Abstract
Cushing’s syndrome (CS) results from chronic exposure to cortisol excess, produced by the adrenal cortex. Hypercortisolism predisposes to psychiatric and neurocognitive disorders, mainly to depression and anxiety disorders. Screening tools to identify psychiatric symptoms are available for clinicians in their daily practice, although a specific diagnosis should be performed by specialists. Even if psychiatric symptoms improve after remission of hypercortisolism, complete recovery may not be achieved. Given the burden of these symptoms, psychiatric or psychological monitoring and treatment should be offered through all phases of CS, with a multidisciplinary approach. The aim of this article is to review data on the prevalence, diagnosis and management of psychiatric symptoms seen in patients with CS and to propose therapeutic approaches that may be followed in clinical practice. The prevalence of different psychiatric disorders has been described in both the active phase and after CS remission. Patients may not talk spontaneously about psychiatric symptoms they present, thus clinicians should ask directly about them. We recommend the use of screening tools in clinical practice to detect and treat these symptoms promptly. Even if reference endocrinologists cannot perform a definite psychiatric diagnosis, it will be important to ask patients directly about the presence of symptoms and refer if necessary to a psychiatrist. Additionally, patient information and educational programmes could be useful to manage psychiatric symptoms and to improve quality of life in patients with CS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The hypothalamic–pituitary–adrenal (HPA) axis plays an essential role in stress responses and stress-related psychiatric disorders. Repetitive exposure to uncontrollable stressors has been associated with changes in HPA axis function in both preclinical and clinical studies [1, 2] and is known to be a risk factor for the precipitation of psychiatric disorders in vulnerable individuals [3].
The central nervous system is rich in glucocorticoid receptors, especially the hippocampus, which is critical for learning and memory and therefore particularly vulnerable to glucocorticoid excess [4]. Chronic hypercortisolism during stress response leads to a cascade of processes in the brain, which determine region-specific alterations in dendrite and spine morphology and a reduction in neurogenesis [5, 6]. Literature demonstrating smaller hippocampal volumes in patients with affective disorders compared with control patients is quite vast [7, 8]. In fact, patients with depression frequently have a disturbed HPA-axis regulation [9].
Cushing’s syndrome (CS) is the result of a chronic exposure to an excess of cortisol produced by the adrenal cortex. Cushing’s syndrome is probably a good human model to evaluate the effects of cortisol excess on the brain. The same endocrine conditions that are elicited by both chronic stress and CS may induce brain atrophy and hippocampus volume reductions [10] and facilitate the development of neuropsychiatric disorders, especially in individuals who carry a genetic risk [8, 11].
Cushing’s syndrome can be caused by a pituitary adenoma-secreting adrenocorticotropic hormone (ACTH) [also known as Cushing’s disease], adrenocortical tumours secreting cortisol, or by bilateral adrenal hyperplasia or dysplasia. Less frequently it is caused by an extrapituitary tumour (ectopic ACTH-secretion syndrome), or very rarely by a tumour secreting corticotropin-releasing hormone (ectopic corticotropin-releasing hormone-secretion syndrome). Available treatments for CS include surgery, radiotherapy or medical therapy [17]. Patients with CS experience multiple co-morbidities, including cardiovascular (hypertension, atherosclerosis, changes in heart functionality), metabolic (dyslipidemia, central obesity, diabetes mellitus), as well as thrombotic disorders, bone disorders, and cognitive and neuropsychological impairment related to cortisol excess [6, 12–17], some of which can still be present after remission of CS.
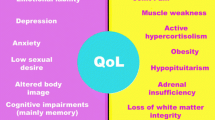
Indeed, patients with CS demonstrate deficits in memory, not totally reversible after biochemical cure, and a wide range of cognitive impairments and mood disorders [18, 19]. Hypercortisolism predisposes to psychopathology, mainly to depression and anxiety symptoms [20]. In fact, most patients with CS have depression or emotional lability [21], especially if they are older, female and have severe hypercortisolism [21, 22]. Anxiety and maladaptative personality traits have also been reported in patients in remission from CS in some studies [23–25]. Finally, endogenous hypercortisolism has been suggested to determine premature ageing because cognitive performance similar to control patients aged 15 years older has been observed in patients with CS [26].
Health-related quality of life (HRQoL) in patients with CS is also severely affected, especially in active disease [27], and is still impaired after cure of hypercortisolism [28, 29]. Impaired HRQoL is the result of a multifactorial scenario, including physical and psychiatric symptoms, as well as the necessity to undergo repeated testing and check-ups and the need for long-term medication, despite long-term remission of CS.
Few data are available on the management of psychiatric symptoms in CS. The aim of this article is to review data on psychiatric symptoms in CS and to propose management approaches that could be followed in clinical practice.
2 Prevalence
Different explanations could be suggested to explain the high prevalence of neuropsychiatric disorders in patients with CS. Chronic hypercortisolism and HPA axis dysfunction in these patients can lead to structural and functional changes in the central nervous system, developing brain atrophy, particularly in the hippocampus, which may determine the high prevalence of psychiatric disorders, such as affective and anxiety disorders or cognitive dysfunctions [6–8]. Another explanation is that CS is a chronic disease that causes serious dysfunction and impairs HRQoL. Consequent difficulties in the process of adaptation to this situation frequently cause the development of depressive and anxiety symptoms.
In this sense, it is important to note that some studies on the prevalence of neuropsychiatric symptoms in subjects with CS only detected the presence or absence of symptoms rather than providing a complete major diagnosis through structured interviews to that effect. The presence of depressive symptoms does not always indicate the diagnosis of a major depressive disorder. For instance, a poor quality of life and serious physical symptoms of CS could cause sadness and anxiety but not lead to a complete affective disorder. Therefore, in this review, we differentiate those studies that evaluate psychiatric symptoms from those evaluating well-defined psychiatric disorders.
As prevalence rates may differ between patients with active disease and patients in remission of CS, this section is split into two subsections, according to the two stages of the disease. Table 1 summarises the different findings on psychiatric disorders in CS, evaluated with psychiatric criteria and highlighting the diagnostic instruments used.
2.1 Active Disease
Although CS has repeatedly been related to psychopathology in the scientific literature, not many articles have addressed the specific issue of prevalence of the different psychiatric symptoms associated with the disease, except for depression. Depression is the most commonly described psychiatric co-morbidity in CS [15, 20, 30–32]. According to some authors, major depressive disorder can be considered an early manifestation of CS, being present in up to 25% of the patients at the onset of the disease or even immediately before [31, 33–37].
However, not all studies include a standardised assessment of major depressive disorder by specific diagnostic criteria. Regarding specific symptoms related to depression, Starkman et al. in the beginning phase of a longitudinal study of 35 patients found that in up to 17% of patients, suicidal thoughts could be encountered and a minority (5.7%) presented with suicide attempts. Patients also complained of hopelessness (43%) and crying (63%) [21]. Depression during the active phase of the disease has been associated with older age, female sex, a more severe clinical condition, absence of detectable pituitary adenoma, higher pretreatment urinary cortisol levels and past adverse life events [15, 31, 38]. It is also a predictive factor of unsuccessful pituitary surgery and future relapse [39]. No differences have been found in depression between pituitary-dependent and pituitary-independent forms of CS [37], suggesting that hypercortisolism itself is the cause. Regarding studies including specific diagnostic criteria for major depressive disorder, a literature overview indicates that major depressive disorder occurs in 50–80% of patients with active disease, with a higher prevalence in older studies [15, 20, 30, 37, 40, 41]. More details on these studies can be found in Table 1.
Anxiety is also a common feature in patients with active disease. Higher anxiety scores have been reported when comparing patients with CS and control patients [32, 42]. Two studies report the percentage of patients presenting with anxiety without using specific diagnosis criteria, with varying prevalences. The first one establishes that it was present in up to 66% of the patients [21], while the other described “pathological anxiety” in 12% [31]. A few studies have reported the presence of anxiety disorders using specific diagnostic criteria, finding that generalised anxiety disorder was present in 79% of patients [36] and panic disorder in 3–37% of patients, being associated with a more chronic stage of the disease [30, 36, 41].
Cushing’s syndrome can also be associated with other psychiatric disorders. Mania or hypomania has been reported in 3–30% of patients [31, 40, 41]. Psychotic disorders have been diagnosed in 8% of patients, being more common in adrenal carcinomas (40% of patients), where the degree of hypercortisolism can be extreme [31].
Finally, CS can be associated with other psychiatric symptoms that, even if they do not meet the criteria for a psychiatric disorder, may deserve consideration owing to the effect that they have on patients’ lives. Reduced libido has been found in up to 50–69% in three studies (first study 35 patients, prospective; second study 209 patients, cross-sectional; third study 481 patients, prospective) [21, 31, 43] and irritability in up to 86% of the patients [21]. The latter seemed to be the earliest symptom to show up in the course of the disease [21]. Many patients present with middle insomnia (69%), late insomnia (57%) or early insomnia (29%); and emotional lability is present in 17% of the patients [21]. Overall, psychiatric morbidity seems to be related to a greater degree of hypercortisolism, with higher ACTH and/or cortisol levels [21].
2.2 Patients in Remission
After cortisol normalisation leading to remission of CS, improvements of previous psychiatric alterations may occur to some extent gradually over time, although there is controversy as to complete recovery. A longitudinal study of 33 patients reported that psychopathology rates measured with the Symptom Checklist-90 questionnaire decreased from 67% at baseline (active disease) to 54% at 3 months after remission of CS by surgery, 36% at 6 months after surgery and 24% at 12 months after surgery [44]. It is important to note that after remission of CS (3, 6 and 12 months follow-up), 18.2% of the patients were frequently visiting a therapist, 21% were taking antidepressants or anxiolytics, and 6.1% had psychiatric hospitalisations. Interestingly, in the same study, 13.7% of the patients, who had never had a prior psychiatric diagnosis, exhibited a disorder after correction of hypercortisolism, indicating that even after cure new psychopathology may emerge.
Regarding depression, a cross-sectional study including 66 patients found that up to 70% of the patients presenting with depression recovered after endocrine cure [37]. Several studies indicate improvement of depression after treatment [44–48]. However, some patients do not fully recover. Even mild depressive symptoms may have an important impact on the quality of life in patients in remission of CS [49], by interfering with daily tasks, healthy habits and following a doctor’s instructions, which affects the recovery process, as found in patients with other diseases [50, 51].
Several studies not using diagnostic criteria report that patients in remission of CS have higher depression scores than control patients when measured with the Beck Depression Scale II, Comprehensive Psychopathological Rating Scale, Symptom Rating Test, Hospital Anxiety and Depression Scale (HADS), Montgomery-Åsberg Depression Rating Scale or Inventory of Depression Symptomatology [23, 24, 32, 49, 52, 53].
One study found that mean depression scores in the HADS subscale (5.6 ± 4.5) did not indicate mood alterations, as they were below the cut-off score for clinical depression (HADS >8); however, 26% of the patients had scores >8, indicating possible clinical psychopathology [24]. Therefore, it is important to consider that depressive symptoms may still be present in patients with CS, even after cure.
Dorn et al. assessed major depressive disorder rates using diagnostic criteria in patients in remission. They found a gradual reduction in major depressive disorder over time after disease remission, although at 12 months post-treatment 6.9% of the patients still met criteria for major depressive disorder and 17.2% for atypical depression (and 3.5% had suicidal ideation) [44].
Anxiety disorders also tend to improve over time [44–46]. However, studies using questionnaires have shown that patients in remission of CS still have higher anxiety scores than control patients measured by the State-Trait Anxiety Inventory Questionnaire (both current State and Trait scores), Comprehensive Psychopathological Rating Scale, Symptom Rating Test, HADS or Beck Anxiety Inventory [23, 24, 32, 49, 52, 53]. Similar to findings for depression, in patients in long-term remission of CS, mean anxiety scores in the HADS anxiety subscale (6.2 ± 4.2) were below the cut-off score (HADS >8), suggesting no anxiety alterations; however, 20% of the patients had scores >8, indicating possible pathological anxiety [24]. These results indicate that even if anxiety disorders improve after disease remission, subclinical anxiety may remain.
Regarding research dealing with specific diagnostic criteria, Dorn et al. observed that 3 months after treatment (which consisted of surgery for 85% of the patients, and irradiation or medical therapy in 15%), 7.1% of the patients met criteria for anxiety disorders, while no patient presented with anxiety disorders after 6 or 12 months [44]. However, even if no specific anxiety disorders may be present after disease remission, subclinical symptoms should not be neglected.
Regarding personality, little is known about the correlation between CS and personality disturbances. There have been no studies of the prevalence of any specific personality disorder evaluated with structured interviews and by Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria in CS samples. Nevertheless, some studies have described maladaptative or anxiety-related personality traits from a dimensional perspective, when comparing patients with CS with control patients or individuals with non-functioning pituitary adenomas [24, 25], while others have not [23]. Dimopoulou et al. found that patients with CS presented with higher neuroticism scores and harm avoidance but reduced scores in externalising traits (novelty-seeking behaviours or extraversion) than control patients [25]. Interestingly, another study found that neuroticism scores seem to decrease after treatment [46].
Several possible explanations for the association between CS and personality disturbances could be suggested. (1) Chronic cortisol excess might affect the central nervous system, especially decreasing hippocampal volume, affecting the modulation of personality [24, 25], in line with results of Martin et al., who found a correlation between hippocampal volume and novelty seeking and extraversion in healthy subjects [54]. (2) These personality traits could be maladaptative consequences of the presence of a chronic illness with poor HRQoL. (3) The presence of other associated psychiatric symptoms such as depression or anxiety might have influenced the self-reported personality dimensions’ assessments. (4) These findings could be the result of pre-existing personality traits prior to CS. In this sense, a link between genetic vulnerability of the HPA axis and borderline personality disorder symptoms has recently been suggested [55].
Finally, other symptoms have been reported in patients in remission of CS, although no specific diagnostic criteria were used to evaluate them. In a cross-sectional study of 25 patients in long-term remission of Cushing’s disease, higher scores in social phobia and apathy than in control patients (measured with the Fear Questionnaire and the Apathy Scale) have been reported [53]. Regarding libido, in a sample of 61 patients including patients with active disease and in remission (74% in remission), 50% may have a hypoactive sexual desire disorder as measured with the Female Sexual Function Questionnaire and the Female Sexual Functioning Inventory [56]. Even if the exact percentage was not specified, patients also reported insomnia and hypersomnia, and up to 61% reported loss of emotional stability [56, 57].
Some clinical correlates of affective symptoms have been reported in patients in remission of CS [49]. Low brain-derived neurotrophic factor and salivary cortisone levels have been related to high degrees of anxiety and depression, while delay to diagnosis has positively been related to depressive symptoms [49]. Concomitant hypopituitarism is another predisposing factor for depression and anxiety symptoms [58].
3 Diagnosis
Psychiatric diagnosis is not easy to establish, and should be performed by specialists. Therefore, in many cases when psychopathology is suspected, it will be essential that the patients are evaluated by trained mental health professionals with experience in clinical evaluation. It is important to note that some symptoms (for instance, feelings of sadness) may not necessarily imply a clinical diagnosis (for instance, major depressive disorder), and a proper diagnosis is essential to choose the most suitable treatment. Table 2 shows a summary of the main symptoms for some of the most common psychiatric diagnoses that can be found in CS (according to DSM-5) [59]. It is important to mention that when most of the studies on psychiatric symptoms in CS were performed DSM-5 [59] was not available yet. Therefore, previous versions available at that time were used. However, it is important to note that future studies and clinical evaluations assessing diagnostic criteria should use the most recent version available. The International Statistical Classification of Diseases and Related Health Problems is also a standardised classification system widely used, and its last version available at the moment is the tenth revision. The criteria for diagnosing depressive episodes in DSM and the International Statistical Classification of Diseases and Related Health Problems overlap considerably.
Different instruments can be used for the evaluation of psychiatric symptoms and disorders. Clinical interviews are useful tools to establish diagnosis and evaluate outcome after treatment, although some training may be necessary to use them. Examples are the International Neuropsychiatric Interview or the Structured Clinical Interview for DSM-5.
Clinicians can also use screening tools (mainly self-reported questionnaires) in their clinical practice, to identify possible psychiatric symptoms. These instruments can provide valuable information to decide if a more complete psychiatric evaluation is necessary. Table 3 shows a list of possible screening instruments that may be used in daily practice to evaluate anxiety and depression, which are the most common symptoms in CS.
Even if some clinicians (for instance, reference endocrinologists) cannot perform a definite psychiatric diagnosis, it will be important that they ask patients directly for the presence of symptoms described in the former section. It will help to have a more complete picture of the patient’s symptoms, and most importantly, will also help them feel understood, able to ask questions and more comfortable with the therapeutic relationship.
4 Management
Management of psychiatric symptoms is a major concern in CS. The literature has not shown significant differences between pituitary-dependent and pituitary-independent forms of CS in relation to psychopathology [31, 60]. The severity of the disease or higher levels of cortisol (in active disease) seem to be more important for psychiatric symptoms than the aetiology or treatment of the disease. In fact, improvements in depression, anxiety and other affective alterations have been described after reducing cortisol levels with medical treatment (ketoconazole, metyrapone and mifepristone), radiotherapy and/or surgery [60], including bilateral adrenalectomy [61].
Some authors report that in the different phases of CS, affective disorders may have a negative role on quality of life and social functioning, also increasing healthcare utilisation and reducing compliance. In fact, they are even associated with higher mortality [33, 62]. Quality of life is an important outcome in the follow-up of patients with CS who do not recover completely after normalisation of cortisol levels [63]. There are multiple factors, including psychiatric symptoms that can affect quality of life of patients with CS. Indeed, a European multicentre study suggests that depression plays a pivotal role in affecting quality of life [43]. Therefore, the regular monitoring of psychiatric symptoms is necessary to maintain a good quality of life in these patients.
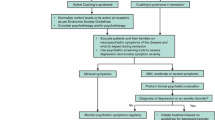
Moreover, health professionals cannot forget that CS is a chronic disease. Therefore, it demands continued evaluation or screening for symptoms and complications typically present in these patients, including psychiatric symptoms. It should be highlighted that psychiatric disorders, although they generally improve, may persist or even worsen after long-term remission of CS, contributing to the persistent impairment of quality of life [63]. Long-term follow-up of psychiatric symptoms should always be considered in the management of CS both in the active phase and after disease remission. Figure 1 shows a proposed algorithm to manage anxiety and depression symptoms in CS. For a better understanding, this section is divided according to different disease status.
4.1 Active Disease
As reported in the previous sections, in most cases, psychopathology improves after cortisol normalisation in CS. Therefore, the first recommendation would be a prompt treatment of hypercortisolism [64]. As recommended by the guidelines of the Endocrine Society, the first-line treatment would be resection of the lesion underlying CS. When surgery is not possible or not effective, second-line therapies include a second surgery, radiotherapy, medical therapy and bilateral adrenalectomy [65].
If surgery is delayed, or not possible, glucocorticoid-lowering drugs can also lead to an improvement in psychiatric symptoms, especially depression [48, 66]. This is especially important in patients with a more severe CS [48].
During this phase, proper patient information will be essential to help patients feel they have some control over the disease. Patient education programmes can also be helpful [56]. Because cortisol normalisation may take a long time, support psychotherapy and psychoeducation may be helpful.
Psychotropic drug treatment may also be recommended in some cases, especially for moderate or severe symptoms or in cases with a past history of major depressive disorder prior to CS. However, it would be desirable first to normalise cortisol levels if possible (achieved by surgical or medical treatment) before prescribing an antidepressant. It is important to note that patients may not respond properly to antidepressants until cortisol normalises [20, 62, 64].
If antidepressive treatment is required, it is important to note that according to some authors, selective serotonin reuptake inhibitors (SSRI) can have a partial effect on depression during active disease, while tricyclic antidepressants have very little effect [64]. However, corticosteroid synthesis inhibitors (as ketoconazole, metyrapone or aminoglutethimide) may be more effective in improving depressive symptoms during active hypercortisolism [20, 62].
Some authors report using in their clinical experience low-dose clonazepam for treating anxiety [20]. Even if unusual, psychosis in CS can be difficult to treat, and in most cases, there will be little or no response to antipsychotic medication when CS is active [67]. Case reports indicate that symptoms may resolve some weeks after surgical resection of the tumour causing CS [68–70]. However, little information is available on treatment recommendations for acute crisis. Information from case reports can be found in Table 4. One case study describing a pituitary patient with an acute paranoid syndrome indicated that clozapine (an atypical antipsychotic) was found to be very effective for psychotic symptoms (while cortisol-lowering drugs, haloperidol or fluphenazine had not been effective) [71]. Other authors indicate that despite some controversy because of its use in inducing abortion, mifepristone can be useful in acute psychotic crises in CS because it rapidly blocks the cortisol receptor [72, 73]. Case reports indicate that cortisol-induced psychosis improved after using this drug in two patients with adrenal carcinomas (within 24 h) [74] and also in a patient with a pituitary macroadenoma who had an incomplete tumour resection [75]. Another case report described improvements in psychotic symptoms combining both mifepristone and etomidate (an anaesthetic only available as an intravenous drug) in a patient with an ectopic ACTH-producing lung carcinoma [76]. Even if further evidence is necessary to confirm these indications, clozapine and mifepristone (alone and/or combined with etomidate) are possible alternatives for severe psychosis in patients with active disease. For CS with severe psychotic symptoms or a psychotic depression, another option would be to consider using symptomatic antipsychotic medication, for example, risperidone, aripiprazole, olanzapine or quetiapine (although in some patients with active disease they may not be effective). When using these drugs, it is recommended to monitor weight, lipid and glucose levels, and side effects such as extrapyramidal or prolactin-related side effects.
When a rapid control of cortisol secretion is required, mifepristone can be useful for severe psychiatric symptoms in patients with CS, especially if they are refractory to other therapies. Its use in CS has mostly been explored in case reports and/or small retrospective studies [73–75, 77–80]. It is important to note that hypokalemia is a common side effect [73–75, 77–80] and endometrium thickening with cystically dilated endometrial glands is another possible co-morbidity [81]. Therefore, the long-term use of this drug is controversial. There is only one open-label, prospective, multicentre 6-month study conducted with mifepristone in patients with endogenous CS refractory to other therapies [82], and the presence of psychiatric symptoms was an inclusion criterion. The authors concluded that even if depression improved at week 24, together with some clinical and metabolic benefits, the long-term efficacy and safety remain to be determined, particularly with regard to the need for potassium supplementation and/or mineralocorticoid receptor blockade and endometrial monitoring. Furthermore, glucocorticoid receptor blockade prevents cortisol levels being used to adapt the mifepristone dose, having to rely instead on indirect markers of disease activity such as severity of diabetes or hypertension.
4.2 Patients in Remission
In some cases, improvement can be dramatic after cortisol normalisation. In others, psychiatric symptoms may persist or even appear for the first time. A case report described a patient with long-standing depression and psychotic features who gradually recovered and even discontinued psychotropic medication after successful pituitary surgery [34]. However, improvement may not mean complete recovery, and further psychopathology can appear after endocrine cure. Several authors highlight the relevance of psychiatric or psychological monitoring and treatment in CS through all phases of the disease, which may be accomplished by a multidisciplinary approach [42, 60, 62, 67]. This is especially relevant when there is persistence or even worsening of the psychiatric symptoms [62]. In fact, the Endocrine Society guidelines on treatment for CS recommend that patients are monitored and treated for psychiatric disorders and symptoms until they resolve [65].
Once normalisation of hypercortisolism is achieved, reassessing the presence of psychiatric and neuropsychiatric symptoms is essential for a proper management. Patients may not talk spontaneously of some of the symptoms they present (such as reduced libido, irritability, insomnia and depression), thus clinicians should ask directly [64]. We recommend the use of screening tools in clinical practice to detect and treat these symptoms promptly, as well as screening for anxiety and depression in patients who present some signs. Some questionnaires such as the Beck Depression Inventory II or Beck Anxiety Inventory provide a range to establish if the symptoms are minimum, mild, moderate or severe. When mild, moderate or severe symptoms are found, a psychiatric evaluation is recommended, and if necessary, treatment considered. It is important to note that patients may under-estimate their symptoms. In our experience, some patients initially reporting not being anxious or sad did in fact present with psychiatric symptoms. Therefore, in case of doubt, the use screening tools to identify symptoms is recommended, and if necessary, a psychiatric evaluation should be performed.
Regarding therapeutic options for psychiatric symptoms in patients with CS in remission, recommendations could be very similar to those for patients without CS [83]. Psychotherapeutic interventions may be helpful [42]. In fact, one study reported having recommended psychotherapy in up to 36.4% of the patients [44]. Cognitive-behavioural therapies may be prescribed, as they usually work in affective disorders [20], although other psychotherapeutic techniques may also be helpful. Even for minor affective symptoms, psychoeducational programmes and physical activity or group therapeutic interventions may help. Additionally, positive psychotherapy or third-wave psychotherapies may be useful to help these patients to accept the consequences of this chronic disorder, with may determine impaired HRQoL.
When an antidepressant is required, the choice of treatment should be discussed with the patient including adverse events and potential interactions. The first option is usually SSRIs because they have a favourable risk-benefit and are equally effective as other antidepressants. Selective serotonin reuptake inhibitors are now the most commonly prescribed group of antidepressants [83] and all have a similar effect. Any SSRI could be recommended, for example, citalopram, escitalopram, fluoxetine, paroxetine, sertraline or venlafaxine at doses recommended by guidelines, which may be adjusted during follow-up [83]. If patients present with severe depression or do not respond adequately to antidepressant treatment, consider referring to specialist mental health services for collaborative care. Different alternatives may be suggested at this point such as switching to another antidepressant, combinations of antidepressants, combination of psychological and drug treatment, or a dose increase. If patients present with moderate-to-severe anxiety symptoms, short-term benzodiazepine treatment may be considered, although there is a risk of dependence.
Another consideration could be from a psychiatry perspective. Approximately one-third of patients treated for depression do not respond satisfactorily to first-line antidepressants and even up to 10% remain depressed after multiple treatments. For these cases of refractory depression, monitoring cortisol levels to rule out possible undiagnosed CS is worth remembering. Appropriate treatment for CS could also improve refractory depression symptoms. Even without CS, HPA-axis abnormalities and glucocorticoids influence multiple aspects of serotoninergic neurotransmission and have been postulated to be central to the development of depression. These data suggest a potential mechanism by which anti-glucocorticoid strategies can improve the effectiveness of antidepressants in clinical practice. A Cochrane review [84] demonstrated the efficacy of metyrapone in treatment-resistant depression. Nevertheless, there is currently insufficient evidence on the efficacy of glucocorticoid antagonists, either alone or to enhance antidepressants for the treatment of major depressive disorder.
Adrenal insufficiency, which often occurs after surgery for CS, should also be considered in the management of CS. Patients usually require substitution therapy with hydrocortisone. There is little information available on the impact of adrenal insufficiency on psychiatric symptoms in CS. One case report on acute psychosis while treating an acute adrenal crisis with hydrocortisone in a patient with secondary adrenal insufficiency after CS has been published [85]. The authors stated the risk of psychosis when treating an adrenal crisis with high doses of hydrocortisone. They concluded that when high doses of corticosteroids are prescribed, evaluation of risk factors for mental disturbances and the cooperation of endocrinologists and psychiatrists are recommended, to increase safety and identify early any psychiatric complications [85].
With regard to primary adrenal insufficiency (Addison’s disease) and psychiatric symptoms, neuropsychiatric manifestations are uncommon. They include depressive symptoms, irritability, sleep disturbances, apathy, cognitive impairment and hallucinations [86, 87]. They are rarely seen as the initial and isolated clinical manifestations of the disease, and they usually accompany the cardinal symptoms of adrenal insufficiency, being correlated with disease severity [87].
Depression is the most common symptom associated with adrenal insufficiency, with usually mild mood symptoms, a decrease in motivation and altered behaviour [88, 89]. Psychosis, delirium, catatonia and disorientation are less frequently seen [90, 91]. Psychosis has been observed in Addisonian crisis and severe manifestations of the disease; only a few case reports have described a psychotic manifestation during the initial presentation of adrenal insufficiency [92, 93]. The aetiology of psychiatric disorders in Addison’s disease is not well understood.
4.3 Considerations for Active Patients and Patients in Remission
There are other important issues to consider when dealing with psychiatric symptoms in CS. A cross-sectional study on the patient perspective including 62 patients revealed that 56% consider patient education helpful [57]. Giving information to the patients with CS (as booklets for instance) can help them feel less afraid and in more control of the disease. In a review of Starkman on neuropsychiatric findings in CS, it was stated that knowing that neuropsychiatric symptoms are common in CS, related to biochemical changes, and that they usually improve over time can be very relieving for patients and their families [64]. Regarding psychopathology, a study showed that most patients consider that they were not given proper information on psychological alterations related to CS [94]. Therefore, patient information and education is recommended during medical care. In fact, a prospective randomised study including 61 patients demonstrated that an educational programme for patients with CS was useful to improve impaired sleeping patterns, a healthy lifestyle and physical activity, and also led to a reduction in the use of health resources [56].
Family involvement is also relevant. Some authors report that both patients and their families should be informed on the possible persistence of psychopathology, even after biochemical cure, and that quality of life and psychological improvement may take a long time to occur (at least 6 months) [20, 44]. It is also important that families are informed on the consequences of the disease, as up to 80% of 62 patients with CS contacted by mail reported that the illness affected their families [57]. In fact, the Endocrine Society Guidelines recommend educating both patients with CS and their families on the clinical features of remission of CS [65].
Support groups can also be helpful for dealing with CS, as considered by 20% of 62 patients included in a cross-sectional study on the patient’s perspective [57]. There are currently many options, which can be face-to-face or online (via websites, facebook, whatsapp). If local groups are available, clinicians may facilitate contacts to the patients. Furthermore, social support can be very helpful to deal with the disease, as it may influence behaviours and lifestyles [62]. Maintaining usual activities and routines (even work, if possible) and having a healthy lifestyle can also positively influence psychiatric symptoms [20].
Finally, it is important for clinicians to consider the impact that CS may have on patients’ lives. In a study focusing on the patient’s perspective, 71% reported that CS had affected their lives greatly, and 20% a lot [57]. Just reaching the correct diagnosis involves consulting a mean of 4.6 doctors (ranging from 1 to 30 specialists) [95]. Apart from psychiatric disturbances, all the co-morbidities of the disease can lead to a low quality of life, even after biochemical cure [28, 29, 58]. A cross-sectional study including the feedback of 62 patients on the effects of CS on their lives showed that they may feel weak and impotent, even when they are in remission [57]. Therefore, clinicians should be aware of all these issues and note that just listening to patients experiences and being supportive can be very helpful for the patients.
In summary, the management of psychiatric co-morbidities will usually require a multidisciplinary approach. In active disease, the main recommendation is prompt treatment of hypercortisolism, which may lead to a rapid improvement of the symptoms. Patients may also benefit from having support from their friends, families and clinicians, participating in support groups, receiving education on the disease and its co-morbidities, and following a healthy lifestyle. Psychotherapy and/or medication may be recommended to some patients. After disease control, patients should be routinely monitored for psychiatric symptoms, considering psychological and/or medical treatment if necessary.
5 Conclusions
Cushing’s syndrome is associated with a series of psychiatric symptoms that should not be neglected. Depression is the most common and well-established feature, occurring in 50–80% of the cases, although other psychiatric symptoms are also often present. Some may still remain after successful treatment of CS or even worsen, having an important impact on patients’ quality of life. Therefore, long-term follow-up should be considered in the management of patients with CS, even after long-term remission of CS.
References
Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;50:200–4.
Carpenter L, Carvalho JP, Tyrka AR, et al. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62:1080–7.
Joëls M, Karst H, Krugers HJ, Lucassen PJ. Chronic stress: implications for neuronal morphology, function and neurogenesis. Front Neuroendocrinol. 2007;28:72–96.
Resmini E, Santos A, Gómez-Anson B, et al. Verbal and visual memory performance and hippocampal volumes, measured by 3-Tesla magnetic resonance imaging, in patients with Cushing’s syndrome. J Clin Endocrinol Metab. 2012;97:663–71.
Wang J, Barak LS, Mook RA Jr, Chen W. Glucocorticoid hedgehog agonists in neurogenesis. Vitam Horm. 2011;87:207–15.
Tata DA, Marciano VA, Anderson BJ. Synapse loss from chronically elevated glucocorticoids: relationship to neuropil volume and cell number in hippocampal area CA3. J Comp Neurol. 2006;498:363–74.
Gold PW, Drevets WC, Charney DS. New insights into the role of cortisol and the glucocorticoid receptor in severe depression. Biol Psychiatry. 2002;52:381–5.
de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–75.
Goodwin G. Neurobiological aetiology of mood disorders. In: Gelder MG, Lopez-Ibor JJ, Andreasen N, editors. New Oxford textbook of psychiatry. Oxford: Oxford University Press; 2000. p. 711–9.
Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–43.
Resmini E, Santos A, Aulinas A, et al. Reduced DNA methylation of FKBP5 in Cushing’s syndrome. Endocrine. 2016;54:768–77.
De Leo M, Pivonello R, Auriemma RS, et al. Cardiovascular disease in Cushing’s syndrome: heart versus vasculature. Neuroendocrinology. 2010;92:50–4.
Jacoby RC, Owings JT, Ortega T, et al. Biochemical basis for the hypercoagulable state seen in Cushing syndrome. Arch Surg. 2001;136:1003–6.
Kaltsas G, Makras P. Skeletal diseases in Cushing’s syndrome: osteoporosis versus arthropathy. Neuroendocrinology. 2010;92:60–4.
Sonino N, Fava GA. Psychosomatic aspects of Cushing’s disease. Psychother Psychosom. 1998;67:140–6.
Pereira AM, Tiemensma J, Romijn JA. Neuropsychiatric disorders in Cushing’s syndrome. Neuroendocrinology. 2010;92:65–70.
Bolland MJ, Holdaway IM, Berkeley JE, et al. Mortality and morbidity in Cushing’s syndrome in New Zealand. Clin Endocrinol (Oxf). 2011;75:436–42.
Santos A, Resmini E, Gómez-Ansón B, et al. Cardiovascular risk and white matter lesions after endocrine control of Cushing’s syndrome. Eur J Endocrinol. 2015;173:765–75.
Forget H, Lacroix A, Somma M, Cohen H. Cognitive decline in patients with Cushing’s syndrome. J Int Neuropsychol Soc. 2000;6:20–9.
Sonino N, Fava GA. Psychiatric disorders associated with Cushing’s syndrome: epidemiology, pathophysiology and treatment. CNS Drugs. 2001;15:361–73.
Starkman MN, Schteingart DE, Schork MA. Depressed mood and other psychiatric manifestations of Cushing’s syndrome: relationship to hormone levels. Psychosom Med. 1981;43:3–18.
Bourdeau I, Bard C, Forget H, et al. Cognitive function and cerebral asessment in patients who have Cushing’s syndrome. Endocrinol Metab Clin N Am. 2005;34:357–69.
Sonino N, Bonnini S, Fallo F, et al. Personality characteristics and quality of life in patients treated for Cushing’s syndrome. Clin Endocrinol (Oxf). 2006;64:314–8.
Tiemensma J, Biermasz NR, Middelkoop HA, et al. Increased prevalence of psychopathology and maladaptive personality traits, after long-term cure of Cushing’s disease. J Clin Endocrinol Metab. 2010;95:129–41.
Dimopoulou C, Ising M, Pfister H, et al. Increased prevalence of anxiety-associated personality traits in patients with Cushing’s disease: a cross-sectional study. Neuroendocrinology. 2013;97:139–45.
Michaud K, Forget H, Cohen H. Chronic glucocorticoid hypersecretion in Cushing’s syndrome exacerbates cognitive aging. Brain Cogn. 2009;71:1–8.
Webb SM, Badia X, Barahona MJ, et al. Evaluation of health-related quality of life in patients with Cushing’s syndrome with a new questionnaire. Eur J Endocrinol. 2008;158:623–30.
Lindsay JR, Nansel T, Baid S, et al. Long-term impaired quality of life in Cushing’s syndrome despite initial improvement after surgical remission. J Clin Endocrinol Metab. 2006;91:447–53.
Hawn MT, Cook D, Deveney C, Sheppard BC. Quality of life after laparoscopic bilateral adrenalectomy for Cushing’s disease. Surger. 2002;132:1064–8.
Dorn LD, Burgess ES, Dubbert B, et al. Psychopathology in patients with endogenous Cushing’s syndrome: atypical or melancholic features. Clin Endocrinol. 1995;43:433–42.
Kelly WF. Psychiatric aspects of Cushing’s syndrome. Q J Med. 1996;89:543–51.
Santos A, Resmini E, Crespo I, et al. Small cerebellar cortex volume in patients with active Cushing’s syndrome. Eur J Endocrinol. 2014;171:461–9.
Cosci F, Fava GA, Sonino N. Mood and anxiety disorders as early manifestations of medical illness: a systematic review. Psychother Psychosom. 2015;84:22–9.
Rasmussen SA, Rosebush PI, Smyth HS, Mazurek MF. Cushing disease presenting as primary psychiatric illness: a case report and literature review. J Psychiatr Pract. 2015;21:449–57.
Tang A, O’Sullivan AJ, Diamond T, et al. Psychiatric symptoms as a clinical presentation of Cushing’s syndrome. Ann Gen Psychiatry. 2013;12:23.
Loosen PT, Chambliss B, de Bold CR, et al. Psychiatric phenomenology in Cushing’s disease. Pharmacopsychiatry. 1992;25:192–8.
Sonino N, Fava GA, Belluardo P, et al. Course of depression in Cushing’s syndrome: response to treatment and comparison with Graves’ disease. Horm Res. 1993;39:202–6.
Sonino N, Fava GA, Raffi AR, et al. Clinical correlates of major depression in Cushing’s disease. Psychopathology. 1998;31:302–6.
Sonino N, Zielezny M, Fava GA, et al. Risk factors and long-term outcome in pituitary-dependent Cushing’s disease. J Clin Endocrinol Metab. 1996;81:2647–52.
Haskett RF. Diagnostic categorization of psychiatric disturbance in Cushing’s syndrome. Am J Psychiatry. 1985;142:911–6.
Hudson JI, Hudson MS, Griffing GT, et al. Phenomenology and family history of affective disorder in Cushing’s disease. Am J Psychiatry. 1987;144:951–3.
Sablowski N, Pawlik K, Lüdecke DK, Herrmann HD. Aspects of personality in patients with pituitary adenomas. Acta Neurochir (Wien). 1986;83:8–11.
Valassi E, Santos A, Yaneva M, ERCUSYN Study Group, et al. The European Registry on Cushing’s syndrome: 2-year experience: baseline demographic and clinical characteristics. Eur J Endocrinol. 2011;165:383–92.
Dorn LD, Burgess ES, Friedman TC, et al. The longitudinal course of psychopathology in Cushing’s syndrome after correction of hypercortisolism. J Clin Endocrinol Metab. 1997;82:912–9.
Starkman MN, Giordani B, Gebarski SS, Schteingart DE. Improvement in mood and ideation associated with increase in right caudate volume. J Affect Disord. 2007;101:139–47.
Kelly WF, Kelly MJ, Faragher B. A prospective study of psychiatric and psychological aspects of Cushing’s syndrome. Clin Endocrinol (Oxf). 1996;45:715–20.
Cohen SI. Cushing’s syndrome: a psychiatric study of 29 patients. Br J Psychiatry. 1980;136:120–4.
Jeffcoate WJ, Silverstone JT, Edwards CR, Besser GM. Psychiatric manifestations of Cushing’s syndrome: response to lowering of plasma cortisol. Q J Med. 1979;48:465–72.
Valassi E, Crespo I, Keevil BJ, et al. Affective alterations in patients with Cushing’s syndrome in remission are associated with decreased BDNF and cortisone levels. Eur J Endocrinol. 2017;176:221–31.
Ziegelstein RC, Fauerbach JA, Stevens SS, et al. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med. 2000;160:1818–23.
Katon WJ. Epidemiology and treatment of depresssion in patients with chronic medical illness. Dialog Clin Neurosci. 2011;13:7–23.
Ragnarsson O, Berglund P, Eder DN, Johannsson G. Long-term cognitive impairments and attentional deficits in patients with Cushing’s disease and cortisol-producing adrenal adenoma in remission. J Clin Endocrinol Metab. 2012;97:1640–8.
Andela CD, van der Werff SJ, Pannekoek JN, et al. Smaller grey matter volumes in the anterior cingulate cortex and greater cerebellar volumes in patients with long-term remission of Cushing’s disease: a case–control study. Eur J Endocrinol. 2013;169:811–9.
Martin SB, Covell DJ, Joseph JE, et al. Human experience seeking correlates with hippocampus volume: convergent evidence from manual tracing and voxel-based morphometry. Neuropsychologia. 2007;45:2874–82.
Martín-Blanco A, Ferrer M, Soler J, et al. The role of hypothalamus-pituitary-adrenal genes and childhood trauma in borderline personality disorder. Eur Arch Psychiatry Clin Neurosci. 2016;266:307–16.
Martínez-Momblán MA, Gómez C, Santos A, et al. A specific nursing educational program in patients with Cushing’s syndrome. Endocrine. 2016;53:199–209.
Gotch PM. Cushing’s syndrome from the patient perspective. Endocrinol Metab Clin N Am. 1994;23:607–17.
van Aken MO, Pereira AM, Biermasz NR, et al. Quality of life in patients after long-term biochemical cure of Cushing’s disease. J Clin Endocrinol Metab. 2005;90:3279–86.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington: American Psychiatric Association; 2014.
Pivonello R, Simeoli C, De Martino MC, et al. Neuropsychiatric disorders in Cushing’s syndrome. Front Neurosci. 2015;9:129.
Ritzel K, Beuschlein F, Mickisch A, Osswald A, et al. Clinical review: outcome of bilateral adrenalectomy in Cushing’s syndrome: a systematic review. J Clin Endocrinol Metab. 2013;98:3939–48.
Sonino N, Fallo F, Fava GA. Psychosomatic aspects of Cushing’s syndrome. Rev Endocr Metab Disord. 2010;11:95–104.
Santos A, Crespo I, Aulinas A, et al. Quality of life in Cushing’s syndrome. Pituitary. 2015;18:195–200.
Starkman MN. Neuropsychiatric findings in Cushing’s syndrome and exogenous glucocorticoid administration. Endocrinol Metab Clin N Am. 2013;42:477–88.
Nieman LK, Biller BM, Findling JW, et al. Treatment of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:2807–31.
Lau D, Rutledge C, Aghi MK. Cushing’s disease: current medical therapies and molecular insights guiding future therapies. Neurosurg Focus. 2015;38:E11.
Bratek A, Kozmin-Burzynska A, Gorniak E, Krysta K. Psychatric disorders associated with Cushing’s syndrome. Psychiatr Danub. 2015;27:S339–43.
Saad MF, Adams F, Mackay B, et al. Occult Cushing’s disease presenting with acute psychosis. Am J Med. 1984;76:759–66.
Zielasek J, Bender G, Schlesinger S, et al. A woman who gained weight and became schizophrenic. Lancet. 2002;360:1392.
Baba M, Ray D. Severe psychosis due to Cushing’s syndrome in a patient with a carcinoid tumour in the lung: a case report and review of the current management. World J Surg Oncol. 2015;13:165.
Górniak M, Rybakowski J. Paranoid syndrome in the course of Cushing’s disease. Post Psychiatr Neurol. 2005;14:18–20.
Johanssen S, Allolio B. Mifepristone (RU 486) in Cushing’s syndrome. Eur J Endocrinol. 2007;157:561–9.
Castinetti F, Fassnacht M, Johanssen S, et al. Merits and pitfalls of mifepristone in Cushing’s syndrome. Eur J Endocrinol. 2009;160:1003–10.
van der Lely AJ, Foeken K, Van Der Mast RC, Lamberts SW. Rapid reversal of acute psychosis in the Cushing syndrome with the cortisol-receptor antagonist mifepristone (RU 486). Ann Intern Med. 1991;114:143–4.
Chu JW, Matthias DF, Belanoff J, et al. Successful long-term treatment of refractory Cushing’s disease with high-dose mifepristone (RU 486). J Clin Endocrinol Metab. 2001;86:3568–73.
Bilgin YM, van der Wiel HE, Fischer HR, De Herder WW. Treatment of severe psychosis due to ectopic Cushing’s syndrome. J Endocrinol Investig. 2007;30:776–9.
Beaufrere B, de Parscau L, Chatelain P, et al. RU 486 administration in a child with Cushing’s syndrome. Lancet. 1987;2:217.
Bertagna X, Bertagna C, Laudat MH, et al. Pituitary-adrenal response to the antiglucocorticoid action of RU 486 in Cushing’s syndrome. J Clin Endocrinol Metab. 1986;63:639–43.
Chrousos GP, Laue L, Nieman LK, et al. Clinical applications of RU 486, a prototype glucocorticoid and progestin antagonist. In: Mantero F, Takeda R, Scoggins BA, et al., editors. The adrenal and hypertension: from cloning to clinic. New York: Raven Press; 1989. p. 273–84.
Cassier PA, Abou-Amara-Olivieri S, Artru P, et al. Mifepristone for ectopic ACTH secretion in metastatic endocrine carcinomas: report of two cases. Eur J Endocrinol. 2008;158:935–8.
Mutter GL, Bergeron C, Deligdisch L, et al. The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod Pathol. 2008;21:591–8.
Fleseriu M, Biller BM, Findling JW, SEISMIC Study Investigators, et al. Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing’s syndrome. J Clin Endocrinol Metab. 2012;97:2039–49.
National Collaborating Centre for Mental Health (UK). Depression: the treatment and management of depression in adults (updated edition). National Institute for Health and Clinical Excellence: guidance. Leicester: British Psychological Society; 2010.
Gallagher P, Malik N, Newham J, et al. Antiglucocorticoid treatments for mood disorders. Cochrane Database Syst Rev. 2008;1:CD005168.
Brykalski J, Papierska L, Załuska M. Acute psychosis in the course of treatment of acute adrenal crisis with hydrocortisone in the patient with secondary adrenal insufficiency: a case study. Psychiatr Pol. 2015;49:673–81.
Løvas K, Husebye ES. High prevalence and increasing incidence of Addison’s disease in western Norway. Clin Endocrinol (Oxf). 2002;56:787–91.
Erichsen MM, Løvås K, Skinningsrud B, et al. Clinical, immunological, and genetic features of autoimmune primary adrenal insufficiency: observations from a Norwegian registry. J Clin Endocrinol Metab. 2009;94:4882–90.
Anglin RE, Rosebush PI, Mazurek MF. The neuropsychiatric profile of Addison’s disease: revisiting a forgotten phenomenon. J Neuropsychiatry Clin Neurosci. 2006;18:450–9.
Schultebraucks K, Wingenfeld K, Heimes J, et al. Cognitive function in patients with primary adrenal insufficiency (Addison’s disease). Psychoneuroendocrinology. 2015;55:1–7.
Thomsen AF, Kvist TK, Andersen PK, Kessing LV. The risk of affective disorders in patients with adrenocortical insufficiency. Psychoneuroendocrinology. 2006;31:614–22.
Holtzman CW, Trotman HD, Goulding SM, et al. Stress and neurodevelopmental processes in the emergence of psychosis. Neuroscience. 2013;249:172–91.
Farah Jde L, Lauand CV, Chequi L, et al. Severe psychotic disorder as the main manifestation of adrenal insufficiency. Case Rep Psychiatry. 2015;2015:512430.
Tatsuzawa Y, Ono Y, Takahashi T, et al. Case of isolated adrenocorticotropic hormone deficiency mimicking major depressive disorder. Psychiatry Clin Neurosci. 2011;65(3):302.
Martínez MA, Gómez C, Santos A, et al. Quina informació van rebre els malalts amb síndrome de Cushing i risc cardiovascular? Ag Inf. 2012;16:17–22.
Kreitschmann-Andermahr I, Psaras T, Tsiogka M, et al. From first symptoms to final diagnosis of Cushing’s disease: experiences of 176 patients. Eur J Endocrinol. 2015;172:285–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this review.
Conflict of interest
Alicia Santos, Eugenia Resmini, Juan Carlos Pascual, Iris Crespo and Susan Webb have no conflicts of interest directly relevant to the content of this review.
Rights and permissions
About this article
Cite this article
Santos, A., Resmini, E., Pascual, J.C. et al. Psychiatric Symptoms in Patients with Cushing’s Syndrome: Prevalence, Diagnosis and Management. Drugs 77, 829–842 (2017). https://doi.org/10.1007/s40265-017-0735-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-017-0735-z