Abstract
Nanoparticle albumin-bound paclitaxel (Abraxane®) [hereafter referred to as nab-paclitaxel] is a taxane developed to avoid some of the toxicities associated with solvent-bound (sb) paclitaxel. Nab-paclitaxel, in combination with carboplatin, is indicated for the first-line treatment of non-small cell lung cancer (NSCLC) in patients who are not candidates for curative surgery and/or radiation therapy. This article summarizes pharmacological, efficacy and tolerability data relevant to the use of nab-paclitaxel in this indication. Compared with sb-paclitaxel plus carboplatin, nab-paclitaxel plus carboplatin significantly improved the objective response rate (ORR), but did not prolong progression-free survival or overall survival (OS), in the overall population of patients with advanced NSCLC in a multinational phase III trial. The nab-paclitaxel regimen also provided benefit over the sb-paclitaxel regimen in certain patient subgroups, including patients with squamous cell histology (in terms of ORR) and patients who were elderly (in terms of OS). Nab-paclitaxel plus carboplatin had a manageable tolerability profile with some benefits over sb-paclitaxel plus carboplatin, including lower rates of grade ≥3 neutropenia, peripheral neuropathy, arthralgia and myalgia, although was associated with more grade ≥3 anaemia and thrombocytopenia. Given its efficacy and tolerability, intravenous nab-paclitaxel plus carboplatin is a valuable first-line treatment option for patients with advanced NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Albumin-stabilized nanoparticle formulation developed to minimize the toxicities associated with solvent-bound paclitaxel |
Microtubule inhibitor, administered weekly via a 30 min intravenous infusion |
Improves objective response rates in patients with non-small cell lung cancer, particularly those with squamous cell histology |
Manageable tolerability profile |
1 Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide [1]. Non-small cell lung cancer (NSCLC) accounts for 85–90 % of all lung cancer diagnoses [2], and the majority of patients are diagnosed with advanced disease at presentation [3]. Advanced NSCLC carries a poor prognosis, as most patients are unsuitable for curative treatment [4]. Platinum-based doublet chemotherapy has been shown to improve quality of life and prolong survival in patients with advanced or metastatic NSCLC and a good performance status [2, 3]. The combination of a taxane (either paclitaxel or docetaxel) with a platinum compound (cisplatin or carboplatin) is often used for the first-line treatment of advanced NSCLC [3].
Paclitaxel is poorly soluble in water, and conventional formulations require the use of polyoxyethylated castor oil solvent (Cremophor EL®) and ethanol as vehicles for intravenous delivery [5, 6]. However, the amount of Cremophor EL® required for paclitaxel is relatively high and is responsible for many of the side effects observed with solvent-bound (sb) paclitaxel, including serious acute hypersensitivity reactions and neurological toxicity [6]. Nanoparticle albumin-bound paclitaxel (hereafter referred to as nab-paclitaxel) was developed in order to minimize the toxicities associated with Cremophor EL®-based paclitaxel formulations [7].
Nab-paclitaxel (Abraxane®) has been approved in several countries, including the EU, the USA and Japan, as first-line treatment in combination with carboplatin in patients with advanced NSCLC who are not candidates for curative surgery and/or radiation therapy. This article reviews the efficacy and tolerability of intravenous nab-paclitaxel in the treatment of patients in this population and briefly summarizes its pharmacology. The focus of discussion is on the approved dose (100 mg/m2 in NSCLC; see Sect. 6) wherever possible, except where data are limited. Nab-paclitaxel is also approved for the treatment of metastatic breast cancer and as first-line treatment in combination with gemcitabine in patients with metastatic pancreatic cancer [8, 9]; discussion of these indications is outside the scope of this review.
2 Pharmacodynamics of Albumin-Bound Paclitaxel
Paclitaxel promotes the assembly of microtubules and stabilizes them by stopping their depolymerization, thereby inhibiting the normal reorganization of the microtubular network required for interphase and mitosis [8, 9]. Paclitaxel also induces the formation of abnormal microtubule arrays throughout the cell cycle and the formation of multiple microtubule asters during mitosis [8, 9].
Nab-paclitaxel is formed by homogenizing paclitaxel with human serum albumin under high pressure, resulting in a nanoparticle colloidal suspension [10]. Albumin mediates endothelial caveolar transcytosis of plasma constituents, and has been shown to enhance transport of paclitaxel across endothelial cells [9]. In vitro, endothelial binding and transcytosis of paclitaxel were 9.9- and 4.2-fold higher with nab-paclitaxel than with Cremophor EL®-based paclitaxel (both p < 0.0001) [7]. Transcytosis of nab-paclitaxel was completely suppressed by methyl-β-cyclodextrin, a known functional inhibitor of gp60 receptor/caveolar transport, suggesting that transendothelial cell transport of albumin is mediated by the gp60 (albondin) receptor. Cremophor EL® dose-dependently inhibited endothelial cell binding of paclitaxel [50 % maximal inhibitory concentration (IC50) 0.010 %]. Inhibition was complete at a concentration of 0.1 %. Cremophor EL® also inhibited paclitaxel binding to albumin (IC50 0.0017 %); this concentration was lower than those typically seen after infusion of Cremophor EL®-based paclitaxel [7].
In vivo, nab-paclitaxel induced tumour regression and prolonged survival in murine xenograft models of cancer [7]. Of the solid tumour cell lines studied (lung, breast, ovarian, prostate and colon), the lung tumour xenograft was the most sensitive to both nab-paclitaxel and Cremophor EL®-based paclitaxel, with the antitumour activity of the two agents not significantly differing in this setting [7].
The antitumour effects of nab-paclitaxel (alone or in combination with platinum-based therapies) have also been observed in early-phase clinical trials in patients with advanced solid tumours [10–22], including NSCLC [14–22]. The largest of the NSCLC trials [21] demonstrated a favourable efficacy and tolerability profile for nab-paclitaxel 100 mg/m2 weekly plus carboplatin; this regimen was subsequently taken into a large phase III trial in patients with NSCLC (Sect. 4).
SPARC (secreted protein, acidic and rich in cysteine) is overexpressed in a number of cancers [23]. In a study that assessed tissue samples from NSCLC patients, SPARC was expressed more frequently in squamous cell carcinoma than in adenocarcinoma, and was shown to be an independent prognostic factor for overall survival [24]. The ability of SPARC to bind albumin is thought to enhance tumoural accumulation of albumin-bound drugs [25] and, consistent with this, intratumoural accumulation of nab-paclitaxel has been shown to be 33 % greater than that of sb-paclitaxel in mouse xenograft models [7]. Despite the enhanced accumulation seen with albumin-bound drugs, nab-paclitaxel was associated with a better antitumour response than sb-paclitaxel in both SPARC-expressing tumours as well as some SPARC-negative tumours [26]. A phase II study is currently underway to further explore the potential role of SPARC as a predictive or prognostic biomarker of nab-paclitaxel-based therapy [27].
3 Pharmacokinetics of Albumin-Bound Paclitaxel
Nab-paclitaxel consists of nanoparticles of paclitaxel (≈130 nm) stabilized with human serum albumin [8, 9]. The particles are present in a non-crystalline, amorphous state, allowing for rapid dissolution into smaller (≈10 nm), soluble, albumin-bound paclitaxel complexes following intravenous administration [9].
Plasma concentrations of paclitaxel decline in a biphasic manner following intravenous administration of nab-paclitaxel [8]. The exposure to nab-paclitaxel is dose proportional across a dose range of 80–300 mg/m2 [8, 9] and the pharmacokinetics of nab-paclitaxel are independent of the duration of intravenous administration [8]. Nab-paclitaxel 100 mg/m2 produced maximum plasma concentration (Cmax) values of 1275–4253 ng/mL and area under the concentration-time curve from time 0 to infinity (AUC∞) values of 1756–4141 h·ng/mL in patients with various cancers [13, 14, 28]. When the pharmacokinetic properties of nab-paclitaxel and sb-paclitaxel were compared in patients with solid tumours, mean Cmax values were significantly (p < 0.001) greater for nab-paclitaxel 260 mg/m2 than for sb-paclitaxel 175 mg/m2 (3.8- [11] and 6.5- [29] fold), while mean AUC∞ values did not differ significantly between formulations [11, 29].
Nab-paclitaxel is highly (94 %) plasma protein bound, and is evenly distributed into blood cells [9]. The total volume of distribution of paclitaxel is relatively large (≈1741 L), suggesting extensive extravascular distribution and/or tissue binding of paclitaxel [8, 9]. The volume of distribution of paclitaxel was significantly (p = 0.003) higher with nab-paclitaxel 260 mg/m2 than with sb-paclitaxel 175 mg/m2 in patients with solid tumours [11]. In addition, the mean fraction of unbound paclitaxel in plasma was significantly higher with nab-paclitaxel than with sb-paclitaxel (6.3 vs. 2.4 %; p < 0.001) [11].
In a population pharmacokinetic analysis of data from eight studies in patients with various solid tumours (n = 150), the pharmacokinetics of nab-paclitaxel over a dose range of 80–375 mg/m2 were adequately described by a three-compartment model with both saturable distribution and saturable elimination [30].
Paclitaxel is metabolized largely by cytochrome P450 (CYP) 2C8 to 6α-hydroxypaclitaxel, with minor metabolism by CYP3A4 to 6α-3′-p-dihydroxypaclitaxel and 3′-p-hydroxypaclitaxel [8, 9]. Following a 30-min infusion of nab-paclitaxel 260 mg/m2 in patients with metastatic breast cancer, the mean cumulative recovery of the unchanged drug and its metabolites in the urine was <5 % [8, 9]. Elimination of paclitaxel is primarily via hepatic metabolism and biliary excretion [9] and ≈20 % of the total dose is excreted in the faeces [8]. Over the dose range of 80–300 mg/m2, paclitaxel has a mean terminal half-life of 13–27 h and a mean plasma clearance of 13–30 L/h/m2 [8, 9].
Because paclitaxel is metabolized by CYP2C8 and CYP3A4, caution is advised when nab-paclitaxel is coadministered with inhibitors (e.g. ketoconazole) or inducers (e.g. rifampicin) of CYP2C8 or CYP3A4 [8, 9]. No clinically relevant pharmacokinetic interactions were seen between nab-paclitaxel and carboplatin in Japanese patients with NSCLC [14]. In addition, alteration of the infusion sequence (i.e. nab-paclitaxel followed by carboplatin in cycle 1 and vice versa in cycle 2) did not affect the pharmacokinetics of nab-paclitaxel in patients with solid tumours [13].
According to the US prescribing information, gender, age (24–85 years), race (Asian vs. White) bodyweight (40–143 kg), body surface area (1.3–2.4 m2) and type of solid tumour do not alter the pharmacokinetics of nab-paclitaxel to a clinically relevant extent [8]. The EU summary of product characteristics states that paclitaxel AUC is reduced by ≈25 % in patients weighing 50 kg compared with those weighing 75 kg, although the clinical relevance of this finding is unknown [9].
The pharmacokinetics of paclitaxel were not affected by mild or moderate renal impairment [8, 9]. Mild hepatic impairment had no clinically relevant effect on the pharmacokinetics of paclitaxel. The maximum elimination rate of paclitaxel was decreased by 22–26 % and the mean paclitaxel AUC was increased by ≈20 % in patients with moderate or severe hepatic impairment, compared with patients with normal hepatic function [8, 9].
4 Therapeutic Efficacy of Albumin-Bound Paclitaxel
This section focuses on the efficacy of nab-paclitaxel plus carboplatin as first-line therapy in patients with advanced NSCLC, as assessed in a large randomized, open-label, multicentre, phase III study [31]. The trial enrolled adults with histologically or cytologically confirmed non-resectable stage IIIB or IV NSCLC who were previously untreated for metastatic disease and had not received radiotherapy during the previous 4 weeks. At baseline, 49 % of patients had adenocarcinoma, 43 % had squamous cell carcinoma and 8 % had other tumour histologies. The majority of patients were male (75 %), white (81 %), previous or current smokers (73 %), had an Eastern Cooperative Oncology Group performance score of 0 or 1 (99 %) and had stage IV disease (79 %). The median age of patients was 60 years. Patients were randomized to receive either nab-paclitaxel 100 mg/m2 (via 30 min intravenous infusion) weekly (i.e. days 1, 8 and 15) plus carboplatin AUC 6 mg/mL/min every 3 weeks or sb-paclitaxel 200 mg/m2 (via 3 h intravenous infusion) plus carboplatin every 3 weeks. Randomization was stratified by disease stage, sex, histology and geographic region. Treatment continued for ≥6 cycles or until disease progression or unacceptable toxicity; the median number of cycles was 6 in each treatment group [31]. Some data are presented as abstracts [32–36].
4.1 Objective Response
First-line therapy with nab-paclitaxel plus carboplatin was associated with a significantly greater (by 31 %) objective response rate (ORR) than sb-paclitaxel plus carboplatin in the overall patient population, as assessed by independent radiological assessment using RECIST criteria (primary endpoint analysis; Table 1). With the exception of one patient in the sb-paclitaxel group who had a complete response, all ORRs were partial responses [31]. Similar findings were reported for ORR when determined by the investigator [38 % of nab-paclitaxel recipients vs. 30 % of sb-paclitaxel recipients; response rate ratio (RRR) 1.274; 95 % CI 1.076–1.509; p = 0.005] [31].
Independent radiological assessment of ORR according to NSCLC histology found a significantly greater ORR with nab-paclitaxel plus carboplatin versus sb-paclitaxel plus carboplatin in the subset of patients with squamous cell histology, while the ORR was not significantly different between treatment groups in patients with non-squamous NSCLC (Table 1), including adenocarcinoma (26 vs. 27 %), large-cell carcinoma (33 vs. 15 %) and undifferentiated histology (24 vs. 15 %) [31, 37].
Several additional subgroup analyses were also performed. Neither age (≥ or <70 years) [38] nor region (Japan [39], North America, Russia/Ukraine [35]) impacted ORR outcome (nab- vs. sb-paclitaxel), as indicated by logistic regression analysis (p-value for treatment interaction by age was non-significant) [38] or multivariant analysis [35]. The nab-paclitaxel regimen was associated with a significantly (p < 0.05) greater ORR than the sb-paclitaxel regimen in patients with diabetes (52 vs. 27 %; RRR 1.935) [32] and ≥4 metastatic sites [33] but not in patients with mild [creatinine clearance (CLCR) >50 to ≤80 mL/min] or moderate (CLCR ≤50 mL/min) renal impairment [40]. However, some subgroups were small (n = 53–724) and analyses may therefore have lacked statistical power [32, 39, 40].
4.2 Other Clinical Outcomes
According to independent radiological assessment, stable disease (≥16 weeks) was observed in 20 % of patients receiving nab-paclitaxel plus carboplatin and 24 % of those receiving sb-paclitaxel plus carboplatin, while the proportion of patients experiencing disease progression was 16 % in both treatment groups [31].
In the overall ITT population, first-line therapy with nab-paclitaxel plus carboplatin was non-inferior to sb-paclitaxel plus carboplatin with regard to median progression-free survival [PFS; 6.3 vs. 5.8 months; hazard ratio (HR) 0.902; 95 % CI 0.767–1.060] and median overall survival (OS; 12.1 vs. 11.2 months; HR 0.922; 95 % CI 0.797–1.066), according to independent radiological assessment [31]. Similarly, there were generally no significant between-treatment differences in either of these survival outcomes when data were assessed by strata (i.e. region, sex, age, histology or disease stage) [31, 35, 38, 39] or in patients with renal impairment [40]. However, median OS was significantly (p < 0.01) longer for nab-paclitaxel plus carboplatin than sb-paclitaxel plus carboplatin in North American patients (12.7 vs. 9.8 months; HR 0.622) [31] and in patients aged ≥70 years (19.9 vs. 10.4 months; HR 0.583) [31, 38], with both age and region displaying a significant interaction with the treatment effect [35, 38]. In addition, patients with diabetes had a significantly longer PFS with nab-paclitaxel plus carboplatin than with sb-paclitaxel plus carboplatin (10.9 vs. 4.9 months; p = 0.016; n = 31 and 30, respectively); with this difference remaining significant after adjusting for age, disease stage, histology and region (p ≤ 0.026) [32]. In the subgroup of patients with squamous NSCLC who received >4 cycles of nab-paclitaxel plus carboplatin (n = 138), median PFS was 3.4 months and median OS was 13.8 months; survival at 1 year was 59 % [36].
A further analysis using quality-adjusted time without symptoms or toxicity (Q-TWiST) methodology confirmed the favourable benefit/risk profile of first-line therapy with nab-paclitaxel plus carboplatin among older patients aged ≥60 (n = 546) or ≥70 (n = 154) years [41]. Nab-paclitaxel plus carboplatin yielded a clinically relevant and statistically significant (p < 0.05) Q-TWiST survival gain of 1.4 months in patients aged ≥60 years, with a percent gain (relative to the OS of sb-paclitaxel plus carboplatin) of 10.8 %. The corresponding Q-TWiST difference among patients aged ≥70 years was 2.0 months, with a relative gain of 16.2 %; this difference was not statistically significant [41]. In another Q-TWiST analysis, a statistically significant (p value not reported) and clinically relevant Q-TWiST survival gain of 2.5 months in favour of nab-paclitaxel plus carboplatin was observed in North American patients, with a relative gain of 23.3 % [34].
First-line therapy with nab-paclitaxel plus carboplatin appeared to have a positive impact on health-related quality of life, according to an exploratory analysis of patient- and physician-assessed symptoms [42]. Patient-reported symptoms were assessed using the Functional Assessment of Cancer Therapy (FACT)-Taxane questionnaire. For instance, during 8 cycles of treatment, the nab-paclitaxel regimen was associated with significantly less worsening of peripheral neuropathy (p < 0.001), neuropathic pain in the hands and feet (p < 0.001) and hearing loss (p = 0.002) than the sb-paclitaxel regimen. Physician assessment of peripheral neuropathy by cycle also revealed a significant (p < 0.001) benefit of nab-paclitaxel plus carboplatin over sb-paclitaxel plus carboplatin [42].
5 Tolerability of Albumin-Bound Paclitaxel
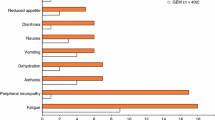
First-line nab-paclitaxel plus carboplatin had a manageable tolerability profile in patients with advanced NSCLC in the phase III trial [31] discussed in Sect. 4. Nab-paclitaxel plus carboplatin was associated with significantly (p < 0.05) lower rates of some grade ≥3 treatment-related adverse events than sb-paclitaxel plus carboplatin, including neutropenia (47 vs. 58 %), peripheral neuropathy (3 vs. 12 %; see Sect 5.1), arthralgia (0 vs. 2 %) and myalgia (<1 vs. 2 %). However, the incidence of some others, including anaemia (27 vs. 7 %) and thrombocytopenia (18 vs. 9 %), was significantly (p < 0.001) greater with nab-paclitaxel plus carboplatin than with sb-paclitaxel plus carboplatin, although these adverse events were generally manageable (a single blood transfusion was used for correction of anaemia) and without sequelae (thrombocytopenia was not associated with increased rates of haemorrhages) [31].
The incidence of the most common treatment-related grade ≥3 adverse events (febrile neutropenia, fatigue, anorexia and nausea) was not significantly different between treatment groups [31]. Taxane dose delays and dose reductions occurred in 82 and 46 % of patients in the nab-paclitaxel plus carboplatin group, compared with 54 and 23 % of patients in the sb-paclitaxel plus carboplatin group. Most dose reductions were due to neutropenia (29 vs. 10 %); potential reasons for this finding are discussed in Sect. 7. Overall, 4 % of patients in the nab-paclitaxel plus carboplatin group and 5 % of patients in the sb-paclitaxel plus carboplatin group withdrew from treatment because of adverse events. Treatment was discontinued due to unacceptable toxicity in 12 % of patients in both groups. Two deaths (one in each treatment group) were considered to be related to the study drug [31]. There have been rare reports of severe hypersensitivity reactions with nab-paclitaxel, including very rare but sometimes fatal anaphylactic reactions [8, 9].
5.1 Peripheral Neuropathy
Peripheral neuropathy is a common adverse event associated with taxane therapy. In the phase III trial [31], peripheral neuropathy of any grade occurred in significantly (p < 0.001) fewer nab-paclitaxel than sb-paclitaxel recipients (46 vs. 62 %). Corresponding rates of grade 3 peripheral neuropathy were 3 and 11 %, respectively; no patients in the nab-paclitaxel plus carboplatin group experienced grade 4 peripheral neuropathy (vs. two sb-paclitaxel plus carboplatin recipients) [31, 43]. Peripheral neuropathy led to taxane dose reductions and dose delays in 2 and 3 % of nab-paclitaxel plus carboplatin recipients versus 7 and 8 % of sb-paclitaxel plus carboplatin recipients (available as an abstract) [43].
Peripheral neuropathy of any grade occurred significantly later with nab-paclitaxel plus carboplatin than with sb-paclitaxel plus carboplatin (median time to onset 49 vs. 38 days; p < 0.001) [43], although the median time to grade ≥3 peripheral neuropathy onset did not differ significantly between the treatment groups (121 vs. 106 days) [43]. There was no significant between-group difference in the median time taken for grade ≥3 sensory neuropathy to improve to grade 1 (38 vs. 104 days) [31, 43].
6 Dosage and Administration
For patients with NSCLC who are not candidates for curative surgery and/or radiation therapy, the recommended dosage of nab-paclitaxel in the EU and the USA is 100 mg/m2 administered via intravenous infusion over 30 min on days 1, 8 and 15 of each 21-day cycle [8, 9]. Concomitant carboplatin should be administered on day 1 of each 21-day cycle, immediately following the completion of nab-paclitaxel administration. Nab-paclitaxel should not be substituted with or for other formulations of paclitaxel [8, 9].
Dose reductions and/or interruptions may be necessary to manage neurological, haematological or non-haematological toxicities [8, 9]. No dosage adjustment is necessary in patients aged ≥65 years in the EU [9]. The efficacy and tolerability of nab-paclitaxel in paediatric patients (aged ≤18 years [9]) has not been established [8, 9].
Dosage adjustments are not required in patients with mild to moderate renal impairment (Sect. 3); data in patients with severe renal impairment or end-stage renal disease are insufficient for dosage recommendations to be provided [8, 9]. Nab-paclitaxel should be administered with caution in patients with hepatic impairment, and such patients should be carefully monitored for the development of profound myelosuppression [8, 9]. In the USA, a reduced starting dosage of nab-paclitaxel is recommended in patients with moderate or severe hepatic impairment [8]. Nab-paclitaxel should not be administered to patients with total bilirubin >5 × the upper limit of normal (ULN) or aspartate aminotransferase >10 × ULN because hepatic impairment may increase the toxicity of paclitaxel [8, 9]. No dosage adjustments are required in patients with mild hepatic impairment (Sect. 3) [8, 9].
The US prescribing information contains a boxed warning regarding neutropenia [8]. Nab-paclitaxel is contraindicated in patients with baseline neutrophil counts of <1500 cells/mm3 [8, 9]. Complete blood cell counts should be performed frequently in all patients to monitor for bone marrow suppression (primarily neutropenia) [8, 9].
Local prescribing information should be consulted for comprehensive information on dosage adjustments, contraindications, warnings, precautions and use in special patient populations.
7 Current Status of Albumin-Bound Paclitaxel in Non-Small Cell Lung Cancer
The taxane nab-paclitaxel is an albumin-stabilized nanoparticle formulation of paclitaxel designed to overcome insolubility issues associated with conventional sb-paclitaxel. Nab-paclitaxel offers several practical advantages over sb-paclitaxel, including a shorter infusion time (30 min vs. 3 h) and the potential for fewer hypersensitivity reactions (given that it is Cremophor EL®-free), which may negate the need for the antihistamine and steroid premedications routinely used with Cremophor EL®-based paclitaxel formulations [10, 44, 45]. Severe hypersensitivity reactions have occurred with nab-paclitaxel, albeit rarely (Sect. 5). Although it has a shorter infusion time than sb-paclitaxel, patients have to receive nab-paclitaxel every week rather than every 3 weeks, which they may find less convenient. Some patients may prefer longer but less frequent infusions. However, more frequent administration may be beneficial in terms of managing or avoiding adverse events such as neutropenia, as the frequency gives more options to delay or interrupt the regimen as necessary based on blood count monitoring.
Nab-paclitaxel in combination with carboplatin is approved in the EU [9], the USA [8] and some other countries worldwide for the first-line treatment of patients with advanced NSCLC who are unfit for surgery and/or radiation therapy. The regimen demonstrated efficacy in this indication in a large, multinational phase III trial in which it was associated with a significantly higher ORR than sb-paclitaxel plus carboplatin (Sect. 4.1) and was non-inferior to the sb-paclitaxel regimen in terms of PFS and OS (Sect. 4.2). Some concerns have been raised regarding the use of ORR alone as the primary outcome measure, and the clinical relevance of an 8 % absolute between-group difference in ORR with no survival benefit has been questioned [46]. Nevertheless, survival outcomes in this study were generally comparable with historic results from trials in patients with advanced NSCLC [31].
The beneficial effects of nab-paclitaxel plus carboplatin on ORR were seen the prespecified subgroup of patients with squamous cell histology (Sect. 4.1). It should be noted that the increased ORR seen with nab-paclitaxel plus carboplatin versus sb-paclitaxel plus carboplatin in patients with squamous cell histology is the highest reported in a phase III trial in patients with squamous NSCLC [31]. In light of these findings, studies designed to investigate potential prognostic biomarkers in patients with squamous NSCLC would be of interest. A phase II trial comparing the efficacy of nab-paclitaxel plus carboplatin with gemcitabine plus carboplatin as first-line therapy in patients with advanced squamous cell carcinoma of the lung is ongoing; this study will investigate the predictive value of SPARC and caveolin-1 in PFS and OS (exploratory endpoints) (Sect. 2) [27]. In addition, a phase III study (ABOUND.sqm) investigating the efficacy and tolerability of nab-paclitaxel maintenance therapy following nab-paclitaxel plus carboplatin induction therapy is currently underway in patients with squamous NSCLC; this study plans to assess tumour biomarker response as an exploratory endpoint [47].
OS was prolonged with nab-paclitaxel plus carboplatin versus sb-paclitaxel plus carboplatin in patients from North America and in patients aged ≥70 years (Sect. 4.2). It is possible that differences between regions in baseline characteristics and standard of care may have played a role in the observed regional differences in survival outcomes [31]. In addition, the survival benefit of nab-paclitaxel seen in elderly patients, a subgroup who are typically undertreated, may have been related to the improved toxicity seen with nab-paclitaxel [38]. Further studies to confirm these findings in elderly patients would be of interest. A randomized phase II study in elderly patients with NSCLC (ABOUND.70+; NCT02151149) is currently underway.
In general, first-line nab-paclitaxel plus carboplatin had a manageable tolerability profile in patients with advanced NSCLC (Sect. 5). The nab-paclitaxel regimen demonstrated some benefits over the sb-paclitaxel regimen, including lower rates of grade ≥3 neutropenia, peripheral neuropathy, arthralgia and myalgia, but was associated with more grade ≥3 anaemia and thrombocytopenia [31]. Peripheral neuropathy of any grade occurred more with sb-paclitaxel plus carboplatin than with nab-paclitaxel plus carboplatin; this finding was supported by the results of the FACT-Taxane questionnaire, which demonstrated less worsening of neuropathy, pain and hearing loss with nab-paclitaxel plus carboplatin (Sect. 4.2). The reduction in peripheral neuropathy observed with nab-paclitaxel plus carboplatin may have been due to the absence of Cremophor EL®. The weekly dosing schedule of nab-paclitaxel plus carboplatin may have contributed to the higher incidence of dose delays and dose reductions compared with sb-paclitaxel plus carboplatin, which was administered every 3 weeks [31]. It is also possible that the open-label study design may have biased the reporting of safety data [46].
The costs associated with chemotherapy in patients with lung cancer constitute a substantial economic burden [48]. In a US cost-effectiveness analysis from a payer perspective, the incremental cost per life-year gained with nab-paclitaxel plus carboplatin relative to sb-paclitaxel plus carboplatin in patients with advanced NSCLC was estimated to be over the willingness-to-pay threshold of $US100,000; however, this was reduced to $US83,000 and $US23,000 in patients from North America or aged ≥70 years [49]. Another analysis [50] also estimated nab-paclitaxel plus carboplatin to be cost effective versus bevacizumab plus sb-paclitaxel and carboplatin in patients aged ≥70 years with advanced NSCLC. Both analyses are available as abstracts; further well-designed pharmacoeconomic analyses would be beneficial.
To conclude, current evidence indicates that nab-paclitaxel plus carboplatin is an effective therapy with a manageable tolerability profile for use in patients with advanced NSCLC, and is therefore a valuable first-line option in this setting.
Data selection sources:
Relevant medical literature (including published and unpublished data) on albumin-bound paclitaxel was identified by searching databases including MEDLINE (from 1946), PubMed (from 1946) and EMBASE (from 1996) [searches last updated 5 Oct 2015], bibliographies from published literature, clinical trial registries/databases and websites. Additional information was also requested from the company developing the drug.
Search terms: Albumin-bound paclitaxel, nab-paclitaxel, nab-PTX, nab-PAC, Abraxane
Study selection: Studies in patients with non-small cell lung cancer who received albumin-bound paclitaxel. When available, large, well designed, comparative trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
References
World Health Organization. Cancer: fact sheet no. 297. 2015. http://www.who.int. Accessed 5 Oct 2015.
Reck M, Popat S, Reinmuth N, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii27–39.
Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. 2012;10(10):1236–71.
Besse B, Adjei A, Baas P, et al. 2nd ESMO Consensus Conference on Lung Cancer: non-small-cell lung cancer first-line/second and further lines of treatment in advanced disease. Ann Oncol. 2014;25(8):1475–84.
Robinson DM, Keating GM. Albumin-bound paclitaxel: in metastatic breast cancer. Drugs. 2006;66(7):941–8.
Gelderblom H, Verweij J, Nooter K, et al. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590–8.
Desai N, Trieu V, Yao Z, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12(4):1317–24.
Celgene Corporation. Abraxane® for injectable suspension (paclitaxel protein-bound particles for injectable suspension) [albumin-bound]: US prescribing information. 2014. http://www.fda.gov/. Accessed 5 Oct 2015.
European Medicines Agency. Abraxane 5 mg/ml powder for suspension for infusion: summary of product characteristics. 2015. http://www.ema.europa.eu. Accessed 5 Oct 2015.
Ibrahim NK, Desai N, Legha S, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8(5):1038–44.
Gardner ER, Dahut WL, Scripture CD, et al. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin Cancer Res. 2008;14(13):4200–5.
Yamada K, Yamamoto N, Yamada Y, et al. Phase I and pharmacokinetic study of ABI-007, albumin-bound paclitaxel, administered every 3 weeks in Japanese patients with solid tumors. Jpn J Clin Oncol. 2010;40(5):404–11.
Stinchcombe TE, Socinski MA, Walko CM, et al. Phase I and pharmacokinetic trial of carboplatin and albumin-bound paclitaxel, ABI-007 (Abraxane) on three treatment schedules in patients with solid tumors. Cancer Chemother Pharmacol. 2007;60(5):759–66.
Okamoto I, Yamamoto N, Kubota K, et al. Safety and pharmacokinetic study of nab -paclitaxel plus carboplatin in chemotherapy-naive patients with advanced non-small cell lung cancer. Invest New Drugs. 2012;30(3):1132–7.
Bertino EM, Williams TM, Shilo K, et al. Phase 2 trial of nab-paclitaxel plus carboplatin for advanced NSCLC in patients at risk of bleeding from VEGF directed therapies [abstract no. 193]. Int J Radiat Oncol Biol Phys. 2014;90(5 Suppl 1):S45.
Fang Y, Wang L, Xia GH, et al. Clinical investigation of efficacy of albumin bound paclitaxel plus platinum compounds as first-line chemotherapy for stage III/IV squamous non-small cell lung cancer. Asian Pac J Cancer Prev. 2014;15(17):7453–7.
Green MR, Manikhas GM, Orlov S, et al. Abraxane, a novel cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann Oncol. 2006;17(8):1263–8.
Lin L, Chen H, Huang X, et al. Nab-paclitaxel (Abraxane) based chemotherapy for pretreated elderly advanced NSCLC patients: an open-label, single center, randomized phase II trial [abstract no. P1.10-013]. J Thorac Oncol. 2013;8(Suppl 2):S566–7.
Paik PK, James LP, Riely GJ, et al. A phase 2 study of weekly albumin-bound paclitaxel (Abraxane) given as a two-hour infusion. Cancer Chemother Pharmacol. 2011;68(5):1331–7.
Reynolds C, Barrera D, Jotte R, et al. Phase II trial of nanoparticle albumin-bound paclitaxel, carboplatin, and bevacizumab in first-line patients with advanced nonsquamous non-small cell lung cancer. J Thorac Oncol. 2009;4(12):1537–43.
Socinski MA, Manikhas GM, Stroyakovsky DL, et al. A dose finding study of weekly and every-3-week nab-paclitaxel followed by carboplatin as first-line therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2010;5(6):852–61.
Yang J, Huang C, Song Y, et al. A phase II trial of first-line nab-paclitaxel/carboplatin versus gemcitabine/carboplatin in advanced squamous cell carcinoma of the lung (CTONG1002) [abstract no. 8085]. J Clin Oncol. 2014;32(15 Suppl 1).
Podhajcer OL, Benedetti LG, Girotti MR, et al. The role of the matricellular protein SPARC in the dynamic interaction between the tumor and the host. Cancer Metastasis Rev. 2008;27(4):691–705.
Huang Y, Zhang J, Zhao YY, et al. SPARC expression and prognostic value in non-small cell lung cancer. Chin J Cancer. 2012;31(11):541–8.
Merlot AM, Kalinowski DS, Richardson DR. Unraveling the mysteries of serum albumin-more than just a serum protein. Front Physiol. 2014;5:299.
Shao H, Tang H, Salavaggione OE, et al. Improved response to nab-paclitaxel compared with cremophor-solubilized paclitaxel is independent of secreted protein acidic and rich in cysteine expression in non-small cell lung cancer. J Thorac Oncol. 2011;6(6):998–1005.
Yang JJ, Huang C, Chen GY, et al. A randomized phase II clinical trial of nab-paclitaxel and carboplatin compared with gemcitabine and carboplatin as first-line therapy in locally advanced or metastatic squamous cell carcinoma of lung. BMC Cancer. 2014;14(1):684.
Ando M, Yonemori K, Katsumata N, et al. Phase I and pharmacokinetic study of nab-paclitaxel, nanoparticle albumin-bound paclitaxel, administered weekly to Japanese patients with solid tumors and metastatic breast cancer. Cancer Chemother Pharmacol. 2012;69(2):457–65.
Sparreboom A, Scripture CD, Trieu V, et al. Comparative preclinical and clinical pharmacokinetics of a Cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in cremophor (Taxol). Clin Cancer Res. 2005;11(11):4136–43.
Chen N, Li Y, Ye Y, et al. Pharmacokinetics and pharmacodynamics of nab-paclitaxel in patients with solid tumors: disposition kinetics and pharmacology distinct from solvent-based paclitaxel. J Clin Pharmacol. 2014;54(10):1097–107.
Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30(17):2055–62.
Hirsh V, Owen SP, Ko A, et al. Analysis of outcomes in diabetic patients in a phase 3 trial of nab-paclitaxel (nab-P) plus carboplatin (C) in the first-line treatment of advanced non-small cell lung cancer (NSCLC) [abstract no. 137]. Int J Radiat Oncol Biol Phys. 2014;90(5 Suppl 1):S21.
Hirsh V, Page RD, Ko A, et al. Analysis of predictive factors in a phase 3 trial of nab-paclitaxel (nab-P) plus carboplatin (C) as first-line therapy for patients (pts) with advanced non-small cell lung cancer (NSCLC) [abstract no. 189]. Int J Radiat Oncol Biol Phys. 2014;90(5 Suppl 2):S43.
Langer CJ, Hirsh V, Wan Y, et al. A quality-adjusted time without symptoms or toxicity (Q-TWiST) analysis comparing nab-paclitaxel plus carboplatin (nab-PC) with solvent-based paclitaxel plus carboplatin (sb-PC) in first-line advanced non-small cell lung cancer (NSCLC) [abstract no. e19004]. J Clin Oncol. 2014;32(15 Suppl 1).
Socinski M, Okamoto I, Hon JK, et al. Nab-paclitaxel in combination with carboplatin as first-line therapy in patients (pts) with advanced non-small cell lung cancer (NSCLC): analysis of pt characteristics and clinical treatment patterns by region [abstract no. 183]. J Thorac Oncol. 2012;9(Suppl 4):S242–3.
Socinski MA, Ko A, Renschler MF. Nab-paclitaxel plus carboplatin in patients (pts) with squamous cell (SCC) non-small cell lung cancer (NSCLC): analysis of pts treated beyond 4 cycles in a pivotal phase 3 trial [abstract no. MO24.07]. J Thorac Oncol. 2013;8(Suppl 2):S404.
Socinski MA, Okamoto I, Hon JK, et al. Safety and efficacy analysis by histology of weekly nab-paclitaxel in combination with carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24(9):2390–6.
Socinski MA, Langer CJ, Okamoto I, et al. Safety and efficacy of weekly nab(R)-paclitaxel in combination with carboplatin as first-line therapy in elderly patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24(2):314–21.
Satouchi M, Okamoto I, Sakai H, et al. Efficacy and safety of weekly nab-paclitaxel plus carboplatin in patients with advanced non-small cell lung cancer. Lung Cancer. 2013;81(1):97–101.
Langer CJ, Hirsh V, Ko A, et al. Weekly nab-paclitaxel in combination with carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: analysis of safety and efficacy in patients with renal impairment. Clin Lung Cancer. 2015;16(2):112–20.
Langer CJ, Hirsh V, Okamoto I, et al. Survival, quality-adjusted survival, and other clinical end points in older advanced non-small-cell lung cancer patients treated with albumin-bound paclitaxel. Br J Cancer. 2015;113(1):20–9.
Hirsh V, Okamoto I, Hon JK, et al. Patient-reported neuropathy and taxane-associated symptoms in a phase 3 trial of nab-paclitaxel plus carboplatin versus solvent-based paclitaxel plus carboplatin for advanced non-small-cell lung cancer. J Thorac Oncol. 2014;9(1):83–90.
Sakai H, Ko A, Renschler MF, et al. Nab-paclitaxel in combination with carboplatin as first-line therapy in patients with advanced non-small cell lung cancer (NSCLC): analysis of peripheral neuropathy [abstract no. P1.10-018]. J Thorac Oncol. 2013;8(Suppl 2):S569–70.
Gupta N, Hatoum H, Dy GK. First line treatment of advanced non-small-cell lung cancer—specific focus on albumin bound paclitaxel. Int J Nanomed. 2014;9:209–21.
Socinski MA. Update on taxanes in the first-line treatment of advanced non-small-cell lung cancer. Curr Oncol. 2014;21(5):e691–703.
Levine MN, Juergens R. Method to our madness or madness in our methods? Pitfalls in trial methodology. J Clin Oncol. 2012;30(17):2025–6.
Spigel DR, Ko A, Ong TJ, et al. ABOUND.sqm: a phase 3 randomized study of maintenance nab-paclitaxel (NAB-P) after induction therapy with NAB-P plus carboplatin (C) in patients (pts) with squamous cell (SCC) non-small cell lung cancer (NSCLC) [abstract no. 1221TiP]. Ann Oncol. 2014;25:iv417–iv425.
Vera-Llonch M, Weycker D, Glass A, et al. Healthcare costs in patients with metastatic lung cancer receiving chemotherapy. BMC Health Serv Res. 2011;11:305.
Spigel D, Harwin W, Dranitsaris G, et al. Nab-paclitaxel in combination with carboplatin as first-line therapy in patients (pts) with advanced non-small cell lung cancer (NSCLC): an economic analysis [abstract no. P3.10-043]. J Thorac Oncol. 2013;8(Suppl 2):S1176–7.
Hirsh V, Berger A, Binder G, et al. Cost effectiveness of nab-paclitaxel plus carboplatin (nab-PC) relative to bevacizumab plus solvent-based paclitaxel and carboplatin (B+sb-PC) in elderly patients with advanced non-small cell lung cancer (NSCLC) [abstract no. 238]. Int J Radiat Oncol Biol Phys. 2014;90(5 Suppl 1):S61–S2.
Acknowledgments
During the peer review process, the manufacturer of tolvaptan was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Hannah Blair and Emma Deeks are salaried employees of Adis/Springer, are responsible for the article content and declare no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: M. M. Fukuoka, Izumi Municipal Hospital, Kinki University School of Medicine, Osaka, Japan; S. Harada, University of Alabama at Birmingham, Birmingham, AL, USA; C. Rolfo, Department of Oncology, Antwerp University Hospital, Edegem, Belgium; K. Yamada, Division of Respirology, Neurology, and Rheumatology, Department of Internal Medicine, Kurume University School of Medicine, Fukuoka, Japan.
Rights and permissions
About this article
Cite this article
Blair, H.A., Deeks, E.D. Albumin-Bound Paclitaxel: A Review in Non-Small Cell Lung Cancer. Drugs 75, 2017–2024 (2015). https://doi.org/10.1007/s40265-015-0484-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0484-9