Abstract
Galcanezumab-gnlm (Emgality™; Eli Lilly and Company), hereafter galcanezumab, is a humanized monoclonal antibody against the calcitonin gene-related peptide (CGRP) ligand. A potent vasodilator, CGRP is implicated in nociceptive transmission and migraine pathogenesis. In September 2018, the US FDA approved galcanezumab as a once-monthly subcutaneous injection for the preventive treatment of migraine in adults. In the same month, the EMA issued a positive opinion for galcanezumab for the prophylaxis of migraine in adults who have at least 4 migraine days per month. Galcanezumab is also undergoing phase III evaluation for the preventive treatment of cluster headache in North America and Europe. This article summarizes the milestones in the development of galcanezumab leading to its first approval for the preventive treatment of migraine in adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Galcanezumab (Emgality™), a humanized monoclonal antibody that selectively binds to and neutralizes calcitonin gene-related peptide (CGRP) [1], is being developed by Eli Lilly as a subcutaneous formulation for the preventive treatment of migraine and cluster headache. CGRP is a potent vasodilatory neuropeptide that is widely distributed in the central and peripheral nervous system [2, 3]. Involved in nociceptive transmission and modulation, CGRP has been firmly implicated in the pathophysiology of migraine and has recently emerged as a therapeutic target for the prophylaxis of migraine and related conditions [3].

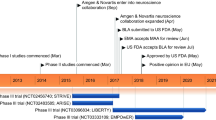
Key milestones in the development of galcanezumab for the preventive treatment of migraine in adults, focussing on phase III trials. BLA Biologics License Application, CHMP Committee for Medicinal Products for Human Use
Galcanezumab was recently approved in the USA for the prevention of migraine in adults [4]. It is available as a 120 mg/mL injection (single-dose prefilled pen or syringe) for subcutaneous use and is intended for patient self-administration [5]. The recommended regimen is 240 mg (administered as two consecutive 120 mg injections) once as a loading dose, followed by a once-monthly dose of 120 mg [5]. In the EU, galcanezumab has received a positive opinion for the prophylaxis of migraine in adults who have at least 4 migraine days per month [6]. Galcanezumab is undergoing phase III development for the preventive treatment of cluster headache in North America and Europe, and has been granted Fast Track Designation for cluster headache by the US FDA [7]. While galcanezumab has also been evaluated in patients with osteoarthritis, development in this indication appears to have been discontinued.
1.1 Company Agreements
In January 2014, Eli Lilly announced that it had acquired all developmental and commercial rights for galcanezumab back from Arteaus Therapeutics, following a successful phase II proof-of-concept trial [8]. Eli Lilly had licensed the worldwide rights to develop galcanezumab to Arteaus Therapeutics in October 2011 [8, 9]. Under the terms of this agreement, Arteaus Therapeutics in collaboration with Eli Lilly was to develop the antibody through phase II trials to demonstrate proof of concept in the prevention of migraine [9]. Upon completion of this, Eli Lilly would have the option of continuing drug development at prenegotiated terms, including milestones and royalties [9].
2 Scientific Summary
2.1 Pharmacodynamics
Galcanezumab is a highly specific and potent humanized antibody to CGRP [1]. In vitro, galcanezumab binds to human CGRP with high affinity (KD 31 pmol/L), neutralizing CGRP-induced receptor activation (average IC50 0.35 nmol/L). Galcanezumab inhibited cAMP production induced by either human or rat CGRP in a concentration-dependent manner, with inhibition reaching 100% at the highest concentrations of galcanezumab [1].
Galcanezumab inhibits capsaicin-induced vasodilation in vivo [10]. Galcanezumab 75–600 mg significantly (p < 0.05) inhibited capsaicin-induced dermal blood flow (DBF) response relative to placebo at all postdose timepoints (i.e. days 3, 14, 28 and 42) in healthy volunteers administered a single subcutaneous dose. Inhibition of capsaicin-induced DBF response was greater at higher galcanezumab plasma concentrations. In a multi-dose cohort of healthy volunteers administered four subcutaneous doses of galcanezumab (150 mg every 2 weeks), galcanezumab was associated with a rapid and durable suppression of capsaicin-induced DBF; there was an inverse correlation between galcanezumab exposure and DBF response from day 14 through to ≥ 176 days after the first dose [10].
2.2 Pharmacokinetics
Galcanezumab has linear pharmacokinetics, with generally dose-proportional increases in exposure over the dose range of 1–600 mg when administered as a single subcutaneous dose [2, 5]. The time to maximum concentration was ≈ 5 days and steady-state concentrations for the 120 mg maintenance dose were achieved by month 1 when a loading dose of 240 mg was administered in patients with migraine [5, 11]. Galcanezumab had a limited distribution (apparent volume of distribution ≈ 7 L) [5, 11]. Absorption was not significantly impacted by injection site location [5].
It is thought that galcanezumab is catabolized into small peptides and amino acids [5]. Galcanezumab had an elimination half-life of ≈ 27 days and an apparent clearance of 0.008 L/h [5, 11].
In a population pharmacokinetics (PPK) analysis, galcanezumab pharmacokinetics were not influenced by age, sex, race, or migraine subtype (episodic or chronic) [5]. Body weight did not impact galcanezumab pharmacokinetics to any clinically relevant degree. While no dedicated clinical studies have evaluated the effects of hepatic or renal impairment on galcanezumab pharmacokinetics, PPK analyses found that galcanezumab clearance was not significantly influenced by bilirubin concentration and that galcanezumab pharmacokinetics were not affected by mild or moderate renal impairment (creatinine clearance ≥ 30 mL/min); patients with severe renal impairment (creatinine clearance < 30 mL/min) have not been evaluated [5].
As galcanezumab is not metabolized by cytochrome P450 (CYP450) enzymes, galcanezumab is unlikely to interact with concomitant drugs that are substrates, inducers or inhibitors of CYP450 enzymes [5].
Features and properties of galcanezumab
Alternative names | Galcanezumab-gnlm; Emgality; LY2951742 |
Class | Antimigraines; monoclonal antibodies |
Mechanism of action | Humanized monoclonal antibody that binds to calcitonin gene-related peptide (CGRP) ligand and blocks its binding to the receptor |
Route of administration | Subcutaneous |
Pharmacodynamics | Binds to calcitonin gene-related peptide (CGRP) with high affinity (KD 31 pmol/L), neutralizing CGRP-induced receptor activation; inhibits capsaicin-induced dermal blood flow response |
Pharmacokinetics | Linear pharmacokinetics; slow absorption (time to maximum concentration ≈ 5 days) and long elimination half-life (≈ 27 days) |
Adverse reactions | Injection-site reactions (including pain, erythema and pruritus) |
ATC codes | |
WHO ATC code | N02C-X08 (galcanezumab) |
EphMRA ATC code | N2C (anti-migraine preparations) |
Chemical name | Immunoglobulin G4, anti-(human calcitonin gene-related peptide) (human-Mus musculus clone III heavy chain), disulfide with human-Mus musculus clone III kappa-chain, dimer |
2.3 Therapeutic Trials
2.3.1 Migraine
Galcanezumab provided effective preventive treatment for episodic migraine in the EVOLVE-1 (NCT02614183) [12] and EVOLVE-2 (NCT02614196) [13] trials. In EVOLVE-1, galcanezumab 120 and 240 mg (once-monthly) were associated with significantly greater overall reductions in monthly migraine headache days than placebo during the double-blind treatment phase [least-squares mean (LSM) change from baseline − 4.7 and − 4.6 vs. − 2.8; p < 0.001 for both comparisons] (primary endpoint) [12]. Onset of effect was month 1 in both treatment groups (p < 0.001 vs. placebo for both doses) [12] and a benefit was seen as early as week 1 in post-hoc analyses of weekly migraine headache days (p < 0.001 for galcanezumab recipients vs. placebo recipients) in this study (abstract data) [14]. Both doses were significantly more effective than placebo in reducing the number of monthly headache days with acute medication use during double-blind treatment (LSM change − 4.0 and − 3.8 vs. − 2.2; p < 0.001) [12]. Compared with patients receiving placebo, significantly greater proportions of patients receiving galcanezumab 120 or 240 mg achieved a ≥ 50% (62.3 and 60.9% vs. 38.6%), ≥ 75% (38.8 and 38.5% vs. 19.3%) or 100% (15.6 and 14.6% vs. 6.2%) reduction from baseline in monthly migraine headache days during treatment (p < 0.001 for all comparisons). Both doses of galcanezumab were associated with significantly greater mean improvements from baseline in patient-reported health outcomes [Migraine-Specific Quality of Life Questionnaire (MSQ) Role Function-Restrictive domain scores and Patient Global Impression of Severity (PGI-S) scores] than placebo during treatment (average of months 4, 5 and 6) [p ≤ 0.008 for all comparisons] [12].
In EVOLVE-2, galcanezumab 120 and 240 mg (once-monthly) were also associated with significantly greater overall reductions in monthly migraine headache days than placebo during double-blind treatment (LSM change from baseline − 4.3 and − 4.2 vs. − 2.3; p < 0.001 for both comparisons) [primary endpoint] [13]. Again, onset of effect was month 1 in both treatment groups (p < 0.001 vs. placebo for both doses) [13] and a benefit was seen as early as week 1 in post-hoc analyses of weekly migraine headache days (p < 0.001 for galcanezumab recipients vs. placebo recipients) in this study (abstract data) [14]. There were significantly greater reductions in the number of monthly headache days with acute medication use during double-blind treatment with galcanezumab 120 or 240 mg than with placebo (LSM change − 3.7 and − 3.6 vs. − 1.9; p < 0.001) [13]. Compared with placebo recipients, significantly greater mean proportions of galcanezumab 120 and 240 mg recipients achieved a ≥ 50% (59.3 and 56.5% vs. 36.0%), ≥ 75% (33.5 and 34.3% vs. 17.8%) or 100% (11.5 and 13.8% vs. 5.7%) reduction from baseline in monthly migraine headache days during treatment (p < 0.001 for all comparisons). With respect to patient-reported health outcomes (MSQ Role Function-Restrictive domain scores and PGI-S scores), mean improvements from baseline during treatment (average of months 4, 5 and 6) were significantly greater with galcanezumab 120 and 240 mg than with placebo (p ≤ 0.012 for all comparisons) [13].
EVOLVE-1 [12] and -2 [13] were multicentre, randomized, double-blind, placebo-controlled phase III trials that enrolled adults aged 18–65 years with a diagnosis of migraine (with or without aura) for ≥ 1 year prior and migraine onset before 50 years of age. To be eligible for enrollment, they were required to experience 4–14 migraine headache days (including probable migraine headache days [13]) and ≥ 2 migraine attacks per month during the baseline period [12, 13]. Eligible patients received galcanezumab 120 mg (n = 213 in EVOLVE-1 and n = 231 in EVOLVE-2), galcanezumab 240 mg (n = 212 and 223, respectively) or placebo (n = 433 and 461, respectively), administered by subcutaneous injection once per month for 6 months. Patients randomized to galcanezumab 120 mg received a loading dose of 240 mg (two 120 mg injections). At baseline, the mean number of migraine headache days per month was 9.1–9.2 across treatment groups in both trials [12, 13]. In EVOLVE-1, 60.0% of patients had received prior preventive treatment and 18.5% of patients had failed ≥ 1 prior preventive treatment in the previous 5 years due to lack efficacy (4.9% of patients had failed ≥ 2) [12]. In EVOLVE-2, 65.5% of patients had received prior preventive treatment and 14.3% of patients had failed ≥ 2 prior preventive treatments [13].
Galcanezumab also provided effective migraine prevention in patients with chronic migraine [15]. Relative to placebo, galcanezumab 120 and 240 mg (once-monthly) were associated with significantly greater reductions from baseline in monthly migraine headache days (overall LSM change − 4.8 and − 4.6 vs. − 2.7; p < 0.001 for both comparisons) during the 3-month double-blind treatment phase (primary endpoint) of the multinational phase III REGAIN trial (NCT02614261) in adults with chronic migraine [15]. Significant reductions in number of monthly migraine headache days were evident from month 1 onwards (p < 0.01 vs. placebo for each dose at months 1, 2 and 3) [15, 16]. The mean proportion of patients with a ≥ 50% reduction in monthly migraine headache days over months 1–3 was significantly higher with galcanezumab than placebo (p < 0.001 for both doses) [15]. Compared with placebo, galcanezumab 240 mg (but not 120 mg, after multiplicity adjustment) was also associated with a significantly higher mean proportion of patients achieving a ≥ 75% reduction in monthly migraine headache days over months 1–3, a significantly greater overall mean reduction in monthly migraine headache days requiring acute migraine treatment and significantly greater mean improvements in patient-reported health outcomes at month 3 (MSQ Role Function-Restrictive domain scores and PGI-S ratings) [all p < 0.01, and statistically significant vs. placebo after multiplicity adjustment] [15, 16].
REGAIN enrolled patients aged 18–65 years with chronic migraine (≥ 15 headache days per month, with ≥ 8 meeting criteria for migraine) [15]. Eligible patients were randomized to receive subcutaneous galcanezumab 120 mg (n = 278; initial dose was a loading dose of 240 mg [16]), galcanezumab 240 mg (n = 277) or placebo (n = 558) once per month for 3 months [15]. At baseline, the mean number of migraine headache days per month was 19.4, 19.2 and 19.6 with galcanezumab 120 mg, 240 mg and placebo, respectively [16]. Across treatment arms, 75.9–79.4% of patients had received prior preventive treatment and 24.5–35.0% of patients had failed ≥ 2 prior preventive treatments [16].
Galcanezumab had a durable beneficial effect on migraine headache day frequency [17]. Galcanezumab 120 mg and 240 mg significantly reduced monthly migraine headache days from baseline to month 12 (reductions of 5.6 and 6.5 days; p < 0.001 for both doses) in a multinational, open-label, phase III safety study (NCT02614287) in adults with episodic or chronic migraine. Patients were randomized to receive subcutaneous galcanezumab 120 or 240 mg once-monthly for 12 months (initial dose was 240 mg in both groups). They had an average of 10.6 migraine days per month at baseline [17].
Galcanezumab 120 mg significantly reduced migraine headache days per month (i.e. 28-day period) relative to placebo [change from baseline to month 3 of − 4.8 vs. − 3.7 days; 99.6% posterior probability (pre-specified superiority threshold 95%)] (Bayesian analysis; primary endpoint) in a multicentre, double-blind, placebo-controlled phase IIb trial (NCT02163993) in patients with episodic migraine [18]. This was the only assessed galcanezumab dose to meet the superiority threshold [19]. Patients in this trial were adults aged 18–65 years with a history of migraine (with or without aura) for ≥ 1 year prior to enrollment [18]. To be enrolled, they had to experience 4–14 migraine headache days and ≥ 2 migraine attacks in a 28-day period during the prospective baseline phase of the study. Eligible patients were randomized to receive galcanezumab 5 mg (n = 68), 50 mg (n = 68), 120 mg (n = 70), 300 mg (n = 67) or placebo (n = 137), administered by subcutaneous injection once per month for 3 months. At baseline, the mean number of migraine headache days per month was 6.7 in galcanezumab recipients and 6.6 in placebo recipients [18].
Compared with placebo, galcanezumab was associated with a significantly greater reduction in the frequency of migraine headache days per month (i.e. 28-day period) from baseline to week 12 [− 4.2 vs. − 3.0 days; LSM difference − 1.2 (90% CI − 1.9 to − 0.6); p = 0.003] (primary endpoint) in the multicentre, randomized, double-blind, placebo-controlled phase II proof-of-concept study (NCT01625988) in patients with migraine [20]. The trial enrolled adults aged 18–65 years with a ≥ 1-year history of migraine and 4–14 migraine headache days per month; those with a history of chronic migraine were excluded. Patients received subcutaneous galcanezumab 150 mg (n = 107) or placebo (n = 110) every 2 weeks for 12 weeks. During the baseline period, the mean number of migraine headache days per month was 6.7 with galcanezumab and 7.0 with placebo [20].
2.3.2 Cluster Headache
Galcanezumab was associated with a significantly greater reduction in weekly cluster headache attack frequency than placebo [overall mean change from baseline across weeks 1–3 of − 8.7 vs. − 5.2; difference − 3.5 (95% CI − 6.7 to − 0.2); p = 0.036] in a multinational, double-blind, placebo-controlled phase III trial (NCT02397473) [primary endpoint; abstract data] [21]. The proportion of patients achieving a ≥ 50% reduction in weekly cluster headache frequency at week 3 was significantly higher with galcanezumab than with placebo (76 vs. 57%; p = 0.04). The proportion of patients reporting that they were very much/much better based on the Patient Global Impression of Improvement was significantly greater with galcanezumab than with placebo at week 4 (73 vs. 46%; p = 0.016) but not week 8 (72 vs. 66%; p = 0.58). The trial enrolled adults with episodic cluster headache randomized to receive subcutaneous galcanezumab 300 mg (n = 49) or placebo (n = 57) once-monthly for 2 months. During a prospective baseline period, the mean number of weekly cluster headache attacks was 17.8 in patients who went on to receive galcanezumab and 17.3 in patients who received placebo [21].
Relative to placebo, galcanezumab did not significantly reduce weekly cluster headache attack frequency from baseline across weeks 1–12 (primary endpoint) in the multinational, double-blind, placebo-controlled, phase III trial (NCT02438826) in patients with chronic cluster headache (n = 237) [media release] [22]. In this trial, patients were randomized to receive subcutaneous galcanezumab 300 mg or placebo once-monthly for 3 months. At baseline, patients had an average of 18.8 cluster headache attacks per week [22].
Key clinical trials of galcanezumab (Eli Lilly and Company)
Drug(s) | Indication | Phase | Status | Location(s) | Identifier |
|---|---|---|---|---|---|
Galcanezumab, placebo | Treatment-resistant migraine (episodic or chronic) | III | Recruiting | Multinational | CONQUER; NCT03559257; 16670; I5Q-MC-CGAW; EudraCT2018-000600-42 |
Galcanezumab, placebo | Migraine (episodic; patients aged 6–17 years) | III | Recruiting | USA, Puerto Rico | REBUILD; NCT03432286; I5Q-MC-CGAS; EudraCT2017-004351-23 |
Galcanezumab | Migraine (episodic or chronic) | III | Active, not recruiting | Japan | NCT02959190; 16108; I5Q-JE-CGAP |
Galcanezumab, placebo | Migraine (chronic) | III | Completed | Multinational | REGAIN; NCT02614261; 15769; I5Q-MC-CGAI; EudraCT2015-001883-21 |
Galcanezumab, placebo | Migraine (episodic) | III | Completed | Multinational | EVOLVE-2; NCT02614196; 15768; I5Q-MC-CGAH, EudraCT2015-001882-17 |
Galcanezumab, placebo | Migraine (episodic) | III | Completed | USA, Canada, Puerto Rico | EVOLVE-1; NCT02614183; 15767; I5Q-MC-CGAG |
Galcanezumab | Migraine (episodic or chronic) | III | Completed | Multinational | NCT02614287; 15770; I5Q-MC-CGAJ; EudraCT2015-001884-38 |
Galcanezumab, placebo | Migraine (episodic) | IIb | Completed | USA | NCT02163993; 15414; I5Q-MC-CGAB |
Galcanezumab, placebo | Migraine (episodic) | II | Active, not recruiting | Japan | NCT02959177; 15796, I5Q-JE-CGAN |
Galcanezumab, placebo | Migraine | II | Completed | USA | NCT01625988; ART-01 |
Galcanezumab | Cluster headache (episodic or chronic) | IIIb | Enrolling by invitation | Multinational | NCT02797951; 16351; I5Q-MC-CGAR; EudraCT2015-005234-21 |
Galcanezumab, placebo | Cluster headache (chronic) | III | Active, not recruiting | Multinational | NCT02438826; 15781; I5Q-MC-CGAM; EudraCT2014-005429-11 |
Galcanezumab, placebo | Cluster headache (episodic) | III | Completed | Multinational | NCT02397473; 15780; I5Q-MC-CGAL; EudraCT2015-000149-22 |
2.4 Adverse Events
Subcutaneous galcanezumab was generally well tolerated in adult patients with episodic or chronic migraine in clinical trials [12, 13, 15, 20, 23]. In an analysis of pooled data from placebo-controlled phase III clinical trials in episodic or chronic migraine (n = 705 once-monthly galcanezumab 120 mg recipients and n = 1451 placebo recipients; ≤ 6 months of treatment), injection-site reactions was the most common adverse reaction reported with galcanezumab (occurring in 18% of galcanezumab recipients and 13% of placebo recipients) [5]. This was the only adverse reaction to occur with an incidence of ≥ 2% with galcanezumab and ≥ 2% greater than with placebo [5]. Where reported, most injection-site reactions were of mild to moderate severity [12, 13]. Adverse events led to treatment discontinuation in 1.8% of patients during the double-blind period of the pooled trials [5].
Serious adverse events were infrequent with galcanezumab therapy in placebo-controlled phase III trials in episodic or chronic migraine [12, 13, 16]. Serious adverse events were reported in 2.9% of galcanezumab 120 mg recipients and in no galcanezumab 240 mg recipients (vs. 1.2% of placebo recipients) in EVOLVE-1 [12]. In EVOLVE-2, serious adverse events were reported in 2.2, 3.1 and 1.1% of patients receiving galcanezumab 120 mg, galcanezumab 240 mg and placebo, respectively (no significant difference with either dose vs. placebo) [13]. In each trial, no specific serious adverse event was reported in more than one galcanezumab recipient [12, 13]. In the phase III REGAIN trial in chronic migraine, serious adverse events were reported in 0.4, 1.4 and 0.7% of patients receiving galcanezumab 120 mg, galcanezumab 240 mg and placebo, respectively [16].
In a longer-term (12-month) open-label phase III trial in adults with episodic or chronic migraine (n = 270) randomized to receive once-monthly galcanezumab 120 or 240 mg (following a 240 mg starting dose), the most common adverse events (≥ 10%) included injection-site pain, nasopharyngitis and upper respiratory tract infection (media release data) [17]. Serious adverse events occurred in 3 patients receiving galcanezumab 120 mg and 7 patients receiving galcanezumab 240 mg [17]. Adverse events led to treatment discontinuation in 4.8% of patients [17].
The safety profile of subcutaneous galcanezumab in adult patients with episodic cluster headache was consistent with that in those with migraine (abstract data) [21]. Aside from injection-site pain being reported in significantly more galcanezumab recipients than placebo recipients (8.2 vs. 0%; p = 0.043), there were no clinically meaningful differences between once-monthly galcanezumab 300 mg (n = 49) and placebo (n = 57) arms in tolerability or safety parameters [21].
2.4.1 Immunogenicity
In placebo-controlled clinical trials (≤ 6 months in duration), anti-drug antibodies (ADAs) to galcanezumab developed in 4.8% (33/688) of patients receiving once-monthly galcanezumab [5]. The majority of these patients (32/33) had in vitro neutralizing activity. In a 12-month open-label study, up to 12.5% (16/128) of once-monthly galcanezumab recipients developed ADAs to galcanezumab. Most ADA-positive patients tested positive for neutralizing antibodies. While data are limited, ADA development did not appear to affect the pharmacokinetics, efficacy or safety of galcanezumab [5].
2.5 Ongoing Clinical Trials
Subcutaneous galcanezumab is being evaluated in several ongoing clinical trials sponsored by Eli Lilly and Company. The multinational, randomized, double-blind, placebo-controlled, phase III CONQUER trial (NCT03559257) is currently recruiting, and will assess the safety and efficacy of galcanezumab in adults with treatment-resistant episodic or chronic migraine. In the USA and Puerto Rico, the randomized, double-blind, placebo-controlled, phase III REBUILD trial (NCT03432286) in paediatric patients (aged 6–17 years of age) with episodic migraine is also currently recruiting. In Japan, a randomized, long-term, open-label phase III trial (NCT02959190) is evaluating two doses of galcanezumab in patients with episodic and chronic migraine. A randomized, double-blind, phase II trial (NCT02959177) comparing galcanezumab and placebo in Japanese patients with episodic migraine is also nearing completion.
Galcanezumab is being studied in chronic cluster headache in an ongoing open-label extension phase of NCT02438826. Patients who have completed either NCT02438826 or NCT02397473 are being invited to enroll in a multinational, open-label phase IIIb safety study (NCT02797951) of galcanezumab in episodic or chronic cluster headache.
3 Current Status
Galcanezumab received its first global approval on 27 September 2018 in the USA for the preventive treatment of migraine in adults.
References
Benschop RJ, Collins EC, Darling RJ, et al. Development of a novel antibody to calcitonin gene-related peptide for the treatment of osteoarthritis-related pain. Osteoarthr Cartil. 2014;22(4):578–85.
Monteith D, Collins EC, Vandermeulen C, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the CGRP binding monoclonal antibody LY2951742 (galcanezumab) in healthy volunteers. Front Pharmacol. 2017;8:740.
Goldberg SW, Silberstein SD. Targeting CGRP: a new era for migraine treatment. CNS Drugs. 2015;29:443–52.
Eli Lilly and Company. Lilly’s Emgality™ (galcanezumab-gnlm) receives U.S. FDA approval for the preventive treatment of migraine in adults [media release]. 5 Oct 2018. http://investor.lilly.com/.
Eli Lilly and Company. EMGALITY (galcanezumab-gnlm) injection, for subcutaneous use: US prescribing information. 2018. http://pi.lilly.com/. Accessed 11 Oct 2018.
European Medicines Agency. Summary of opinion: Emgality (galcanezumab). 2018. http://www.ema.europa.eu/. Accessed 11 Oct 2018.
Eli Lilly and Company. Lilly’s investigational medicine for prevention of migraine met primary endpoint in a Phase 2b study [media release]. 17 June 2015. http://www.lilly.com/.
Eli Lilly and Company. Lilly announces acquisition of CGRP antibody for migraine prevention from Arteaus Therapeutics [media release]. 13 Jan 2014. http://www.lilly.com/.
Atlas Venture. Arteaus Therapeutics, LLC raises $18 M to prevent migraines [media release]. 19 Oct 2011. http://www.atlasventure.com/.
Vermeersch S, Benschop RJ, Van Hecken A, et al. Translational pharmacodynamics of calcitonin gene-related peptide monoclonal antibody LY2951742 in a capsaicin-induced dermal blood flow model. J Pharmacol Exp Ther. 2015;354(3):350–7.
Kielbasa W, O’Brien L, Moser B, et al. Assessment of pharmacokinetics, target engagement and immunogenicity in patients with migraine administered galcanezumab, an anti-CGRP antibody [abstract no. IOR09]. Headache. 2018;58 (Suppl 2):77–8.
Stauffer VL, Dodick DW, Zhang Q, et al. Evaluation of Galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018;75(9):1080–8.
Skljarevski V, Matharu M, Millen BA, et al. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38(8):1442–54.
Aurora S, Detke H, Millen B. Rapid onset of effect of galcanezumab for the prevention of episodic migraine: post-hoc analyses of two phase 3 studies [abstract no. IOR05]. Headache. 2018;58 (Suppl. 2):75.
Detke HC, Wang S, Skljarevski V, et al. A phase 3 placebo-controlled study of galcanezumab in patients with chronic migraine: results from the 3-month double-blind treatment phase of the REGAIN study [abstract no. PS89LB]. Headache. 2017;57(8):1336–7.
Eli Lilly and Company. Summary of the efficacy and safety of galcanezumab in phase 3, randomized, double-blind, placebo-controlled studies. 2017. http://www.headache.mobi/. Accessed 11 Oct 2018.
Eli Lilly and Company. IHC 2017: Lilly’s galcanezumab demonstrates positive long-term safety results for up to 12 months in patients with migraine [media release]. 8 Sep 2017. http://www.lilly.com/.
Skljarevski V, Oakes TM, Zhang Q, et al. Effect of different doses of galcanezumab vs placebo for episodic migraine prevention: a randomized clinical trial. JAMA Neurol. 2018;75(2):187–93.
Eli Lilly and Company. Galcanezumab (LY2951742) CGRP monoclonal antibody for the prevention of migraine: phase 2, randomized, double-blind, placebo-controlled studies, ART01 and CGAB. 2017. http://www.headache.mobi/. Accessed 11 Oct 2018.
Dodick DW, Goadsby PJ, Spierings EL, et al. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2014;13(9):885–92.
Martinez JM, Goadsby PJ, Dodick DW, et al. Study CGAL: a placebo-controlled study of galcanezumab in patients with episodic cluster headache: results from the 8-week double-blind treatment phase [abstract no. 33]. Postgrad Med. 2018;130(Suppl. 1):24–5.
Eli Lilly and Company. Lilly’s galcanezumab meets primary endpoint in phase 3 study evaluating galcanezumab for the prevention of episodic cluster headache [media release]. 15 May 2018. http://www.lilly.com/.
Oakes TMM, Skljarevski V, Zhang Q, et al. Safety of galcanezumab in patients with episodic migraine: a randomized placebo-controlled dose-ranging phase 2b study. Cephalalgia. 2018;38(6):1015–25.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Yvette Lamb is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Lamb, Y.N. Galcanezumab: First Global Approval. Drugs 78, 1769–1775 (2018). https://doi.org/10.1007/s40265-018-1002-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-018-1002-7