] and with lenalidomide in MCL cell lines [11].

Chemical structure of zanubrutinib
In patients with CLL/SLL, zanubrutinib induced favourable changes in immune microenvironment by improving T cell exhaustion, and downregulating checkpoint molecules on suppressor cells and adhesion/homing receptors on B cells [12].
Zanubrutinib showed near complete and sustained BTK occupancy in both peripheral blood mononuclear cells (PBMCs) and lymph nodes in patients with B-cell malignancies in a phase I trial (NCT02343120) [1]. Patients received zanubrutinib 40, 80, 160 or 320 mg once daily or 160 mg twice daily. In PBMCs, > 95% BTK occupancy was achieved with the lowest dose and the occupancy rate remained > 95% 4 h postdose in almost all patients across dosage groups, with no significant differences between the groups [1]. At steady state, the median BTK occupancy in PBMCs was maintained at 100% over 24 h with 320 mg once daily [3]. In lymph nodes, the 160 mg twice-daily dosage was associated with a significantly higher median BTK occupancy (100% vs. 94%) and the proportion of patients with > 95% occupancy (89% vs. 50%) than the 320 mg once-daily dosage. Based on these findings, the recommended phase II regimen was 160 mg twice daily [1].
2.2 Pharmacokinetics
In patients with B-cell malignancies, zanubrutinib maximum plasma concentration (Cmax) and area under the plasma drug concentration-time curve (AUC) increased in a nearly dose-proportional manner over a dosage range of 40–320 mg [1]. Zanubrutinib is rapidly absorbed after oral administration (median time to Cmax ≈ 2 h) and has minimal accumulation after multiple administrations [3]. Food had no clinically relevant effect on zanubrutinib exposure in healthy volunteers. The geometric mean apparent steady-state volume of distribution of zanubrutinib is 881 L. Approximately 94% of zanubrutinib is plasma protein bound, with a blood-to-plasma ratio of 0.7–0.8. The mean half-life of zanubrutinib is approximately 2–4 h after a single oral dose of 160 or 320 mg, and the geometric mean apparent oral clearance is 182 L/h. In healthy volunteers receiving a single radiolabeled zanubrutinib 320 mg dose, ≈ 87% of the dose was recovered in faeces (38% unchanged) and 8% in urine (< 1% unchanged) [3].
Features and properties of zanubrutinib
Alternative names | BGB-3111; BRUKINSA |
Class | Amides, Antineoplastics, Phenyl ethers, Piperidines, Pyrazoles, Pyrimidines, Small molecules |
Mechanism of Action | Bruton’s tyrosine kinase inhibitor |
Route of Administration | Oral |
Pharmacodynamics | Highly selective for BTK; inhibits BTK with potency similar to ibrutinib; lesser off-target kinase inhibition than ibrutinib; associated with near complete and sustained BTK occupancy in PBMCs as well as lymph nodes |
Pharmacokinetics | Median time to Cmax ≈ 2 h; minimal accumulation; volume of distribution at steady state 881 L; mean half-life ≈ 2–4 h; primarily metabolized by CYP3A |
Adverse events | |
Most frequent | ↓ Neutrophil count, ↓ platelet count, upper respiratory tract infection, ↓ white blood cell count ↓ haemoglobin, rash, bruising, diarrhoea and cough |
Occasional | Atrial fibrillation/flutter, major haemorrhage, grade ≥ 3 hypertension |
ATC codes | |
WHO ATC code | L01X-E (Protein kinase inhibitors) |
EphMRA ATC code | L1 (Antineoplastics) |
Chemical name | (7S)-2-(4-Phenoxyphenyl)-7-(1-(prop-2-enoyl)piperidin-4-yl)-4,5,6,7-tetrahydropyrazolo(1,5-a)pyrimidine-3-carboxamide |
Age (19–90 years), sex, race (Asian, Caucasian and other), bodyweight (36–140 kg) and mild or moderate renal impairment had no clinically relevant effect on zanubrutinib pharmacokinetics, while the effect of severe renal impairment (creatinine clearance < 30 mL/min) and dialysis is unknown [3]. Zanubrutinib total AUC increased by 11%, 21% and 60% (unbound AUC by 23%, 43%, and 194%) in subjects with mild, moderate and severe hepatic impairment, respectively, relative to subjects with normal liver function. In patients with severe hepatic impairment, the recommended dosage of zanubrutinib is 80 mg twice daily [3].
Zanubrutinib is primarily metabolized by cytochrome P450(CYP)3A [3]. Zanubrutinib exposure is increased or decreased when coadministered with CYP3A inhibitors or inducers, respectively. The recommended dosage of zanubrutinib is 80 mg once daily and 80 mg twice daily when coadministered with strong and moderate CYP3A inhibitors, respectively. Concomitant use of a moderate or strong CYP3A inducer with zanubrutinib should be avoided. Zanubrutinib decreased exposure to CYP3A and CYP2C19 substrates, and had no clinically relevant effect on warfarin (a CYP2C9 substrate) or rosiglitazone (a CYP2C8 substrate). Zanubrutinib increased exposure to digoxin (a P-gp substrate), and had no clinically relevant effect on rosuvastatin (a BCRP substrate). Zanubrutinib is an inducer of CYP2B6 and is likely to be a substrate of P-gp, according to in vitro studies [3].
2.3 Therapeutic Trials
In clinical trials discussed in this section, zanubrutinib dosage was 160 mg twice daily (or 320 mg daily). In the dose-expansion second part of the NCT02343120 trial (Sect. 2.1), disease-specific cohorts received this dosage [1]. The clinical activity profile of zanubrutinib seen in Chinese patients with B-cell malignancies in a phase I trial (NCT03189524) was consistent with that reported in other trials [13].
Key clinical trials of zanubrutinib (sponsored by BeiGene)
Drug(s) | Indication | Phase | Status | Location(s) | Identifier |
|---|---|---|---|---|---|
Zanubrutinib | B-cell malignancies | III | Not yet recruiting | Multinational | NCT04170283;BGB-3111-LTE1 |
Zanubrutinib, Bendamustine, Rituximab | MCL | III | Recruiting | USA | NCT04002297; BGB-3111-306 |
Zanubrutinib, Ibrutinib | CLL, SLL | III | Active, not recruiting | Multinational | NCT03734016; BGB-3111-305; ALPINE |
Zanubrutinib, Bendamustine, Rituximab, Venetoclax | CLL, SLL | III | Recruiting | Multinational | NCT03336333; BGB-3111-304; SEQUOIA |
Zanubrutinib, Ibrutinib | WM | III | Active, not recruiting | Multinational | NCT03053440; BGB-3111-302; ASPEN |
Zanubrutinib | MZL | II | Recruiting | Multinational | NCT03846427; BGB-3111-214; MAGNOLIA |
Zanubrutinib | CLL, SLL | II | Recruiting | USA | NCT04116437; BGB-3111-215 |
Zanubrutinib | MCL | II | Active, not recruiting | China | NCT03206970; BGB-3111-206 |
Zanubrutinib, rituximab | MZL, FL, DLBCL | II | Active, not recruiting | China | NCT03520920; BGB-3111-213 |
Zanubrutinib | WM | II | Active, not recruiting | China | NCT03332173; BGB-3111-210 |
Zanubrutinib | CLL, SLL | II | Active, not recruiting | China | NCT03206918; BGB-3111-205 |
Zanubrutinib | DLBCL | II | Active, not recruiting | China | NCT03145064; BGB-3111-207 |
Zanubrutinib, obinutuzumab | NHL | II | Recruiting | Multinational | NCT03332017; BGB-3111-212; ROSEWOOD |
Zanubrutinib | B-cell Malignancies | I|II | Not yet recruiting | Japan | NCT04172246; BGB-3111-111 |
Zanubrutinib, tislelizumab | Lymphoma, leukaemia | I | Recruiting | Australia, China | NCT02795182; BGB-3111_BGB-A317_Study_001 |
Zanubrutinib | B-cell malignancies | I | Recruiting | China | NCT03189524; BGB-3111-1002 |
Zanubrutinib | B-cell Malignancies | I | Active, not recruiting | Multinational | NCT02343120; BGB-3111-AU-003 |
Zanubrutinib, obinutuzumab | B-cell malignancies | I | Active, not recruiting | Multinational | NCT02569476; BGB-3111_GA101_Study_001 |
2.3.1 Monotherapy
2.3.1.1 Mantle Cell Lymphoma
Zanubrutinib was effective in patients with MCL who had received at least one prior therapy in a single-arm, open-label phase II trial (NCT03206970) [3, 14]. In 86 evaluable patients, ORR was 84% (95% CI 74–91) [primary endpoint], complete response rate (CRR) was 59% and partial response rate (PRR) was 24%; the median duration of response (DOR) was 19.5 months [3]. The ORR was generally consistent across various subgroups, including those based on Mantle Cell International Prognostic Index, previous therapy and blastoid variant [14]. The median progression-free survival (PFS) was 16.7 months [14]. Tumour response was assessed by an independent review committee (IRC) using 2014 Lugano classification [3].
In the previously-treated MCL cohort (n = 37) in the NCT02343120 trial, ORR was 86.5% (95% CI 71.2–95.5), CRR was 29.7% and PRR was 56.8%, and the median DOR was 17.1 months [median follow-up (MFU) 14.3 months] [15].
2.3.1.2 Waldenstrom Macroglobulinemia
Zanubrutinib was effective in patients with WM and MYD88 wild type (WT) in the nonrandomized cohort of the open-label, multicentre, phase III ASPEN study (NCT03053440) [4]. In 26 evaluable patients (five treatment-naïve, 21 RR), ORR was 76.9%, major response (partial response or better) rate (MRR) was 53.8% and very good PRR was 15.4% (MFU 9.5 months). Median time to first major response was 2.9 months. Median PFS was not reached. Tumour response was assessed by the investigator, based on NCCN WM guidelines and modified Owen criteria [4].
In the WM cohort (24 treatment-naïve, 49 RR) in the NCT02343120 trial, ORR was 92% (95% CI 83–97), MRR was 82% (95% CI 72–90) and very good PRR was 41% (MFU 23.9 months) [16]. In 57 evaluable patients with WM and MYD88 L265P mutation, ORR was 91%, MRR was 86% and very good PRR or CRR was 46% (MFU 21.8 months). The corresponding rates in eight evaluable patients with WM and MYD88 WT were 88%, 63% and 25%, respectively (MFU 24.1 months). Median PFS was not reached; 2-year PFS rate was 81% (95% CI 68–89). Response rates reported are for IRC assessment, based on the modified Owen criteria [16]. The depth of response (i.e. very good PRR) appeared to increase over time up to 1 year [17, 18].
2.3.1.3 Chronic Lymphocytic Leukaemia/Small Lymphocytic Lymphoma
Zanubrutinib treatment was associated with an ORR of 92.2% (95% CI 84.6–96.8) in 90 evaluable treatment-naïve patients with CLL/SLL and del(17p) mutation in the nonrandomized arm of the open-label, international, phase III SEQUOIA trial (NCT03336333) [MFU 7.0 months] [19].
Zanubrutinib provided a treatment benefit in patients with RR CLL (n = 82) or SLL (n = 9), including those with del(17p) or TP53 mutation, in a single-arm, multicentre phase II trial (NCT03206918) [20]. CRR was 4.4% and a best response of partial response with lymphocytosis or better was seen in 91.2% (95% CI 83.4–91.6) of patients, according to the investigator assessment (MFU 15.1 months). In patients with del(17p) or TP53 mutation, ORR was 95.5%. Among responders, the median time to first response was 2.79 months. The estimated 1-year PFS rate was 80.9% (95% CI 67–89) [20].
In the CLL/SLL cohort (n = 120) in the NCT02343120 trial, ORR was 96.7% (95% CI 91.7–99.1) in all patients (n = 120) and 93.8% (95% CI 69.8–99.8) in those with del(17p) mutation (n = 16) [MFU 26.4 months] [21]. ORRs were generally similar between treatment-naïve patients (100%; n = 22) and those with RR disease (95.9%; n = 98). PFS rate at 2 year was 75% (95% CI 40–91) [21].
2.3.1.4 Non-Hodgkin’s Lymphoma
In the NHL cohort (n = 62 evaluable) in the NCT02343120 trial, ORR was 58.1% in all patients, 60.9% in patients with aggressive lymphoma (DLBCL or MCL; n = 46) and 50% in those with indolent lymphoma (FL or MZL; n = 16) [22]. CRR was 12.9%, 15.2% and 6.3%, respectively, and PRR was 45.2%, 45.7% and 43.8% [22].
2.3.2 Combination Therapy
Zanubrutinib in combination with tislelizumab showed promising clinical activity in patients with B-cell malignancies in a phase Ib trial (NCT02795182) [23]. Zanubrutinib in combination with obinutuzumab also showed treatment benefit in a phase Ib trial (NCT02569476) in patients with treatment-naïve (n = 20) or RR (n = 25) CLL/SLL or RR FL (n = 36) [24]. ORR was 96% in the CLL/SLL cohort (100% for treatment-naïve, 92% for RR disease) and 72% in the FL cohort; CRR was 27% and 36% in the respective cohorts (MFU 25.5 and 17.8 months for the CLL/SLL and FL cohorts) [24].
2.4 Adverse Events
Zanubrutinib monotherapy was generally well tolerated in patients with B-cell malignancies in clinical trials [3, 25]. Safety of zanubrutinib was analysed in a pooled analysis (n = 682) of six monotherapy trials (NCT03189524, NCT03206918, NCT03206970, NCT03145064, NCT03332173, NCT02343120) [25]. Almost all patients had received either 160 mg twice-daily or 320 mg once-daily dosages (median duration of treatment 13.4 months). Grade ≥ 3 adverse events (AEs) occurred in 57% of patients (31% treatment-related) and the most common (incidence ≥ 3%) were decreased neutrophil count (14%), anaemia (8%), neutropenia (7%), pneumonia (5%), decreased platelet count (4%), lung infection (4%) and hypertension (3%). Serious AEs were reported in 36% of patients (12% treatment-related) and the most common were pneumonia (5%), lung infection (3%), urinary tract infection (2%), pyrexia (2%), cellulitis (1%), anaemia (1%), and pleural effusion (1%). Overall, 9% of patients discontinued treatment because of AEs, including 3.5% because of treatment-related AEs. Death because of treatment-related AEs occurred in 1.3% of patients. AEs of interest associated with BTK inhibitors, such as atrial fibrillation/flutter (any grade, 1.9%), major haemorrhage (any grade, 2.5%) and hypertension (grade ≥ 3, 3.4%) were infrequent with zanubrutinib; exposure adjusted incidence rate of these AEs was 0.13, 0.17 and 0.24 events/100 patient-months, respectively [25].
In patients with previously-treated MCL (approved indication) who received zanubrutinib in the NCT03206970 and NCT02343120 trials (n = 118), the most common (incidence ≥ 3%) grade ≥ 3 adverse reactions with zanubrutinib were neutropenia and neutrophil count decreased (15%), pneumonia (10%), anaemia and haemoglobin decreased (8%), thrombocytopenia and platelet count decreased (5%), leukopenia and white blood count decreased (5%), hypertension (3.4%), haemorrhage (3.4%) and musculoskeletal pain (3.4%) [3]. Other clinically significant adverse reactions occurring in < 10% of patients included major haemorrhage (5%), hyperuricemia (6%) and headache (4.2%). Serious adverse reactions occurred in 31% of patients, with the most common being pneumonia (11%) and haemorrhage (5%). Death within 30 days of the last dose of zanubrutinib occurred in 7% of patients [3].
In the CLL/SLL and FL cohorts receiving zanubrutinib plus obinutuzumab in NCT02569476, grade ≥ 3 AEs were reported in 71% and 50% of patients, serious AEs in 49% and 33% and AEs leading to zanubrutinib discontinuation in 4% and 6% of patients [24].
2.5 Ongoing Clinical Trials
The following phase III clinical trials are ongoing: zanubrutinib plus rituximab versus bendamustine plus rituximab in previously untreated patients with MCL who are not eligible for stem cell transplantation (NCT04002297); zanubrutinib versus ibrutinib in patients with RR CLL/SLL (NCT03734016; ALPINE); zanubrutinib with or without venetoclax versus bendamustine plus rituximab in patients with previously untreated CLL/SLL (NCT03336333; SEQUOIA); and, zanubrutinib versus ibrutinib in patients with WM (NCT03053440; ASPEN). Preliminary results have been reported for the randomized cohort of 201 patients with a MYD88 mutation in ASPEN, and it didn’t achieve its primary endpoint [26]. Phase II clinical trials with zanubrutinib are ongoing in patients with MCL (NCT03206970), CLL/SLL (NCT04116437, NCT03824483 and NCT03206918), DLBCL, FL or MZL (NCT03520920), DLBCL (NCT03145064), MZL (NCT03846427), NHL (NCT03332017) and WM (NCT03332173). The phase I/II clinical trial in various disease-specific cohorts (NCT02343120) and a number of phase I trials in B-cell malignancies are ongoing.
3 Current Status
Zanubrutinib received its first approval in the USA on 14 November 2019 for the treatment of adult patients with MCL who have received at least one prior therapy.
References
Tam CS, Trotman J, Opat S, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134(11):851–9.
Rickert RC. New insights into pre-BCR and BCR signalling with relevance to B cell malignancies. Nat Rev Immunol. 2013;13(8):578–91.
BeiGene. Brukinsa™ (zanubrutinib) capsules, for oral use: US Prescribing Information; 2019. https://www.brukinsa.com. Accessed 10 Dec 2019.
Dimopoulos M, Opat S, Lee HP, et al. Major responses in Myd88 wildtype (Myd88wt) Waldenstrom macroglobulinemia (WM) patients treated with Bruton tyrosine kinase (BTK) inhibitor zanubrutinib (BGB-3111) [abstract no. PF487]. HemaSphere. 2019;3:196.
BeiGene. BeiGene announces plan to pursue accelerated approval in the U.S. of BTK inhibitor zanubrutinib in Waldenstrm macroglobulinemia (WM) [media release]; 22 Jul 2018. http://www.beigene.com.
BieGene. BieGene Ltd. Form 10-K (28 February 2019); 2019. http://ir.beigene.com. Accessed 10 Dec 2019.
Catalent. Catalent to supply BeiGene’s BTK inhibitor BRUKINSA™ (zanubrutinib) [media release]. 21 Nov 2019. http://www.catalent.com.
Li CJ, Jiang C, Liu Y, et al. Pleiotropic action of novel Bruton’s tyrosine kinase inhibitor BGB-3111 in mantle cell lymphoma. Mol Cancer Ther. 2019;18(2):267–77.
Flinsenberg TWH, Tromedjo CC, Hu N, et al. Differential effects of BTK inhibitors ibrutinib and zanubrutinib on NK cell effector function in patients with mantle cell lymphoma. Haematologica. 2019. https://doi.org/10.3324/haematol.2019.220590.
Tarantelli C, Zhang L, Curti E, et al. The Bruton tyrosine kinase inhibitor zanubrutinib (BGB-3111) demonstrated synergies with other anti-lymphoma targeted agents. Haematologica. 2019;104(7):e307–9.
Hu N, Zhang S, He M, et al. BTK inhibitor BGB-3111 synergizes with lenalidomide in MCL models. Cancer Res. 2016;76:3.
Zou YX, Zhu HY, Li XT, et al. The impacts of zanubrutinib on immune cells in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Hematol Oncol. 2019;37(4):392–400.
Zhu J, Li J, Zhou J, et al. BGB-3111, a highly specific BTK inhibitor, is well tolerated and highly active in Chinese patients with relapsed/refractory B-cell malignancies: initial report of a phase 1 trial in China [abstract]. Blood. 2017;130(Suppl 1).
Song Y, Zhou K, Zou D, et al. Zanubrutinib in patients with relapsed/refractory mantle cell lymphoma [abstract no. 015]. Hematol Oncol. 2019;37(Suppl 2):45–6.
Tam CS, Wang M, Simpson D, et al. Updated safety and efficacy data in the phase 1 trial of patients with mantle cell lymphoma (MCL) treated with bruton tyrosine kinase (BTK) inhibitor zanubrutinib (BGB-3111) [abstract no. 191]. Hematol Oncol. 2019;37(Suppl 2):245–7.
Trotman J, Opat S, Marlton P, et al. Updated safety and efficacy data in a phase 1/2 trial of patients with Waldenstrom macroglobulinaemia (WM) treated with the Bruton tyrosine kinase (BTK) inhibitor zanubrutinib (BGB-3111) [abstract no. PF481]. HemaSphere. 2019;3:192–3.
Trotman J, Tam CS, Marlton P, et al. Improved depth of response with increased follow-up for patients (PTS) with Waldenstrom macroglobulinemia (WM) treated with Bruton’s tyrosine kinase (BTK) inhibitor zanubrutinib [abstract no. PS1186]. HemaSphere. 2018;2(Suppl 2):537–8.
Trotman J, Opat S, Marlton P, et al. Bruton’s tyrosine kinase (BTK) inhibitor BGB-3111 demonstrates high very good partial response (VGPR) rate in patients with Waldenstrom macroglobulinemia (WM) [abstract no. 59]. Hematol Oncol. 2017;35(Suppl 2):70–1.
Tam CS, Robak P, Ghia P, et al. Efficacy and safety of zanubrutinib in patients with treatment-naive chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) with Del(17p): initial results from arm C of the SEQUOIA (BGB-3111-304) trial [abstract no. 499]. In: American Society of Hematology annual meeting and exposition; 2019.
Xu W, Yang S, Zhou K, et al. Zanubrutinib for patients with relapsed or refractory chronic lymphocytic leukemia [abstract no. 049]. Hematol Oncol. 2019;37(Suppl 2):87–8.
Cull G, Simpson D, Opat S, et al. Treatment with the Bruton tyrosine kinase inhibitor zanubrutinib (BGB-3111) demonstrates high overall response rate and durable responses in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): updated results from a phase 1/2 trial [abstract no. 500]. In: American Society of Hematology annual meeting and exposition; 2019.
Tam CS, Simpson D, Opat S, et al. Safety and activity of the highly specific BTK inhibitor BGB-3111 in patients with indolent and aggressive non-Hodgkin’s lymphoma [abstract]. Blood. 2017;130(Suppl 1).
Cull G, Opat S, Trotman J, et al. Safety and activity of the highly specific BTK inhibitor BGB-3111 in combination with the PD-1 inhibitor BGB-A317 in patients with B-cell lymphoid malignancies [abstract]. Blood. 2017;130(Suppl 1).
Tam CS, Quach H, Nicol A, et al. Zanubrutinib plus obinutuzumab in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) or relapsed/refractory (R/R) follicular lymphoma (FL) [abstract no. 075]. Hematol Oncol. 2019;37(Suppl 2):121–2.
Tam CS, Opat S, Zhu J, et al. Pooled analysis of safety data from monotherapy studies of the Bruton tyrosine kinase (BTK) inhibitor, zanubrutinib (BGB-3111), in B-cell malignancies [abstract no. PS1159]. HemaSphere. 2019;3:526.
BeiGene. BeiGene announces results of phase 3 ASPEN trial of zanubrutinib compared to ibrutinib for the treatment of patients with Waldenström’s macroglobulinemia [media release]. 2019. http://ir.beigene.com.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Yahiya Syed is a salaried employee of Adis International Ltd/Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
Enhanced material for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.11344898.
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Syed, Y.Y. Zanubrutinib: First Approval. Drugs 80, 91–97 (2020). https://doi.org/10.1007/s40265-019-01252-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01252-4