Abstract
Chronic rhinosinusitis (CRS) is an inflammatory sinonasal condition with multiple etiologic factors that is associated with a vast economic cost. Treatment is most frequently pharmacologic and has centered on agents that ameliorate inflammation, decrease bacterial or pathogen load, and facilitate egress of mucus or purulence from the sinonasal cavity. Nasal saline irrigations, topical nasal steroids, certain antibiotics, and systemic steroids have shown some efficacy in the management of CRS. Recently, biologic therapeutics that target specific inflammatory pathways associated with subsets of CRS have been developed and evaluated. Early data evaluating these biologic treatments suggest a potential role in treating a subset of CRS with refractory, poorly controlled disease. Additional studies are necessary to identify which patients would benefit most from biologic therapies and to assess the cost of these therapies compared with the benefit they provide. This review describes the pathophysiology of CRS and summarizes both established and novel biologic pharmacologic treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Chronic rhinosinusitis (CRS) is an inflammatory sinonasal condition with multiple etiologic factors. Pharmacologic treatment is the foundation of management for this condition. |
Nasal saline irrigations, topical nasal steroids, certain antibiotics, and systemic steroids have shown some efficacy in the management of CRS. |
Novel biologic therapeutics are under evaluation for the treatment of refractory, poorly controlled CRS with nasal polyps. |
1 Pathophysiology
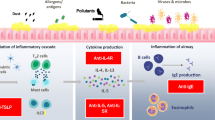
Chronic rhinosinusitis (CRS) affects tens of millions of patients worldwide and has an estimated total national healthcare cost of over$8 billion annually in the United States [1]. Multiple etiologic factors have been linked to the development of CRS, including environmental and occupational factors, infection, allergy, disruptions of the sinonasal mucosal epithelial barrier, genetic abnormalities, anatomic variations, inflammation/osteitis, vitamin D status, biofilms, disturbances of the nasal microbiome, immune status, ciliary dysfunction, superantigens, and systemic diseases [2]. In part due to the many etiologic factors, CRS is not a uniform disease, rather it displays significant phenotypic heterogeneity and likely represents a syndrome consisting of many diseases that have yet to be more clearly defined. While commonly considered to be an infectious disease, most CRS appears to have a primarily inflammatory pathophysiology.
CRS can be grossly divided into disease with (CRSwNP) and without nasal polyposis (CRSsNP), even though there is still significant heterogeneity within these subcatergorizations. Recent evidence supports the presence of distinct underlying endotypes that lead to different disease states, including T helper (Th) 1-driven pathways for CRSsNP and Th2-driven pathways for CRSwNP [3]. The different pathways associated with CRS and molecular mechanisms behind those pathways provide insight into possible new therapeutic targets.
CRSsNP patients typically have an abundance of neutrophils and elevated type 1 cytokines such as interferon (IFN)-γ, while CRSwNP patients have an array of eosinophils, mast cells, basophils, and elevation in type 2 cytokines such as interleukin (IL)-4, IL-5, and IL-13 [4]. IL-5 is essential for the differentiation, survival, and activation of eosinophils, which plays a significant role in not only CRSwNP but also other atopic diseases, including asthma [5]. Nasal polyps are most commonly eosinophilic and are therefore intertwined with IL-5 mechanistically, although a smaller subset of certain neutrophil-expressing polyps demonstrates higher levels of IL-17 and IFN-γ [4, 6]. In the CRSwNP population, IL-4 is a key factor in the underlying immunoglobulin (Ig) E-mediated response [4]. IL-13 and IL-4 suppress bone remodeling, and IL-13 has recently been shown to have a possible association with osteoneogenesis in a subset of patients with CRS [7, 8].
2 Established Treatment Modalities
A variety of pharmacologic treatments focus on ameliorating inflammation, decreasing bacterial or pathogen load, and permitting egress of mucus or purulence from the paranasal sinuses and nasal cavity. These treatments are generally applicable to both CRSsNP and CRSwNP populations, with some exceptions. High-level studies for these interventions are presented below. For a more comprehensive review of established pharmacologic therapies, the reader is directed to the robust International Consensus Statement on Allergy and Rhinology: Rhinosinusitis [2].
2.1 Nasal Saline Irrigation
High-volume (>200 mL) nasal saline irrigation for the treatment of CRS at all stages of disease is supported by high-level evidence. Saline irrigations facilitate the clearance of mucus, improve ciliary beat frequency, enhance removal of biofilms and antigens, and may also contribute to the resolution of intranasal inflammation [9]. A Cochrane systematic review and meta-analysis in 2007 evaluated the use of nasal saline irrigations in eight trials [9]. Analysis included comparisons of saline irrigation versus no treatment or placebo, as an adjunct to intranasal steroids, against intranasal steroids, and comparisons of high-volume hypertonic versus isotonic solutions. Overall, nasal saline irrigations were determined to be effective as both primary and adjunctive treatment for CRS, although nasal saline was found to be less effective than nasal steroids [9]. High-volume saline irrigation is well-tolerated, inexpensive, and does not have major side effects [2, 9, 10]. A comprehensive consensus statement strongly recommended high-volume nasal saline irrigations as an adjunct to therapy for CRS [2].
Conversely, low-volume nasal saline sprays have been shown to be less efficacious for CRS compared with high-volume irrigations. A randomized trial of patients with chronic nasal and sinus symptoms compared high-volume irrigations with low-volume sprays over 8 weeks and demonstrated greater improvement in patient-reported symptom scores in the high-volume irrigation group [11]. A comprehensive consensus statement identified low-volume saline sprays as likely inferior to high-volume saline irrigations [2].
2.2 Intranasal Steroids
Intranasal steroids are widely used for the treatment of both CRSsNP and CRSwNP at all stages of disease to decrease the inflammatory burden. Mometasone furoate is the only US Food and Drug Administration-approved medication for the treatment of CRSwNP, although other topical steroid medications are frequently employed in clinical practice today [12, 13]. Intranasal steroids have been shown to be quite safe. While previous concerns existed about the potential systemic uptake of certain agents, a recent review evaluating a multitude of studies demonstrated no significant impact on the hypothalamic-pituitary-adrenal axis with newer intranasal steroid agents [14].
A Cochrane review published in 2016 analyzed 18 randomized trials evaluating intranasal steroids versus placebo or no treatment, 14 of which included CRSwNP patients and 4 of which included CRSsNP patients [15]. Medications evaluated included fluticasone propionate 400–800 μg/day, with one study additionally including a higher dose treatment arm; beclomethasone propionate 400–800 μg/day; mometasone furoate 100–400 μg/day; and budesonide 128–400 μg/day [15]. There appeared to be improvement for all symptoms (nasal congestion, rhinorrhea, hyposmia/anosmia, facial pain/pressure), with a moderate benefit for nasal congestion and a small benefit for rhinorrhea [15]. A comprehensive consensus statement recommended standard metered dose nasal steroids based on the benefits of improved symptoms and endoscopic disease severity scores for patients with CRSsNP, and improved symptoms, polyp size, endoscopic appearance, quality of life (QoL) scores, and olfaction metrics for patients with CRSwNP [2]. This consensus statement also concluded that using high-volume steroid irrigations (topical steroid mixed in >200 mL of saline) was an option for CRS due to the benefits of improved QoL metrics, symptoms, and endoscopic appearance postoperatively for CRSsNP, with greater benefit from irrigations anticipated postoperatively given the greater accessibility of the sinus mucosa afforded by the surgically created openings [2].
Another recent Cochrane review evaluated four different intranasal steroids (fluticasone propionate, beclomethasone propionate, mometasone furoate, and budesonide) administered via nasal spray, drops, or actuated inhaler in nine randomized trials of CRS patients and determined there was insufficient evidence to suggest that one type of intranasal steroid was more efficacious than another [16]. Local, intranasal administration enables patients to avoid many of the side effects of systemic steroids. However, it should be noted that the risk of epistaxis is increased with intranasal steroids, although it is possible that small streaks of blood reported in some patients are not particularly clinically relevant [15].
2.3 Oral Steroids
Oral steroids, like their topical counterpart, are commonly used pharmacologic agents intended to decrease the inflammation associated with CRS. Robust evidence supports employing short-term courses of oral steroids for CRSwNP, while there is limited evidence to support the routine use of oral steroids in CRSsNP [2]. There are potential significant side effects of oral steroids that must be considered, including hyperglycemia, gastrointestinal ulcers, adrenal suppression, increased bone turnover, and avascular necrosis at higher cumulative doses [2].
A recent Cochrane review evaluated the efficacy of oral steroids in CRSwNP in eight randomized trials [17]. The authors concluded that patients experienced improvements in QoL metrics and symptom severity following a 2- to 3-week course of oral steroid therapy, although this benefit was limited to 3–6 months after therapy. Additionally, side effects, including gastrointestinal disturbances and insomnia, may have been increased during treatment with oral steroids [17]. These conclusions are supported by a recent consensus statement that recommends oral steroids in CRSwNP for short-term management but not long-term or frequent management due to the increased risk of side effects [2]. An evidence-based risk analysis in CRSwNP patients using complication rates from the literature, QoL changes, and US Medicare costs demonstrated that endoscopic sinus surgery (ESS) would be favored over medical management when patients required oral steroids more frequently than once every 2 years [18].
2.4 Antibiotics
Antibiotics are frequently used to mitigate the infectious component of CRS. In addition to standard antimicrobial effects, macrolide antibiotics possess anti-inflammatory and immunomodulatory properties, which may be of additional benefit in treating sinonasal disease [19].
Macrolides have been primarily evaluated in refractory CRSsNP and have been shown to decrease endoscopic disease severity scores and certain symptoms. Based on this evidence, macrolides are a treatment option for CRSsNP, although neither the subgroup of patients who would most likely benefit nor the optimal treatment regimen (dose and length of therapy) has been rigorously studied [2]. Similarly, in CRSwNP, macrolides decrease polyp burden after ESS and reduce symptoms, and may be beneficial postoperatively [2]. A recent Cochrane review on the utility of antibiotics in CRS patients found limited evidence that systemic antibiotics are effective in treating CRS, although there was a moderate, transient QoL improvement in CRSwNP patients receiving 3 months of macrolide antibiotics [20]. Again, this improvement is thought to be due to a combination of anti-inflammatory, immunomodulatory and antibacterial effects [19]. Side effects of macrolides are rare and are usually related to gastrointestinal irritation; very unusual side effects include ototoxicity, liver dysfunction, and the small chance of the cardiac arrhythmia torsades de pointes, which recently prompted an FDA warning cautioning against the use of macrolides in patients with known cardiac risk factors [2, 21].
The use of non-macrolide antibiotics for CRS is more controversial and potential risks and benefits must be evaluated. For the CRSsNP population, evidence for both short- and long-term use of non-macrolide antibiotics is limited, and no recommendation for their use was possible based on an extensive literature review [2]. For the CRSwNP population in a non-acute clinical setting, evidence for the use of short-term non-macrolide antibiotics generally suggests that harms of gastrointestinal upset, the chance of fostering resistant organisms, and the risk of anaphylaxis outweigh the benefits associated with these agents [2]. While controversial, antibiotics are commonly prescribed for CRS; in recent years, provider visits for CRS were among the most frequent reason an antibiotic was prescribed [22]. The potential negative consequences of antibiotic therapy include allergic reactions, cost, adverse effects, and bacterial resistance [22]. Common adverse effects include gastrointestinal irritation, rashes, genitourinary infections, and Clostridium difficile colitis [2].
Intravenous antibiotics are not recommended for routine cases of CRS based on the risk-benefit ratio of complications such as thrombophlebitis, neutropenia, venous thromboembolism, rash, and other adverse events compared with a potential reduction in symptoms, although there may be a role for intravenous agents in complicated, extra-paranasal complications of sinusitis [2].
Topical antibiotics, which may locally deliver a high concentration of active agent to diseased sinonasal mucosa, are not recommended for CRS based on a greater extent of side effects (congestion, epistaxis, and irritability) compared with a lack of long-term benefit in randomized trials [2, 23,24,25]. Although included in the Cochrane review search design, no randomized trials of topical antibiotics were included in the most recent Cochrane review of antibiotics for chronic sinusitis [20].
2.5 Antifungal Agents
Fungal elements may be a contributing etiologic factor in a subset of CRS patients, especially those with eosinophilic disease [26]. However, antifungals have not demonstrated significant benefit in treating CRS. A Cochrane review evaluated six randomized studies of antifungals in the management of CRS and allergic fungal rhinosinusitis, five of which assessed topical antifungals and one of which investigated systemic therapy [27]. Pooled meta-analysis of these studies using original data demonstrated no benefit for either formulation of antifungals over placebo [27]. Other analyses agree with this finding, confirming there is no evidence supporting oral or topical antifungals in the treatment of CRS and recommending against the use of these agents in most cases [2].
2.6 Leukotriene Antagonists
Cysteinyl leukotrienes are mediators of inflammation that originate in eosinophils and mast cells and are closely related to the pathophysiology of CRSwNP and asthma [28, 29]. Leukotriene antagonists (LTAs) function by inhibiting the arachadonic acid inflammatory cascade; montelukast, zarfilukast, and pranlukast inhibit the cysteinyl leukotriene 1 receptor and block downstream effects of this pathway, while zileuton inhibits 5-lipoxygenase and decreases leukotriene synthesis [30].
Overall, studies have demonstrated a mild benefit for LTAs in treating CRSwNP. A systematic review evaluated LTAs in CRSwNP patients among 12 studies, 5 of which were randomized trials [31]. Several of the randomized trials demonstrated improvement in symptom and nasal endoscopy disease severity scores. However, 2 of these 12 studies were combined in a meta-analysis and showed no difference between LTA and intranasal steroid arms when measuring disease severity scores. The authors concluded that LTAs were effective for treating CRSwNP, with limited benefit as adjunctive therapy to intranasal steroids [31]. A separate review of five studies evaluating the utility of montelukast in CRSwNP patients concluded there is moderate evidence that montelukast is an effective adjunct to intranasal or oral steroids [29]. A recent consensus statement describes montelukast as an option for managing CRSwNP patients with or without intranasal steroids [2]; however, a comprehensive European Position Paper from 2012 makes a recommendation to avoid LTAs in CRSwNP [32]. Montelukast has rarely been associated with neuropsychiatric side effects [2], while zileuton has been shown to be effective in patients with asthma-exacerbated respiratory disease but has a less favorable side effect profile than montelukast, including the potential for transaminitis [2, 33].
2.7 Endoscopic Sinus Surgery
Surgical treatment for CRS is indicated when appropriate medical therapy cannot fully control symptoms. While a full discussion of the role and utility of ESS is beyond the scope of this review, the topic is briefly mentioned as ESS is a standard component of treatment for CRS [34,35,36,37,38] and has multiple aims, including the removal of antigenic or inflammatory material, enhancing mucociliary clearance, and facilitating instillation and distribution of topical medications, such as topical steroids, postoperatively [2, 39]. The use of saline irrigations and topical intranasal steroids following ESS reduces symptom severity and improves endoscopic scores. In addition, administration of topical intranasal steroids postoperatively helps decrease the polyp recurrence rate in CRSwNP [40, 41].
3 Biologic Pharmacologic Treatments
Traditional pharmacologic agents and ESS are effective in controlling the symptoms of CRS for the majority of patients; however, a substantial proportion of patients who undergo appropriate medical therapy and ESS have disease recurrence and persistent symptoms. Among CRSwNP patients, those with aspirin-exacerbated respiratory disease, asthma and/or frontal sinus disease are more likely to remain symptomatic and require revision surgery [42]. There is a clear need for additional therapeutic interventions to decrease the inflammatory disease burden in these patients with more complicated disease.
As our understanding of the pathophysiology of CRS endotypes progresses, a personalized treatment approach targeted to the specific molecular pathways involved will be possible. These evolving biologic treatments often originated as treatments for patients with asthma or atopic diseases, including allergic rhinitis, food allergy, atopic dermatitis, and urticaria. Many patients with these diseases display a similar inflammatory profile as patients with CRSwNP, including elevated Th2 cytokines IL-4, IL-5, and IL-13 [43,44,45]. Table 1 lists the current biologic agents being evaluated for the treatment of CRS.
3.1 The Anti-Immunoglobulin E Pathway: Omalizumab and Ligelizumab
Omalizumab, a recombinant humanized monoclonal antibody previously approved for patients with refractory allergic asthma [46], has recently been studied in the CRSwNP population. Asthma and CRSwNP often occur simultaneously and both diseases have been shown to share similar inflammatory mediators [2]. IgE leads to allergic symptoms via binding with a specific high-affinity receptor via the Fc region on mast cells and basophils, subsequently promoting degranulation and allowing the release of inflammatory mediators [47]. Omalizumab complexes with free circulating IgE, which leads to lower expression of IgE on effector cells and subsequently inhibits the stimulation of these cells [4]. The selective binding to IgE also disrupts the binding of IgE to the high-affinity IgE receptor [4, 47]. Omalizumab does not complex with IgE molecules that are already bound to cells, and therefore does not promote mast cell crosslinking and degranulation [47].
Following success in treating asthmatics, controlled studies in the early 2000s demonstrated a benefit for omalizumab in treating patients with allergic rhinitis [48, 49]. Later that decade, case reports posited a potential benefit for symptom reduction for asthmatic CRS patients [50, 51]. These reports were followed by a small retrospective pilot study undertaken in CRSwNP asthmatic patients that demonstrated an improvement in endoscopic nasal polyp scores after treatment with omalizumab compared with a control group who did not receive omalizumab, without improvement in computed tomography (CT) disease severity scores [52].
An initial randomized, double-blind, placebo-controlled trial of omalizumab for CRS was published in 2010 [53]. This study had recruitment challenges and did not distinguish between CRSwNP and CRSsNP, although 12 of 14 patients had CRSwNP and all had failed prior ESS. After subcutaneous injection of omalizumab or placebo every 4 weeks for 6 months, treatment with omalizumab decreased CT disease severity scores (the primary outcome measure) in a non-significant fashion. Secondary outcomes, such as general and disease-specific QoL outcomes, olfactory testing, nasal endoscopic disease severity scores, nasal peak inspiratory flow, and eosinophil count in nasal lavage also did not show differences between the omalizumab and placebo groups. The authors concluded that IgE plays a small role in CRS mucosal inflammation, and suggested that larger controlled studies were necessary to assess the size of any effect [53].
Subsequent high-level evidence demonstrated a greater benefit for treating CRSwNP patients with omalizumab. In a randomized, double-blind, placebo-controlled trial of allergic and non-allergic patients with nasal polyps (N = 24), patients treated with four to eight subcutaneous omalizumab injections (n = 16) experienced an improvement in nasal polyp scores, the primary outcome, compared with the control group (n = 8), starting 8 weeks after treatment and persisting through 16 weeks [54]. The treatment group also demonstrated improvements in CT disease severity scores, most nasal and asthma symptoms, the Short-Form Health Questionnaire (SF-36) general QoL scores, and the sleep and general symptoms domains of the 31-item Rhinosinusitis Outcome Measure instrument (RSOM-31). While the authors acknowledge these benefits, they suggested that due to high costs, omalizumab should be reserved for asthmatic CRSwNP patients who have recalcitrant symptoms despite ESS [54].
A randomized, phase II trial of 27 patients evaluating the utility of subcutaneous omalizumab for the treatment of nasal polyposis has recently been completed; however, full results are not yet available [55]. A recent retrospective chart review in patients with CRS and asthma who received omalizumab demonstrated a decrease in antibiotic prescriptions following omalizumab treatment compared with the period prior to anti-IgE treatment; post-omalizumab steroid use trended toward declining but was not significant [56].
Ligelizumab (QGE031) is a new, investigational anti-IgE antibody with a higher binding affinity to IgE compared with omalizumab. Early-stage testing demonstrated superior pharmacologic effects in atopic and asthmatic patients compared with omalizumab [57, 58]. The role of this agent in treating CRS remains to be discerned.
3.2 The Anti-Interleukin-5 Pathway: Reslizumab, Mepolizumab and Benralizumab
The majority of CRSwNP patients have a Th2 inflammatory profile with significant tissue eosinophilia and IL-5 expression [59], although there is geographic/ethnic variability associated with nasal polyposis and Asian patients have been shown to have a greater propensity toward Th1 profiles compared with Caucasians [60]. IL-5 is one of the most important and specific factors for eosinophil growth, differentiation, and survival [4, 61]. In vitro work has demonstrated that IL-5 localizes to eosinophils, as well as mast cells and lymphocytes, in nasal polyp tissue, and that treatment with an anti-IL-5 antibody led to increased eosinophil apoptosis and mitigated tissue eosinophila [62].
Reslizumab, a humanized monoclonal antibody to IL-5, was the first anti-IL-5 antibody evaluated clinically for the treatment of sinonasal disease, and is currently approved for the treatment of severe asthma [63]. Reslizumab was tested in a randomized, double-blind, placebo-controlled trial in which 24 CRSwNP subjects received a single intravenous infusion of reslizumab 3 mg/kg (n = 8), reslizumab 1 mg/kg (n = 8), or placebo (n = 8) [64]. The primary outcome, nasal polyp severity score, was improved in approximately half of the treated patients for 4 weeks. On post hoc analysis, patients with elevated baseline nasal secretion IL-5 levels (>40 pg/mL) were found to be more likely to respond to anti-IL-5 treatment. Both treatment groups experienced a decrease in blood eosphinophil count within 1 day of dosing that was sustained for 8 weeks. A randomized, double-blind, phase III study evaluating the utility of reslizumab in treating CRS is underway [65].
Mepolizumab, a humanized anti-IL-5 antibody currently approved for severe asthma [66], was subsequently assessed in a randomized controlled trial of 30 patients with advanced nasal polyps in which patients received either two intravenous infusions of mepolizumab 28 days apart (n = 20) or placebo (n = 10) [67]. Compared with the placebo group, mepolizumab-treated patients had improved nasal polyp severity scores 8 weeks after the first treatment, the primary outcome. CT severity scores 8 weeks after treatment improved in the majority of the mepolizumab-treated group, compared with less than one-fifth of the placebo group. There were no significant changes in symptom scores and, unlike the aforementioned reslizumab study [64], there was no difference in baseline nasal IL-5 levels between mepolizumab responders and non-responders [67].
A multicenter, randomized, double-blind trial comparing mepolizumab with placebo as a means to reduce the potential need for surgery in patients with severe nasal polyps has recently been completed [68]. Patients received up to six doses of mepolizumab or placebo at 4-week intervals; however, the results have not yet been published. A separate clinical trial evaluating the effect of mepolizumab for severe bilateral nasal polyps is ongoing [69].
Benralizumab is a humanized monoclonal antibody that binds the IL-5 receptor alpha subunit with high affinity and inhibits IL-5 binding, which leads to eosinophil and basophil apoptosis via antibody-dependent cellular toxicity [70, 71]. While benralizumab has shown utility in treating severe asthma [72], a role in the management of CRSwNP remains to be specifically elucidated. A phase II clinical trial of benralizumab for the treatment of patients with eosinophilic CRS is ongoing [73].
3.3 The Anti-IL-4/IL-13 Pathway: Dupilumab
IL-4 and IL-13 are inflammatory Th2 cytokines with overlapping effects, and therefore an intervention that blocks the downstream effects of both of these molecules could have therapeutic promise [74]. While two different receptors are involved in the pathways of IL-4 and IL-13 signaling, receptors in both pathways include the alpha subunit of the IL-4 receptor [59]. Dupilumab is a fully human monoclonal antibody against the alpha subunit of the IL-4 receptor and thus inhibits the downstream effects from both IL-4 and IL-13 [75]. Dupilumab is currently approved for the treatment of severe atopic dermatitis [76].
In a multinational, randomized, double-blind trial, dupilumab was assessed in 60 patients with CRSwNP refractory to intranasal corticosteroids [77]. Patients received subcutaneous dupilumab (initial 600 mg dose followed by fifteen 300 mg weekly doses; n = 30) or placebo (n = 30); mometasone furoate nasal spray was continued for the treatment period. The change in nasal polyp severity scores, the primary study outcome, was significantly improved in the dupilumab-treated group. Multiple secondary outcomes also showed benefit for dupilumab treatment, including improvement in CT disease severity score, peak nasal inspiratory flow, SNOT-22 scores, olfactory testing, symptom scores, and total serum IgE. Blood eosinophil count showed no difference between groups. The most common adverse events reported were nasopharyngitis, reactions at the injection site, and headache [77]. Additional studies of dupilumab for CRS were recently described at a national European meeting in 2016 but have not yet been published [78]. Furthermore, two phase III trials evaluating the use of dupilumab in the treatment of CRSwNP are ongoing [79, 80]. Other IL-13 inhibitors such as tralokinumab, lebrikizumab, and anrukinzumab have been described as potential targets for asthma but have not been evaluated for CRS [81].
4 Conclusions
Pharmacologic treatment is the foundation of management for CRS. Nasal saline irrigations, topical nasal steroids, certain antibiotics, and systemic steroids have shown some efficacy in the management of CRS. There is no meaningful evidence to support the use of antifungal therapy in the routine management of CRS. Nasal polyposis is a phenotypic subset of CRS that is often associated with specific inflammatory mediators, including cysteinyl leukotrienes, IL-4, IL-5, and IL-13. Antileukotriene therapy has shown mixed results, with modest benefit in some patients with CRSwNP. Recently, novel biologic therapeutics have been evaluated for the management of refractory CRSwNP with severe, poorly controlled disease. Further studies of these novel treatments are warranted, including identifying which subset of CRSwNP patients would most benefit from these therapies and assessing the cost of these treatments compared with the benefits provided.
References
Bhattacharyya N. Incremental health care utilization and expenditures for chronic rhinosinusitis in the United States. Ann Otol Rhinol Laryngol. 2011;120(7):423–7.
Orlandi RR, Kingdom TT, Hwang PH, Smith TL, Alt JA, Baroody FM, et al. International consensus statement on allergy and rhinology: rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(Suppl 1):S22–209.
Akdis CA, Bachert C, Cingi C, Dykewicz MS, Hellings PW, Naclerio RM, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013;131(6):1479–90.
Lam K, Kern RC, Luong A. Is there a future for biologics in the management of chronic rhinosinusitis? Int Forum Allergy Rhinol. 2016;6(9):935–42.
Amini-Vaughan ZJ, Martinez-Moczygemba M, Huston DP. Therapeutic strategies for harnessing human eosinophils in allergic inflammation, hypereosinophilic disorders, and cancer. Curr Allergy Asthma Rep. 2012;12(5):402–12.
Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122(5):961–8.
Oue S, Ramezanpour M, Paramasivan S, Miljkovic D, Cooksley CM, Bassiouni A, et al. Increased IL-13 expression is independently associated with neo-osteogenesis in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2017. doi:10.1016/j.jaci.2017.05.021
Palmqvist P, Lundberg P, Persson E, Johansson A, Lundgren I, Lie A, et al. Inhibition of hormone and cytokine-stimulated osteoclastogenesis and bone resorption by interleukin-4 and interleukin-13 is associated with increased osteoprotegerin and decreased RANKL and RANK in a STAT6-dependent pathway. J Biol Chem. 2006;281(5):2414–29.
Harvey R, Hannan SA, Badia L, Scadding G. Nasal saline irrigations for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev. 2007;18(3):CD006394. doi:10.1002/14651858.CD006394.pub2.
Rudmik L, Hoy M, Schlosser RJ, Harvey RJ, Welch KC, Lund V, et al. Topical therapies in the management of chronic rhinosinusitis: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2013;3(4):281–98.
Pynnonen MA, Mukerji SS, Kim HM, Adams ME, Terrell JE. Nasal saline for chronic sinonasal symptoms: a randomized controlled trial. Arch Otolaryngol Head Neck Surg. 2007;133(11):1115–20.
Liang J, Lane AP. Topical drug delivery for chronic rhinosinusitis. Curr Otorhinolaryngol Rep. 2013;1(1):51–60.
Lexicomp Online. Mometasone (nasal): drug information. https://www-uptodate-com.laneproxy.stanford.edu/contents/mometasone-oral-inhalation-drug-information?source=search_result&search=mometasone&selectedTitle=1~39. Accessed 9 Aug 2017.
Sastre J, Mosges R. Local and systemic safety of intranasal corticosteroids. J Investig Allergol Clin Immunol. 2012;22(1):1–12.
Chong LY, Head K, Hopkins C, Philpott C, Schilder AG, Burton MJ. Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016;4:CD011996.
Chong LY, Head K, Hopkins C, Philpott C, Burton MJ, Schilder AG. Different types of intranasal steroids for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016;4:CD011993. doi:10.1002/14651858.CD011993.pub2.
Head K, Chong LY, Hopkins C, Philpott C, Burton MJ, Schilder AG. Short-course oral steroids alone for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016;4:CD011991. doi:10.1002/14651858.CD011991.pub2.
Leung RM, Dinnie K, Smith TL. When do the risks of repeated courses of corticosteroids exceed the risks of surgery? Int Forum Allergy Rhinol. 2014;4(11):871–6.
Harvey RJ, Wallwork BD, Lund VJ. Anti-inflammatory effects of macrolides: applications in chronic rhinosinusitis. Immunol Allergy Clin North Am. 2009;29(4):689–703.
Head K, Chong LY, Piromchai P, Hopkins C, Philpott C, Schilder AG, et al. Systemic and topical antibiotics for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016;4:CD011994. doi:10.1002/14651858.CD011994.pub2.
Mosholder AD, Mathew J, Alexander JJ, Smith H, Nambiar S. Cardiovascular risks with azithromycin and other antibacterial drugs. N Engl J Med. 2013;368(18):1665–8.
Smith SS, Evans CT, Tan BK, Chandra RK, Smith SB, Kern R. National burden of antibiotic use for adult rhinosinusitis. J Allergy Clin Immunol. 2013;132(5):1230–2.
Desrosiers MY, Salas-Prato M. Treatment of chronic rhinosinusitis refractory to other treatments with topical antibiotic therapy delivered by means of a large-particle nebulizer: results of a controlled trial. Otolaryngol Head Neck Surg. 2001;125(3):265–9.
Wei JL, Sykes KJ, Johnson P, He J, Mayo MS. Safety and efficacy of once-daily nasal irrigation for the treatment of pediatric chronic rhinosinusitis. Laryngoscope. 2011;121(9):1989–2000.
Jervis-Bardy J, Boase S, Psaltis A, Foreman A, Wormald PJ. A randomized trial of mupirocin sinonasal rinses versus saline in surgically recalcitrant staphylococcal chronic rhinosinusitis. Laryngoscope. 2012;122(10):2148–53.
Lanza DC, Dhong HJ, Tantilipikorn P, Tanabodee J, Nadel DM, Kennedy DW. Fungus and chronic rhinosinusitis: from bench to clinical understanding. Ann Otol Rhinol Laryngol Suppl. 2006;196:27–34.
Sacks PL, Harvey RJ, Rimmer J, Gallagher RM, Sacks R. Topical and systemic antifungal therapy for the symptomatic treatment of chronic rhinosinusitis. Cochrane Database Syst Rev. 2011;8:CD008263. doi:10.1002/14651858.CD008263.pub2.
Holgate ST, Peters-Golden M, Panettieri RA, Henderson WR Jr. Roles of cysteinyl leukotrienes in airway inflammation, smooth muscle function, and remodeling. J Allergy Clin Immunol. 2003;111(1 Suppl):S18–34. doi:10.1067/mai.2003.25.
Smith TL, Sautter NB. Is montelukast indicated for treatment of chronic rhinosinusitis with polyposis? Laryngoscope. 2014;124(8):1735–6.
Garcia-Marcos L, Schuster A, Perez-Yarza EG. Benefit-risk assessment of antileukotrienes in the management of asthma. Drug Saf. 2003;26(7):483–518.
Wentzel JL, Soler ZM, DeYoung K, Nguyen SA, Lohia S, Schlosser RJ. Leukotriene antagonists in nasal polyposis: a meta-analysis and systematic review. Am J Rhinol Allergy. 2013;27(6):482–9.
Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50(1):1–12.
Dahlen B, Nizankowska E, Szczeklik A, Zetterstrom O, Bochenek G, Kumlin M, et al. Benefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1187–94.
Hopkins C, Andrews P, Holy CE. Does time to endoscopic sinus surgery impact outcomes in chronic rhinosinusitis? Retrospective analysis using the UK clinical practice research data. Rhinology. 2015;53(1):18–24.
Hopkins C, Rimmer J, Lund VJ. Does time to endoscopic sinus surgery impact outcomes in Chronic Rhinosinusitis? Prospective findings from the National Comparative Audit of Surgery for Nasal Polyposis and Chronic Rhinosinusitis. Rhinology. 2015;53(1):10–7.
Benninger MS, Sindwani R, Holy CE, Hopkins C. Early versus delayed endoscopic sinus surgery in patients with chronic rhinosinusitis: impact on health care utilization. Otolaryngol Head Neck Surg. 2015;152(3):546–52.
Smith KA, Rudmik L. Impact of continued medical therapy in patients with refractory chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4(1):34–8.
Patel ZM, Thamboo A, Rudmik L, Nayak JV, Smith TL, Hwang PH. Surgical therapy vs continued medical therapy for medically refractory chronic rhinosinusitis: a systematic review and meta-analysis. Int Forum Allergy Rhinol. 2017;7(2):119–27.
Chin D, Harvey RJ. Nasal polyposis: an inflammatory condition requiring effective anti-inflammatory treatment. Curr Opin Otolaryngol Head Neck Surg. 2013;21(1):23–30.
Rudmik L, Smith TL. Evidence-based practice: postoperative care in endoscopic sinus surgery. Otolaryngol Clin North Am. 2012;45(5):1019–32.
Rudmik L, Soler ZM, Orlandi RR, Stewart MG, Bhattacharyya N, Kennedy DW, et al. Early postoperative care following endoscopic sinus surgery: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2011;1(6):417–30.
Mendelsohn D, Jeremic G, Wright ED, Rotenberg BW. Revision rates after endoscopic sinus surgery: a recurrence analysis. Ann Otol Rhinol Laryngol. 2011;120(3):162–6.
Fajt ML, Wenzel SE. Biologic therapy in asthma: entering the new age of personalized medicine. J Asthma. 2014;51(7):669–76.
Bachert C, Holtappels G. Pathophysiology of chronic rhinosinusitis, pharmaceutical therapy options. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2015;14:Doc09.
Bachert C, Zhang N. Chronic rhinosinusitis and asthma: novel understanding of the role of IgE ‘above atopy’. J Intern Med. 2012;272(2):133–43.
Strunk RC, Bloomberg GR. Omalizumab for asthma. N Engl J Med. 2006;354(25):2689–95.
Presta LG, Lahr SJ, Shields RL, Porter JP, Gorman CM, Fendly BM, et al. Humanization of an antibody directed against IgE. J Immunol. 1993;151(5):2623–32.
Casale TB, Condemi J, LaForce C, Nayak A, Rowe M, Watrous M, et al. Effect of omalizumab on symptoms of seasonal allergic rhinitis: a randomized controlled trial. JAMA. 2001;286(23):2956–67.
Chervinsky P, Casale T, Townley R, Tripathy I, Hedgecock S, Fowler-Taylor A, et al. Omalizumab, an anti-IgE antibody, in the treatment of adults and adolescents with perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2003;91(2):160–7.
Grundmann SA, Hemfort PB, Luger TA, Brehler R. Anti-IgE (omalizumab): a new therapeutic approach for chronic rhinosinusitis. J Allergy Clin Immunol. 2008;121(1):257–8.
Mendez R. Omalizumab induces resolution of fungal sinusitis in a 15 year old asthmatic patient. J Allergy Clin Immunol. 2006;117(2 Suppl):S8.
Penn R, Mikula S. The role of anti-IgE immunoglobulin therapy in nasal polyposis: a pilot study. Am J Rhinol. 2007;21(4):428–32.
Pinto JM, Mehta N, DiTineo M, Wang J, Baroody FM, Naclerio RM. A randomized, double-blind, placebo-controlled trial of anti-IgE for chronic rhinosinusitis. Rhinology. 2010;48(3):318–24.
Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013;131(1):110–116e1.
Subcutaneous Omalizumab for Treatment of Chronic Rhinosinusitis With Nasal Polyposis. ClinicalTrials.gov Identifier NCT01066104. https://clinicaltrials.gov/ct2/show/results/NCT01066104?term=omalizumab+nasal&rank=2§=X7016. Accessed 20 May 2017.
Chandra RK, Clavenna M, Samuelson M, Tanner SB, Turner JH. Impact of omalizumab therapy on medication requirements for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(5):472–7.
Arm JP, Bottoli I, Skerjanec A, Floch D, Groenewegen A, Maahs S, et al. Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clin Exp Allergy. 2014;44(11):1371–85.
Gauvreau GM, Arm JP, Boulet LP, Leigh R, Cockcroft DW, Davis BE, et al. Efficacy and safety of multiple doses of QGE031 (ligelizumab) versus omalizumab and placebo in inhibiting allergen-induced early asthmatic responses. J Allergy Clin Immunol. 2016;138(4):1051–9.
Bachert C, Zhang L, Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: focus on nasal polyposis. J Allergy Clin Immunol. 2015;136(6):1431–40 (quiz 41).
Cao PP, Li HB, Wang BF, Wang SB, You XJ, Cui YH et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009;124(3):478–84, 484.e1–2.
Hamilos DL, Leung DY, Wood R, Cunningham L, Bean DK, Yasruel Z, et al. Evidence for distinct cytokine expression in allergic versus nonallergic chronic sinusitis. J Allergy Clin Immunol. 1995;96(4):537–44.
Simon H, Yousefi S, Schranz C, Schapowal A, Bachert C, Blaser K. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J Immunol. 1997;158(8):3902–8.
Lexicomp Online. Reslizumab: drug information. https://www-uptodate-com.laneproxy.stanford.edu/contents/reslizumab-drug-information?source=search_result&search=reslizumab&selectedTitle=1~15. Accessed 22 Aug 2017.
Gevaert P, Lang-Loidolt D, Lackner A, Stammberger H, Staudinger H, Van Zele T, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006;118(5):1133–41.
Effect of Reslizumab in Chronic Rhinosinusitis. Clinical Trials.gov Identifier NCT02799446. 2017. https://clinicaltrials.gov/ct2/show/study/NCT02799446?term=reslizumab+nasal&rank=1. Accessed 22 Aug 2017
Lexicomp Online. Mepolizumab: drug information. https://www-uptodate-com.laneproxy.stanford.edu/contents/mepolizumab-drug-information?source=search_result&search=Mepolizumab&selectedTitle=1~27. Accessed 22 Aug 2017.
Gevaert P, Van Bruaene N, Cattaert T, Van Steen K, Van Zele T, Acke F et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128(5):989–95.e1–8.
Mepolizumab in Nasal Polyposis. Clinical Trials.gov Identifier NCT01362244. 2016. https://clinicaltrials.gov/ct2/show/study/NCT01362244?term=mepolizumab+nasal+polyps&rank=2§=X10256. Accessed 20 May 2017.
Effect of Mepolizumab in Severe Bilateral Nasal Polyps. ClinicalTrials.gov Identifier NCT03085797. https://clinicaltrials.gov/ct2/show/record/NCT03085797. Accessed 9 Aug 2017.
Kolbeck R, Kozhich A, Koike M, Peng L, Andersson CK, Damschroder MM et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125(6):1344–53.e2.
Molfino NA, Gossage D, Kolbeck R, Parker JM, Geba GP. Molecular and clinical rationale for therapeutic targeting of interleukin-5 and its receptor. Clin Exp Allergy. 2012;42(5):712–37.
Giannetti MP, Cardet JC. Interleukin-5 antagonists usher in a new generation of asthma therapy. Curr Allergy Asthma Rep. 2016;16(11):80.
Study of Benralizumab (KHK4563) in Patients With Eosinophilic Chronic Rhinosinusitis. ClinicalTrials.gov Identifier: NCT02772419. https://clinicaltrials.gov/ct2/show/study/NCT02772419. Accessed 9 Aug 2017.
Vatrella A, Fabozzi I, Calabrese C, Maselli R, Pelaia G. Dupilumab: a novel treatment for asthma. J Asthma Allergy. 2014;7:123–30.
Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455–66.
Lexicomp Online. Dupilumab: drug information. https://www-uptodate-com.laneproxy.stanford.edu/contents/dupilumab-drug-information?source=search_result&search=Dupilumab&selectedTitle=1~15. Accessed 22 Aug 2017.
Bachert C, Mannent L, Naclerio RM, Mullol J, Ferguson BJ, Gevaert P, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016;315(5):469–79.
Rhinologic disorders: a health priority for the future. 26th Congress of the European Rhinologic Society; 3–7 July 2016; Stockholm.
A Controlled Clinical Study of Dupilumab in Patients With Nasal Polyps (SINUS-24). ClinicalTrials.gov Identifier: NCT02912468. https://clinicaltrials.gov/ct2/show/NCT02912468. Accessed 9 Aug 2017.
Controlled Clinical Study of Dupilumab in Patients With Nasal Polyps (SINUS-52). ClinicalTrials.gov Identifier: NCT02898454. https://clinicaltrials.gov/ct2/show/record/NCT02898454. Accessed 9 Aug 2017.
Bagnasco D, Ferrando M, Varricchi G, Passalacqua G, Canonica GW. A critical evaluation of anti-IL-13 and anti-IL-4 strategies in severe asthma. Int Arch Allergy Immunol. 2016;170(2):122–31.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were directly used for this project.
Conflict of interest
Timothy L. Smith was previously a consultant for Intersect ENT. This relationship ended in 2015. He is supported by a grant for this investigation from the National Institute on Deafness and Other Communication Disorders (NIDCD), one of the National Institutes of Health, Bethesda, MD, USA (R01 DC005805; PI/PD: TL Smith). Public clinical trial registration (http://www.clinicaltrials.gov) ID: NCT01332136. This funding organization did not contribute to the design or conduct of this study; collection, management, analysis, or interpretation of the data; and preparation, review, approval or decision to submit this manuscript for publication.
Rights and permissions
About this article
Cite this article
Beswick, D.M., Gray, S.T. & Smith, T.L. Pharmacological Management of Chronic Rhinosinusitis: Current and Evolving Treatments. Drugs 77, 1713–1721 (2017). https://doi.org/10.1007/s40265-017-0803-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-017-0803-4