Abstract
Fruquintinib (Elunate®) is an orally available, potent and highly selective small molecule inhibitor of VEGFR-1, -2 and -3 that was discovered and developed by Hutchison MediPharma for the treatment of solid tumours. In September 2018, fruquintinib received its first global approval, in China, for use in the treatment of metastatic colorectal cancer (CRC) in patients who have failed at least two prior systemic anti-neoplastic therapies. Fruquintinib is in ongoing phase III clinical development for use in the treatment of advanced NSCLC and advanced gastric cancer. This article summarizes the milestones in the development of fruquintinib leading to this first approval for the treatment of metastatic CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A range of anti-neoplastic agents have been developed that target the pro-angiogenesis vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR) signalling pathway, including targeted monoclonal antibodies [1] and orally available small molecule inhibitors [2]. One issue that can affect small molecule inhibitors, in particular, is poor target selectivity, which may lead to off-target toxicities. Fruquintinib (Elunate®) is an orally available, potent and highly selective small molecule inhibitor of VEGFR-1, -2 and -3 that has been developed by Hutchison MediPharma [a subsidiary of Hutchison China MediTech (Chi-Med)] for the treatment of solid tumours [3]. Based on the findings of the pivotal phase III FRESCO trial [4], on 04 September 2018 fruquintinib was granted its first global approval, by the National Medical Products Administration (NMPA) of China (formerly the China Food and Drug Administration), for the treatment of metastatic colorectal cancer (CRC) patients who have failed at least two prior systemic anti-neoplastic therapies, including fluoropyrimidine, oxaliplatin and irinotecan, with or without prior use of anti-VEGF or anti-epidermal growth factor receptor (-EGFR) therapies [5]. In FRESCO, fruquintinib was administered orally at a dosage of 5 mg once daily on a schedule of 21 days on treatment, 7 days off treatment in repeating 28-day cycles [4]. Treatment was continued until disease progression or unacceptable toxicity.
The approval for fruquintinib in China came following the granting of Priority Review status for fruquintinib in the treatment of metastatic CRC by the NMPA of China in September 2017 [5]. Fruquintinib is in ongoing phase III clinical development in China for use in the treatment of advanced NSCLC (FALUCA trial; NCT02691299) and advanced gastric cancer (FRUTIGA trial; NCT03223376). Additionally, phase I clinical development in patients with solid tumours has been initiated in the USA after the US FDA accepted an Investigational New Drug application for fruquintinib [6].
1.1 Company Agreements and Patent Information
Fruquintinib was originated and developed by Hutchison MediPharma, a subsidiary of (and innovation platform for) Chi-Med. In October 2013, Hutchison MediPharma entered into a licensing agreement with Eli Lilly for the co-development and commercialization of fruquintinib in China [7].

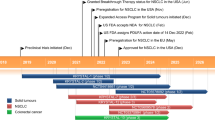
Key milestones in the development of fruquintinib. mCRC metastatic colorectal cancer, NSCLC non-small cell lung cancer
Hutchison MediPharma owns patents in major markets worldwide, including the USA, Europe, Canada, China, Japan and South Korea, for fruquintinib pharmaceutical compositions, and its use for treating angiogenesis-related disorders. Hutchison MediPharma also has a Patent Cooperation Treaty application pending in the major markets worldwide for the crystalline forms of fruquintinib.
2 Scientific Summary
2.1 Pharmacodynamics
Fruquintinib is a potent small molecule tyrosine kinase inhibitor (TKI) with high selectivity for VEGFR-1, -2 and -3 [3]. In an in vitro evaluation of fruquintinib against a panel of 253 kinases, fruquintinib inhibited VEGFR-1, -2 and -3 with IC50 values of 33, 35 and 0.5 nmol/L, respectively; it had weak activity (IC50 values of 128–458 nmol/L) against RET, FGFR-1 and c-kit kinases but had no significant inhibitory effect on any other kinase tested (IC50 values all > 1000 nmol/L) [3].
In in vitro cellular assays, fruquintinib suppressed VEGF/VEGFR signalling and cell proliferation [3]. Furthermore, the anti-angiogenic activity of fruquintinib was demonstrated in in vitro and ex vivo assays, as well as in a tumour xenograft model [3].
In vivo, fruquintinib inhibited tumour growth in multiple human tumour xenograft murine models, including xenograft models of gastric, colon and lung cancer [3]. Of note, an enhanced anti-tumour effect was observed when fruquintinib was administered in combination with chemotherapy agents (docetaxel or oxaliplatin) [3]. A synergistic anti-tumour effect has also been observed in xenograft models when fruquintinib is combined with other targeted therapies, including the EGFR TKIs gefitinib and theliatinib and the c-MET inhibitor savolitinib [8]. Also of note, co-administration of fruquintinib and an anti-PD-L1 antibody provided an enhanced anti-tumour effect in a syngeneic murine tumour model compared with either agent alone [8].
Finally, the anti-tumour activity of fruquintinib has been demonstrated in clinical trials involving patients with a range of solid tumour types, including patients with CRC (Sect. 2.3.1), NSCLC (Sect. 2.3.2), gastric cancer and breast cancer [4, 9,10,11].
2.2 Pharmacokinetics
Fruquintinib displayed linear pharmacokinetics in Chinese patients with solid tumours, with dose-proportional increases in exposure over the dose range of 1–6 mg [9].

Chemical structure of fruquintinib
Inter-individual variation in fruquintinib exposure was low [9].
Features and properties of fruquintinib
Alternative names | Elunate; HMPL-013 |
Class | Anti-neoplastics; small molecules |
Mechanism of action | VEGFR-1 antagonists; VEGFR-2 antagonists; VEGFR-3 antagonists; |
Route of administration | Oral |
Pharmacodynamics | Selectively inhibits VEGFR-1, -2 and -3 with IC50 values of 33, 35 and 0.5 nmol/L, respectively; displays anti-angiogenic activity in in vitro and ex vivo assays and in vivo; displays anti-tumour activity against a range of solid tumours |
Pharmacokinetics | Maximum plasma concentration reached in 2–4 h post-dose; a low volume of distribution; terminal elimination half-life is ~ 42 h; 60% of an administered dose is excreted in urine, 30% in faeces |
Most common treatment-related TEAEs | Hypertension, hand-foot skin reaction, proteinuria, dysphonia, thyroid stimulating hormone level elevated |
ATC codes: | |
WHO ATC code | L01X-E (Protein kinase inhibitors) |
EphMRA ATC code | L1H (Protein kinase inhibitor anti-neoplastics) |
Chemical name | 6-(6,7-dimethoxyquinazolin-4-yl)oxy-N,2-dimethyl-1-benzofuran-3-carboxamide |
Following oral administration, fruquintinib was rapidly absorbed, reaching maximum concentrations in 2–4 hours [9]. There was no clinically relevant food effect on fruquintinib absorption [12]. With a 3-week-on/1-week-off once-daily dosing schedule, steady-state pharmacokinetics were reached after ~ 14 days and maintained through to Day 21, with plasma drug concentrations accumulating approximately threefold [9]. At Day 21 of fruquintinib 5 mg once-daily dosing, mean steady-state maximum (Cmax) and average plasma concentrations were 383 ng/mL and 295 ng/mL, respectively. Over the week off dosing, the fruquintinib plasma concentration fell gradually and steadily, reaching a concentration of < 25 ng/mL by Day 28 [9].
Fruquintinib had a low volume of distribution, indicating relatively low tissue distribution [9]. In a study in healthy Chinese male subjects who were each administered a single radiolabelled dose of fruquintinib, unchanged parent drug was the predominant circulating component found in plasma [13]. Thus, unchanged fruquintinib is considered to be the principal entity responsible for activity. Over a 14-day post-dose collection period, a mean of 90% of the administered radioactivity was recovered (60% in urine, 30% in faeces) [13].
Fruquintinib had a long terminal elimination half-life (~ 42 h in Chinese patients with solid tumours) [9]. Prior to excretion, fruquintinib undergoes extensive metabolism, with multiple enzymes/pathways involved [13]. In the study in healthy subjects, < 6% of the administered dose was excreted in urine or faeces as the parent drug [13]. The major metabolite (M11), formed via N-demethylation on the benzofuran ring, accounted for 17.3% of radioactivity in circulation but lacked activity [13].
2.3 Therapeutic Trials
2.3.1 Metastatic Colorectal Cancer
Fruquintinib as third- or later-line therapy in the treatment of metastatic CRC significantly prolonged overall survival in the randomized, double-blind, placebo-controlled, multicentre phase III FRESCO trial in Chinese patients [4].
Key clinical trials of fruquintinib (Hutchison MediPharma Ltd)
Identifier | Indication | Phase | Drug(s) | Location | Status |
|---|---|---|---|---|---|
FRESCO; NCT02314819 | Metastatic CRC | III | Fruquintinib; placebo | China | Completed |
NCT02196688 | Metastatic CRC | II | Fruquintinib; placebo | China | Completed |
NCT01975077 | Metastatic CRC | I/II | Fruquintinib | China | Completed |
FALUCA; NCT02691299 | Advanced NSCLC | III | Fruquintinib; placebo | China | Ongoing |
NCT02590965 | Advanced NSCLC | II | Fruquintinib; placebo | China | Completed |
NCT02976116 | Advanced NSCLC | II | Fruquintinib; gefitinib | China | Ongoing |
NCT03684967 | Advanced NSCLC | II | Fruquintinib | China | Ongoing |
FRUTIGA; NCT03223376 | Advanced gastric cancer | III | Fruquintinib; paclitaxel; placebo | China | Ongoing |
In FRESCO, eligible patients were randomized (2:1) to receive oral fruquintinib 5 mg or matching placebo administered once daily on a schedule of 21 days on treatment, 7 days off treatment in repeating 28-day cycles [4]. Study treatment was continued until disease progression, unacceptable toxicity or study withdrawal. In addition to fruquintinib or placebo, all patients received best supportive care (BSC) [4].
Median overall survival (primary endpoint) was significantly improved in the fruquintinib group compared with the placebo group [9.3 months (95% CI 8.2–10.5) vs. 6.6 months (95% CI 5.9–8.1); hazard ratio (HR) for death, 0.65 (95% CI 0.51–0.83); p < 0.001] [4]. Consistent with the overall results, in subgroup analyses of overall survival the treatment effect favoured or trended toward fruquintinib over placebo, including in key subgroups based on prior use of VEGF inhibitors [yes (HR 0.68, 95% CI 0.45–1.03) or no (HR 0.60, 95% CI 0.45–0.80)] and K–ras status [wild-type (HR 0.56, 95% CI 0.40–0.78) or mutant (HR 0.75, 95% CI 0.53–1.07) [4].
Additionally, fruquintinib treatment was associated with a significant benefit compared with placebo in key secondary endpoints, including progression-free survival (PFS; 3.7 vs. 1.8 months; p < 0.001), objective response rate [ORR (i.e. a confirmed complete or partial response by RECIST v1.1 criteria); 4.7 vs. 0.0%; p = 0.01] and disease control rate [DCR (i.e. a confirmed complete or partial response or stable disease ≥ 8 weeks); 62.2 vs. 12.3%; p < 0.001] [4]. Subgroup analyses of PFS showed a benefit of fruquintinib treatment versus placebo across all subgroups evaluated [4].
Patients eligible for FRESCO were adults (aged 18–75 years) who had metastatic CRC that had progressed following two or more prior chemotherapy regimens [4]. Prior treatment with VEGF or EGFR inhibitors was permitted (reported in 30.0 and 14.2% of patients, respectively, across groups), but patients who had previously received VEGFR inhibitor therapy were excluded. Patient demographics and baseline disease characteristics and treatment history were generally well matched between the fruquintinib and placebo groups, although the proportion of men was lower in the fruquintinib group (56.8%) than in the placebo group (70.3%). Across both groups, 44.5% of patients had K–ras mutations [4].
2.3.2 Advanced NSCLC
Fruquintinib treatment significantly increased PFS compared with placebo in a randomized, double-blind phase II proof-of-concept trial (NCT02590965) in Chinese patients with advanced NSCLC who had failed two prior lines of chemotherapy [10]. Participants in the trial (n = 91) were randomized (2:1) to receive oral fruquintinib or matching placebo [administered according to the same schedule as used in FRESCO (see Sect. 2.3.1)], both in combination with BSC [10].
Median PFS as assessed by a blinded image central review committee (primary endpoint) was 3.8 months (95% CI 2.8–4.6) in the fruquintinib group versus 1.1 months (95% CI 1.0–1.9) in the placebo group (HR 0.34; 95% CI 0.20–0.57; p < 0.001) [10]. PFS outcomes as assessed by the investigator (secondary endpoint) were near identical to those of the primary endpoint results. Fruquintinib treatment was associated with a significantly higher ORR (13.1 vs. 0.0%; p = 0.041) and DCR (60.7 vs. 13.3%; p < 0.001) compared with placebo. Median overall survival was 7.7 months (95% CI 6.4–15.2) in the fruquintinib group compared with 9.7 months (95% CI 4.3–16.6) in the placebo group (p = 0.152), but the study was not sufficiently powered to test this endpoint. Patients in the fruquintinib group had numerically higher 3-month (90.2 vs. 73.3%) and 6-month (67.2 vs. 58.8%) survival rates [10].
Participants in the trial were adults (aged 18–75 years) with advanced non-squamous NSCLC that had progressed despite prior treatment with two or more standard chemotherapy regimens (the first of which was a platinum-based doublet regimen) [10]. Patients with prior treatment with VEGFR inhibitors were excluded [10].
2.4 Adverse Events
In the FRESCO trial in Chinese patients with metastatic CRC (Sect. 2.3.1), oral fruquintinib had acceptable safety, with a tolerability profile that was largely consistent with that of other inhibitors of the VEGF/VEGFR pathway [4]. With median treatment exposures of 3.7 months and 1.8 months in the fruquintinib and placebo groups, treatment-emergent adverse events (TEAEs) that were considered to be related to study drug treatment occurred in 95.7 and 70.8% of patients in the respective groups. The most common treatment-related TEAEs were hypertension (in 55.4% of fruquintinib recipients vs. 15.3% of placebo recipients), hand-foot skin reaction (49.3 vs. 2.9%), proteinuria (42.1 vs. 24.8%), dysphonia (36.0 vs. 1.5%) and thyroid stimulating hormone level elevated (24.8 vs. 2.2%). TEAEs were mostly of grade 1–2 severity and were usually manageable with dosage modification (treatment interruption or dose reduction) and/or supportive measures. Treatment-related TEAEs of grade ≥ 3 severity were reported in 46.0% of fruquintinib recipients versus 7.3% of placebo recipients, the most common of which were hypertension (in 21.2% of fruquintinib recipients vs. 2.2% of placebo recipients), hand-foot skin reaction (10.8 vs. 0.0%) and proteinuria (3.2 vs. 0.0%) [4].
Serious adverse events (SAEs) were reported in 15.5% of fruquintinib recipients versus 5.8% of placebo recipients, with treatment-related SAEs reported in 6.1 versus 1.5% of patients in the respective groups [4]. TEAEs leading to dosage modification (treatment interruption or dose reduction) occurred in 47.1% of fruquintinib recipients and 13.1% of placebo recipients. The TEAEs most commonly leading to dosage modification in the fruquintinib group were hand-foot skin reaction (13.3% of patients), proteinuria (9.7%) and decreased platelet counts (5.4%). Overall, 15.1% of patients in the fruquintinib group and 5.8% in the placebo group discontinued study drug treatment because of TEAEs. TEAEs resulting in death occurred in nine patients (3.2%) in the fruquintinib group and two patients (1.5%) in the placebo group [4].
Based on available data [10], the safety and tolerability profile of fruquintinib in patients with advanced NSCLC was consistent with that observed in FRESCO.
2.5 Ongoing Clinical Trials
Hutchison MediPharma’s clinical development program for fruquintinib is continuing, with several ongoing clinical trials, including:
-
FALUCA (NCT02691299): a randomized, double-blind, placebo-controlled, multicentre, phase III trial evaluating the efficacy and safety of fruquintinib in patients with advanced non-squamous NSCLC who have failed two prior lines of systemic chemotherapy. The primary endpoint of the trial is overall survival.
-
FRUTIGA (NCT03223376): a randomized, double-blind, placebo-controlled, multicentre, phase III trial evaluating the efficacy and safety of fruquintinib combined with paclitaxel versus paclitaxel alone in patients with advanced gastric or gastroesophageal junction adenocarcinoma who have progressed after first-line standard chemotherapy. The primary endpoint of the trial is overall survival.
-
NCT02976116: a single-arm, multicentre, phase II trial evaluating the efficacy and safety of the combination of fruquintinib and gefitinib as first-line therapy in patients with advanced non-squamous NSCLC harbouring activating EGFR mutations.
-
NCT03684967: an open-label, single-arm, multicentre phase II trial evaluating the efficacy and safety of fruquintinib in high risk patients with advanced NSCLC.
-
NCT03251378: an open-label, multicentre, phase I dose escalation trial to evaluate the safety, tolerability and pharmacokinetics of fruquintinib in patients with advanced solid tumours in the USA. This is the first trial to evaluate fruquintinib in non-Chinese patients.
3 Current Status
Fruquintinib received its first global approval on 04 September 2018, in China, for the treatment of metastatic CRC patients who have failed at least two prior systemic anti-neoplastic therapies, including fluoropyrimidine, oxaliplatin and irinotecan, with or without prior use of anti-VEGF or anti-EGFR therapies [5].
References
Scott AM, Allison JP, Wolchok JD. Monoclonal antibodies in cancer therapy. Cancer Immun. 2012;12(1):14–21.
Zhong H, Bowen JP. Recent advances in small molecule inhibitors of VEGFR and EGFR signaling pathways. Curr Top Med Chem. 2011;11(12):1571–90.
Sun Q, Zhou J, Zhang Z, et al. Discovery of fruquintinib, a potent and highly selective small molecule inhibitor of VEGFR 1, 2, 3 tyrosine kinases for cancer therapy. Cancer Biol Ther. 2014;15(12):1635–45.
Li J, Qin S, Xu RH, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: the FRESCO randomized clinical trial. JAMA. 2018;319(24):2486–96.
Chi-Med. Chi-Med announces the approval of fruquintinib capsules for previously treated colorectal cancer in China [media release]. 2018. http://www.chi-med.com/wp-content/uploads/2018/09/a180905.pdf. Accessed 27 Sep 2018.
Chi-Med. Chi-Med initiates fruquintinib U.S. clinical trials [media release]. 2017. http://www.chi-med.com/wp-content/uploads/2017/12/a171215.pdf. Accessed 27 Sep 2018.
Chi-Med. Cancer therapy collaboration with Lilly [media release]. 2013. http://www.chi-med.com/wp-content/uploads/docArchive/announcements/a131009.pdf. Accessed 27 Sep 2018.
Ren Y, Sun Q, Long J, et al. Evaluation of fruquintinib, a potent and selective oral VEGFR inhibitor, in combination with targeted therapies or immune checkpoint inhibitors in preclinical tumor models [abstract no. 2089 plus poster]. Cancer Res. 2017;77(13 Suppl):2089.
Cao J, Zhang J, Peng W, et al. A Phase I study of safety and pharmacokinetics of fruquintinib, a novel selective inhibitor of vascular endothelial growth factor receptor-1, -2, and -3 tyrosine kinases in Chinese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2016;78(2):259–69.
Lu S, Chang J, Liu X, et al. Randomized, double-blind, placebo-controlled, multicenter phase II study of fruquintinib after two prior chemotherapy regimens in Chinese patients with advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2018;36(12):1207–17.
Xu RH, Li J, Bai Y, et al. Safety and efficacy of fruquintinib in patients with previously treated metastatic colorectal cancer: a phase Ib study and a randomized double-blind phase II study. J Hematol Oncol. 2017;10(1):22.
Li K, Li X, Xia S, et al. Pharmacokinetics of fruquintinib in humans [abstract no. PII-116 plus poster]. Clin Pharmacol Ther. 2018;103 (Suppl S1):S86.
Zhou S, Shao F, Xu Z, et al. A phase I study to investigate the metabolism, excretion, and pharmacokinetics of [14C]fruquintinib, a novel oral selective VEGFR inhibitor, in healthy Chinese male volunteers. Cancer Chemother Pharmacol. 2017;80(3):563–73.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Matt Shirley is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Shirley, M. Fruquintinib: First Global Approval. Drugs 78, 1757–1761 (2018). https://doi.org/10.1007/s40265-018-0998-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-018-0998-z