Abstract
Albiglutide (Eperzan®, Tanzeum®), administered subcutaneously once weekly, is a glucagon-like peptide (GLP)-1 receptor agonist approved for the treatment of type 2 diabetes mellitus in several countries. Albiglutide has a longer half-life than native GLP-1, since it is resistant to degradation by the dipeptidyl peptidase-4 enzyme. As an incretin mimetic, albiglutide enhances glucose-dependent insulin secretion, suppresses inappropriate glucagon secretion, delays gastric emptying and reduces food intake. Several phase III clinical trials have demonstrated the efficacy of albiglutide in terms of improving glycaemic control in patients with inadequately controlled type 2 diabetes, including its use as monotherapy or add-on therapy to other antidiabetic agents (e.g. metformin, sulfonylureas, thiazolidinediones and insulins). In addition to improving glycaemic control, albiglutide had beneficial effects on bodyweight. These improvements in glycaemic control and reductions in bodyweight were maintained during long-term treatment (up to 3 years). Albiglutide was generally well tolerated in clinical trials, with mild to moderate gastrointestinal adverse events seen most commonly. Albiglutide has a convenient once-weekly administration regimen and a low risk of hypoglycaemia (except when used in combination with agents that may be associated with hypoglycaemia, such as sulfonylureas or insulin). Thus, albiglutide is an effective and generally well tolerated treatment option for patients with inadequately controlled type 2 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Glucagon-like peptide-1 receptor agonist with the convenience of once-weekly administration |
Improves glycaemic control when administered as monotherapy or add-on therapy to other antidiabetic agents |
Beneficial effects on bodyweight |
Improvements in glycaemic control and reductions in bodyweight are maintained during long-term therapy |
Generally well tolerated |
1 Introduction
A number of pharmacological options are available for the treatment of type 2 diabetes mellitus [1]. Among the more recently developed antidiabetic agents are incretin-based therapies targeting the gut hormones, glucagon-like peptide (GLP)-1 and glucose-dependent insulinotropic peptide (GIP) [2]. GLP-1 is a 30-amino acid peptide hormone secreted from endocrine cells in the gut in response to food intake [3]. As an incretin hormone, GLP-1 plays an important role in the regulation of blood glucose levels via glucose-dependent stimulation of insulin secretion and suppression of glucagon secretion [2–4]. GLP-1 and GIP also have extrapancreatic actions that may have implications for the prevention and treatment of diabetes-related complications (e.g. neuropathy) and comorbidities (e.g. obesity) independent of glycaemic control [5]. Native GLP-1 is rapidly inactivated by dipeptidyl peptidase (DPP)-4, resulting in a short circulating half-life and limited therapeutic value for type 2 diabetes [3, 4, 6]. Consequently, longer-acting GLP-1 analogues with longer half-lives and resistance to DPP-4 degradation have been developed [4].
Albiglutide (Eperzan®, Tanzeum®) is a once-weekly GLP-1 receptor agonist approved for the treatment of type 2 diabetes in several regions, including the EU [7] and the USA [8]. This article provides a narrative review of the efficacy and tolerability of subcutaneous albiglutide as monotherapy or add-on therapy in patients with inadequately controlled type 2 diabetes. A brief overview of the pharmacological properties of albiglutide is also provided.
2 Pharmacodynamic Properties
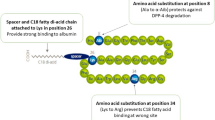
Albiglutide has 97 % homology to the amino acid sequence of endogenous GLP-1 (fragment 7–36) [9]. It is comprised of two tandem copies of modified human GLP-1 genetically fused to recombinant human albumin [8]. A single amino acid substitution (alanine to glycine) at position 8 renders albiglutide resistant to DPP-4-mediated proteolytic degradation [8]. As a result of its fusion to human albumin and DPP-4 resistance, albiglutide has a longer half-life than native GLP-1 and other GLP-1 analogues [4].
Albiglutide is an incretin mimetic, since it is a GLP-1 receptor agonist [10]. In addition to enhancing glucose-dependent insulin secretion and suppressing inappropriate glucagon secretion, albiglutide also delays gastric emptying and reduces food intake by promoting satiety [3, 10, 11].
A single dose of subcutaneous albiglutide 50 mg improved β-cell function in patients with type 2 diabetes (n = 44), according to the results of a stepped glucose clamp study [12]. During the hyperglycaemic plateau, the C-peptide area under the curve (AUC) was significantly (p = 0.02) higher in albiglutide than in placebo recipients. By contrast, C-peptide secretion was suppressed in both albiglutide and placebo recipients during hypoglycaemia. The calculated insulin secretion rate was also significantly higher with albiglutide than with placebo during the hyperglycaemic plateau (p value not stated) [12].
Homeostasis model assessment for β-cell function was significantly (p < 0.01 vs. placebo) improved in patients with type 2 diabetes (n = 221) receiving subcutaneous albiglutide 15 mg once weekly, 30 mg once weekly or 30 mg every 2 weeks in a 16-week study [13]. The proinsulin : insulin ratio was also significantly (p < 0.05 vs. placebo) improved in recipients of albiglutide 30 mg once weekly or 30 mg every 2 weeks. In addition, postprandial suppression of glucagon was seen in patients receiving albiglutide 30 mg every 2 weeks; the least squares mean change from baseline in the postprandial glucagon AUC significantly favoured albiglutide versus placebo (−4.79 vs. +1.94 ng/L; p = 0.0498) [13].
Albiglutide did not impair the counter-regulatory hormone response to hypoglycaemia in patients with type 2 diabetes [12]. In the stepped glucose clamp study, glucagon levels remained low and suppressed in both albiglutide and placebo groups until plasma glucose was clamped at 4.0 mmol/L, at which point the glucagon response appeared earlier in the albiglutide group. The counter-regulatory hormones adrenaline, noradrenaline, cortisol and growth hormone were appropriately suppressed in both groups when plasma glucose levels were >4.0 mmol/L, but increased in response to hypoglycaemia with plasma glucose levels of <3.3 mmol/L. There were no significant differences between albiglutide and placebo in response for any counter-regulatory hormones. Compared with placebo, albiglutide had no significant effect on the recovery time from hypoglycaemia (i.e. glucose levels from 2.8 to 3.9 mmol/L) following the glucose clamp procedure [12].
In patients with type 2 diabetes, the greatest fluctuations in fasting plasma glucose (FPG) were seen with subcutaneous administration of albiglutide 50 mg every 2 weeks or 100 mg monthly [14, 15], with once-weekly administration of albiglutide 15 or 30 mg producing more predictable and consistent results [15]. Based on the results of two phase IIb studies [13, 14], the 30 mg once weekly dose of albiglutide was selected for the phase III programme [16, 17]. Clinically relevant doses of albiglutide 30 mg once weekly significantly reduced FPG levels compared with placebo (p < 0.05) [13–15]. Relative to placebo, once-weekly albiglutide 30 mg also significantly reduced postprandial 2-h glucose at week 16 (p < 0.0001) [13], weighted mean glucose AUC0–4 at day 29 (p < 0.0001) [15] and glycated haemoglobin (HbA1c) at day 43 [15] or week 16 [13, 14] (both p < 0.01). The effects of albiglutide on glycaemic control and other efficacy outcomes in patients with type 2 diabetes receiving albiglutide as monotherapy or add-on therapy in large phase III trials are discussed in Sect. 4.
When administered as a single 100 mg dose in healthy volunteers, albiglutide significantly slowed gastric emptying compared with placebo [7, 8]. Albiglutide increased the gastric emptying half-life (t½) from 0.28 to 0.69 h for liquids (p = 0.0018) and from 1.14 to 2.23 h for solids (p = 0.0112) [7].
In a thorough QT study, albiglutide at clinically relevant doses of up to 50 mg did not produce prolongation of the heart rate-corrected QT interval in healthy volunteers [18]. Albiglutide reduced myocardial infarct size and improved cardiac function and energetics in a rat model of myocardial ischaemia and reperfusion, suggesting that it may have cardioprotective effects [19].
3 Pharmacokinetic Properties
The absolute bioavailability of albiglutide following subcutaneous injection has not been evaluated [8]. However, based on nonclinical studies demonstrating an absolute bioavailability of 50–81 % in monkeys and ≥38 % in mice, albiglutide is expected to have a relatively high bioavailability in humans [9]. After subcutaneous administration of a single 30 mg dose of albiglutide in patients with type 2 diabetes, the mean maximum plasma concentration (Cmax) of albiglutide was achieved 3–5 days post-dose [7, 8]. Plasma concentrations reach steady state within 3–5 weeks of repeated administration of once-weekly albiglutide [7]. Increases in the area under the concentration-time curve (AUC) are dose-proportional at the 30 and 50 mg dose levels, and exposure is similar when albiglutide is injected into the upper arm, abdomen or thigh [7, 8]. Following subcutaneous administration of albiglutide, the mean estimated apparent volume of distribution is 11 L. The plasma protein binding of albiglutide has not been evaluated [7, 8].
Since albiglutide is an albumin fusion protein, it is thought to follow a metabolic pathway similar to native human serum albumin, the catabolism of which occurs predominantly in the vascular endothelium [8]. The expected metabolic pathway for albiglutide is degradation to small peptides and amino acids via proteolysis [7, 8]. The mean clearance of albiglutide in humans is 67 mL/h. The elimination half-life of albiglutide is considered to be ≈5 days [7, 8], although a study in patients with type 2 diabetes reported slightly higher mean geometric half-life values of 6–7 days [20].
The prescribing information states that age, gender, race and bodyweight do not alter the pharmacokinetics of albiglutide to a clinically relevant extent [7, 8]. However, a population pharmacokinetic analysis in 159 Japanese patients with type 2 diabetes [21] found that age and bodyweight were significant predictors of the apparent clearance (CL/F) of albiglutide. Bodyweight was also a significant predictor of the apparent volume of distribution (V/F) of albiglutide [21]. No dosage adjustment of albiglutide is required in type 2 diabetes patients with mild or moderate renal impairment [7, 8]. The use of albiglutide is not recommended in patients with severe renal impairment in the EU [7], although the US prescribing information states that no dosage adjustment is needed in patients with severe renal impairment [estimated glomerular filtration rate (eGFR) 15–30 mL/min/1.73 m2] [8].
The potential for pharmacokinetic drug interactions with albiglutide appears to be low. According to the results of an animal study, albiglutide had little to no effect on hepatic microsomal protein, total cytochrome P450 (CYP) content or CYP1A, CYP2B, CYP2E, CYP3A and CYP4A activity [9]. Since albiglutide slows gastric emptying (Sect. 2), it may impact the absorption of orally administered medications [7, 8]. Caution is advised for drugs requiring clinical monitoring or those with a narrow therapeutic window [7, 8]. In three phase I studies, the pharmacokinetics of digoxin, warfarin and ethinylestradiol/norethisterone were not altered to a clinically relevant extent when these agents were coadministered with albiglutide [22]. However, in another study, concomitant albiglutide decreased simvastatin AUC (by 40 %) and t½ (from ≈7 to 3.5 h) and increased simvastatin Cmax (by 18 %) [7, 8]. Although the clinical relevance of these changes has not been determined, the alterations were not sufficient to warrant dose adjustment of simvastatin [8].
4 Therapeutic Efficacy
The therapeutic efficacy of subcutaneous albiglutide administered once weekly in patients with inadequately controlled type 2 diabetes has been assessed in eight randomized, multicentre studies forming the phase III HARMONY trial programme [23–30]. Trials were double-blind (HARMONY 1 [23], HARMONY 2 [24], HARMONY 3 [25], HARMONY 5 [30] and HARMONY 8 [29]) or open-label (HARMONY 4 [26], HARMONY 6 [27] and HARMONY 7 [28]), and enrolled patients aged ≥18 years [23–26, 28–30] or 18–75 years [27]. Inadequate glycaemic control was defined as a baseline HbA1c level of 7.0–10.0 % [23–26, 28–30] or 7.0–10.5 % [27]. In all trials, patients reaching predefined HbA1c thresholds were eligible to undergo dose titration and/or to receive hyperglycaemic rescue therapy as needed [23–30]. Only hyperglycaemic pre-rescue results were included in the efficacy analyses, including in the longer-term (3 year or 156 week) analyses, with results at the time of rescue carried forward. The primary efficacy endpoint was the change from baseline in HbA1c level at weeks 26 [27, 29], 32 [28], 52 [23, 24, 26, 30] or 104 [25].
4.1 As Monotherapy in Drug-Naïve Patients
Albiglutide monotherapy improved glycaemic control in drug-naïve patients inadequately controlled by diet and exercise alone, according to the results of the HARMONY 2 study (available as an abstract) [24]. Patients were randomized to receive albiglutide 30 mg, albiglutide 50 mg (starting dose of 30 mg, titrated to 50 mg at week 12) or placebo once weekly. At week 52, both dosages of albiglutide were associated with significantly greater reductions from baseline in mean HbA1c and FPG levels than placebo (Table 1). Compared with placebo, a significantly higher proportion of patients in both albiglutide groups achieved HbA1c <7 % (Table 1). Weight loss was observed in all three treatment groups (Table 1) [24].
The beneficial effects of albiglutide on HbA1c and FPG levels were maintained in the longer term in HARMONY 2 (reported as an abstract and poster) [31]. The mean change from baseline in bodyweight at week 156 was −1.32, −2.24 and −2.91 kg in the albiglutide 30 mg, albiglutide 50 mg and placebo groups, respectively. At week 156, the probability of hyperglycaemic rescue was 54.3, 41.8 and 74.4 % in the albiglutide 30 mg, albiglutide 50 mg and placebo groups, respectively. Among patients who completed 3 years of treatment without hyperglycaemic rescue (n = 76), target HbA1c levels of <7 % were achieved by 60.0, 59.4 and 57.1 % of patients in the albiglutide 30 mg, albiglutide 50 mg and placebo groups, respectively [31].
4.2 As Add-On Therapy
Seven trials in the HARMONY programme investigated the efficacy of albiglutide as add-on therapy in patients inadequately controlled with various oral antidiabetic drugs (OADs) [23, 25–30]. In all studies, albiglutide was initiated at a dose of 30 mg once weekly, with optimal up-titration to 50 mg once weekly, if needed, in HARMONY 3–6 and 8 [25–27, 29, 30] and force-titration to 50 mg once weekly in HARMONY 7 [28]. There was no up-titration in HARMONY 1 [23]. Dosages of comparator agents are shown in Table 1. HARMONY 1, 3–5 and 7, which included patients inadequately controlled with OADS alone, are discussed in Sect. 4.2.1. HARMONY 6, which included patients inadequately controlled with insulin alone or in combination with oral therapy, is discussed in Sect. 4.2.2. HARMONY 8, which included patients with renal impairment who were inadequately controlled with OADs, is discussed in Sect. 4.2.3.
4.2.1 In Patients Inadequately Controlled With Oral Therapy
In HARMONY 3, add-on therapy with once-weekly albiglutide was noninferior to once-daily sitagliptin and once-daily glimepiride at reducing HbA1c at week 104 in patients inadequately controlled with metformin, with superiority subsequently shown (Table 1) [25]. Add-on albiglutide was significantly more effective than placebo at reducing HbA1c (Table 1). Reductions from baseline in FPG levels were significantly greater with albiglutide than with all comparators (Table 1). In addition, albiglutide was associated with a significantly greater improvement from baseline in bodyweight than glimepiride, and significantly more albiglutide than placebo recipients achieved an HbA1c goal of <7 % (Table 1). Hyperglycaemic rescue rates at week 104 were also significantly lower with albiglutide relative to placebo (p < 0.00001) and sitagliptin (p = 0.0118); no significant difference was seen between albiglutide and glimepiride [25].
According to the results of the HARMONY 4 study, add-on therapy with once-weekly albiglutide was noninferior to once-daily insulin glargine in terms of the HbA1c-lowering response at week 52 in patients inadequately controlled with metformin alone or in combination with a sulfonylurea (Table 1) [26]. The reduction from baseline in FPG was significantly greater with insulin glargine versus albiglutide, but a significant between-group difference in favour of albiglutide was seen with regard to the change in bodyweight (Table 1). There was no significant between-group difference in the proportion of albiglutide and insulin glargine recipients achieving HbA1c levels of <7 % after 52 weeks of treatment (Table 1). Hyperglycaemic rescue was required by 25.6 % of albiglutide recipients and 23.8 % of insulin glargine recipients [26].
At week 52 in the HARMONY 5 study, add-on therapy with once-weekly albiglutide was more effective than placebo with regard to the change from baseline in HbA1c, but did not demonstrate noninferiority to once-daily pioglitazone, in patients inadequately controlled with metformin and glimepiride (Table 1) [30]. Although the improvement from baseline in FPG was significantly greater with albiglutide versus placebo, pioglitazone reduced FPG levels significantly more than albiglutide (Table 1). Bodyweight decreased from baseline in the albiglutide group but increased in the pioglitazone group; the between-group difference was statistically significant and favoured albiglutide (Table 1). Significantly more pioglitazone than albiglutide recipients achieved target HbA1c levels of <7 % (Table 1) [30].
In the HARMONY 1 trial, add-on therapy with once-weekly albiglutide was more effective than placebo in patients with inadequate glycaemic control on pioglitazone with or without metformin [23]. At week 52, albiglutide was associated with significantly greater improvements from baseline in HbA1c and FPG levels relative to placebo, and significantly more albiglutide than placebo recipients achieved HbA1c levels of <7 % (Table 1). No between-group differences were seen with regard to the change from baseline in bodyweight (Table 1). The time to hyperglycaemic rescue up to week 52 differed significantly between groups (p < 0.0001). Rescue medication was required in 24.4 % of albiglutide and 47.7 % of placebo recipients [23].
According to the results of the HARMONY 7 trial, both once-weekly albiglutide and once-daily liraglutide were associated with clinically meaningful reductions from baseline in HbA1c at week 32 in patients with inadequate glycaemic control on OADs (metformin, a thiazolidinedione, a sulfonylurea or any combination of these), but the criteria for noninferiority of albiglutide were not met. (Table 1) [28]. Relative to albiglutide recipients, liraglutide recipients had significantly greater changes from baseline in FPG levels and bodyweight and significantly more patients achieved HbA1c levels of <7 % (Table 1). In addition, the time to hyperglycaemic rescue was significantly longer with liraglutide versus albiglutide (p = 0.005) and almost twice as many albiglutide as liraglutide recipients required rescue medication by week 32 (15 vs. 8 %) [28]. Data from US patients (n = 728) in the HARMONY 7 study (available as an abstract) [32] found no significant differences between albiglutide and liraglutide with regard to treatment satisfaction. Diabetes Medication Satisfaction (DMS) scores improved from baseline over 32 weeks in both treatment groups, with the greatest improvements seen in the efficacy domain [32].
4.2.1.1 Long-Term Results
Three-year results from the HARMONY 1, 3, 4 and 5 trials have been reported as abstracts and posters [33–36]. Blinding was maintained throughout in HARMONY 1, 3 and 5. In all four trials, add-on albiglutide produced sustained glycaemic control over the entire 3-year study period, as evidenced by durable HbA1c- and FPG-lowering effects [33–36].
In HARMONY 3, the proportion of albiglutide, sitagliptin, glimepiride and placebo recipients who achieved HbA1c levels of <7 % after 3 years was 60.0, 50.0, 43.1 and 43.8 %, respectively [34]. The mean change in bodyweight at week 156 was −2.31 kg with albiglutide versus +0.98 kg with glimepiride (p < 0.0001). Significantly fewer patients in the albiglutide group (29.3 %) required hyperglycaemic rescue at week 156, compared with placebo (49.0 %; p = 0.0002) and sitagliptin (37.7 %; p = 0.0275) [34].
In HARMONY 4, albiglutide was associated with continued weight loss through week 156 (mean −3.5 kg), while mean bodyweight increased by 0.9 kg in the insulin glargine group [36]. At week 156, HbA1c levels of <7 % were achieved by 48.0 % of albiglutide and 52.3 % of insulin glargine recipients. There were no statistically significant differences between albiglutide and insulin glargine regarding the proportion of patients requiring rescue medication (56.2 vs. 48.0 %) [36].
In HARMONY 5, target HbA1c levels of <7 % at week 156 were achieved by 37, 49 and 33 % of albiglutide, pioglitazone and placebo recipients, respectively [35]. Mean bodyweight decreased and remained stable in the albiglutide group over 3 years, but increased in the pioglitazone group (treatment difference –7.9 kg; p < 0.0001). At week 156, the probability of hyperglycaemic rescue was 52, 43 and 80 % in the albiglutide, pioglitazone and placebo groups, respectively [35].
In HARMONY 1, the proportion of patients achieving HbA1c levels of <7 % at week 156 was 59 % with albiglutide and 46 % with placebo [33]. Bodyweight remained stable for 3 years in the albiglutide group (quantitative data not available). The probability of hyperglycaemic rescue at week 156 was 44.8 % in the albiglutide group and 71.6 % in the placebo group, with placebo recipients requiring rescue medication at an earlier median time than albiglutide recipients (p < 0.0001) [33].
4.2.2 In Patients Inadequately Controlled With Insulin ± Oral Therapy
In HARMONY 6, add-on therapy with albiglutide was noninferior to three-times-daily prandial insulin lispro in terms of the HbA1c-lowering response at week 26 in patients with inadequate glycaemic control on insulin glargine alone or in combination with oral metformin and/or a thiazolidinedione (Table 1) [27]. There were no significant between-group differences with regard to the change from baseline in FPG or the proportion of patients achieving HbA1c levels of <7 % (Table 1). Weight loss was observed in the albiglutide group, while bodyweight increased in the insulin lispro group (Table 1). The probability of hyperglycaemic rescue was 22.9 % in the albiglutide group and 24.4 % in the insulin lispro group [27].
Treatment satisfaction improved from baseline over 26 weeks in the albiglutide group, but decreased in the insulin lispro group (available as an abstract) [37]. Compared with insulin lispro recipients, patients receiving albiglutide had significantly higher DMS scores for treatment burden (p < 0.0001), efficacy (p = 0.0264), symptoms (p = 0.0236) and overall satisfaction (p < 0.0001) [37].
Further reductions in HbA1c were seen in patients who completed 52 weeks of treatment, with total mean reductions from baseline of –1.01 and –0.84 % for albiglutide versus insulin lispro (available as an abstract) [38]. After 52 weeks of treatment, 44.6 and 29.8 % of albiglutide and insulin lispro recipients achieved HbA1c levels of <7 %. Albiglutide was associated with a significantly greater mean reduction from baseline in FPG levels than with insulin lispro (–1.48 vs. –0.86 mmol/L; p = 0.0281), and a significant between-group difference was observed for the mean change from baseline in bodyweight (–0.96 vs. +1.66 kg; p < 0.0001). Hyperglycaemic rescue was required in 43.6 and 38.8 % of albiglutide and insulin lispro recipients [38].
4.2.3 In Patients With Renal Impairment
In the HARMONY 8 study, once-weekly albiglutide was noninferior to once-daily sitagliptin at reducing HbA1c at week 26 in patients with renal impairment (eGFR 15–89 mL/min/1.73 m2) and inadequate glycaemic control on diet and exercise and/or oral therapy (metformin, a thiazolidinedione, a sulfonylurea or any combination of these), with superiority subsequently shown (Table 1) [29]. Albiglutide was significantly more effective than sitagliptin with regard to the changes from baseline in FPG and bodyweight, and the proportion of patients achieving an HbA1c target of <7 % (Table 1). In patients with mild (eGFR 60–89 mL/min/1.73 m2; n = 256), moderate (eGFR 30–59 mL/min/1.73 m2; n = 203) or severe (eGFR 15–29 mL/min/1.73 m2; n = 36) renal impairment, differences between albiglutide and sitagliptin in the least squares mean change from baseline in HbA1c were –0.13 % (95 % CI –0.37 to 0.11), –0.53 % (95 % CI –0.80 to –0.26) and –0.47 % (95 % CI –1.12 to 0.18), respectively, and in FPG were –0.83 mmol/L (95 % CI –1.54 to –0.12), –1.60 mmol/L (95 % CI –2.40 to –0.81) and –1.56 mmol/L (95 % CI –3.44 to 0.31), respectively. A significant difference in the mean time to hyperglycaemic rescue was observed between albiglutide and sitagliptin and favoured albiglutide (p = 0.0017). After 52 weeks of treatment, the probability of hyperglycaemic rescue was 20 % with albiglutide and 34 % with sitagliptin [29].
4.3 Pooled Analyses
Data were pooled from the HARMONY 1–7 trials (n = 4,400) to investigate the efficacy of once-weekly albiglutide in the African/African-American [39] and Hispanic/Latino [40] subpopulations (available as abstracts and posters). In the African/African-American subpopulation (n = 596), once-weekly albiglutide was associated with significantly greater improvements in HbA1c than placebo and pooled OADs, but not insulin [39]. In the Hispanic/Latino subpopulation (n = 1,204), HbA1c levels were significantly reduced with albiglutide relative to placebo, but not pooled OADs or insulin [40]. Statistical significance was based on 95 % CIs for between-group differences.
5 Tolerability
Unless specified otherwise, tolerability analyses in the HARMONY trials included both pre- and post-hyperglycaemic rescue results.
5.1 General Profile
Subcutaneous, once-weekly albiglutide was generally well tolerated when administered as monotherapy in drug-naïve patients with inadequate glycaemic control on diet and exercise alone, according to the results of the HARMONY 2 study described in Sect. 4.1 [24, 31], with tolerability maintained in the long term [31]. At week 156, the proportion of patients experiencing on-therapy treatment-related adverse events was 38.6, 43.4 and 23.8 % in the albiglutide 30 mg, albiglutide 50 mg and placebo groups, respectively [31]. The corresponding incidence of serious adverse events was 14.9, 14.1 and 15.8 %, respectively. The most common on-therapy adverse events (irrespective of rescue medication) were headache, nausea, diarrhoea, upper respiratory tract infection, hypertension, back pain and injection-site reaction (Fig. 1). Most injection-site reactions were of mild intensity, and lasted <2 weeks. There were four deaths in the albiglutide 50 mg group, one of which (severe metastatic pancreatic carcinoma) was considered to be drug-related [31].
Tolerability of subcutaneous albiglutide monotherapy in drug-naïve patients with type 2 diabetes participating in the HARMONY 2 trial [31]. Most common (≥8 % in either albiglutide group) on-therapy adverse events occurring up to week 156. URTI upper respiratory tract infection
Once-weekly albiglutide was also generally well tolerated when administered as add-on therapy in patients with inadequate glycaemic control, according to the results of the HARMONY 1 and 3–8 trials discussed in Sect. 4.2.
In a pooled analysis of the HARMONY 1–7 trials (2,116 albiglutide recipients), the most frequently reported adverse events occurring in albiglutide monotherapy or add-on therapy recipients included hypoglycaemia (mostly in patients receiving concomitant sulfonylureas or insulin; see Sect. 5.2), diarrhoea, nausea, upper respiratory infection and injection-site reaction (e.g. rash, erythema, itching); most injection-site reactions were mild in severity [7, 8, 16].
Once-weekly albiglutide also had a favourable gastrointestinal (GI) tolerability profile, according to another pooled analysis (available as an abstract and poster) [41] of data from several studies (HARMONY 1–3, 5, 7 and 8, as well as a phase IIb dose-ranging trial with an exenatide reference arm [14]; total n = 1,391). The most common GI adverse events reported in patients receiving albiglutide (monotherapy or add-on therapy) were diarrhoea (13.1 vs. 10.5 % of placebo recipients), nausea (11.1 vs. 9.6 %), constipation (4.6 vs. 5.1 %) and vomiting (4.2 vs. 2.6 %); most events were of mild or moderate severity and of short duration (median 3–4 days) [41]. The incidence of nausea and/or vomiting was 29.0 % with albiglutide 30 mg once weekly and 45.7 % with exenatide 5–10 µg once daily [14]. The frequency of GI adverse events was generally not increased when albiglutide was added to metformin [41]. Compared with add-on liraglutide, the rates of nausea (9.9 vs. 29.2 %; p < 0.0001) and vomiting (5.0 vs. 9.3 %; p = 0.0154) were significantly lower with add-on albiglutide [28].
Add-on therapy with once-weekly albiglutide was associated with a numerically higher incidence of GI and injection-site reactions than insulin [26, 27, 36]. Among patients receiving albiglutide or insulin lispro in combination with insulin glargine for 26 weeks in the HARMONY 6 trial (Sect. 4.2.2), diarrhoea occurred in 13.0 versus 4.3 %, nausea occurred in 11.2 versus 1.4 %, vomiting occurred in 6.7 versus 1.4 % and injection-site reactions occurred in 9.5 versus 5.3 % [27]. Among patients receiving albiglutide or insulin glargine in combination with OADs for 3 years in HARMONY 4 (Sect. 4.2.1), nausea occurred in 13.3 versus 7.5 %, diarrhoea occurred in 10.9 versus 7.9 % and injection-site reactions occurred in 9.9 versus 3.3 % [36].
In the HARMONY 8 trial [29], the incidence of GI adverse events increased with declining renal function [8]. Diarrhoea occurred in 6, 13 and 21 % of patients with mild, moderate or severe renal impairment. Corresponding rates of nausea were 3, 5 and 16 %, respectively. The US PI recommends caution when initiating or escalating doses of albiglutide in patients with renal impairment [8].
5.2 Hypoglycaemia
In HARMONY 2, monotherapy with once-weekly albiglutide was associated with low rates of hypoglycaemia in drug-naïve patients inadequately controlled on diet and exercise alone [31]. At week 156, documented pre-rescue symptomatic hypoglycaemia (plasma glucose ≤3.9 mmol/L or ≤70 mg/dL and the presence of hypoglycaemic symptoms) occurred in 2, 1 and 3 % of patients receiving albiglutide 30 mg, albiglutide 50 mg and placebo, respectively. No severe hypoglycaemic events were reported [31].
Albiglutide was generally associated with low rates of hypoglycaemia when administered once weekly as add-on therapy in the HARMONY 1 and 3–8 trials [23, 25–30, 33–36]. Across all studies, overall rates of documented pre-rescue symptomatic hypoglycaemia among albiglutide recipients ranged from 3.0 to 17.5 %. There were few (<2 %) cases of severe hypoglycaemia [23, 25–30, 33–36]. Patients receiving albiglutide and concomitant sulfonylureas appeared more likely to experience hypoglycaemia [26, 28–30, 35, 36]. For example, in HARMONY 4, the incidence of documented pre-rescue symptomatic hypoglycaemia was 21.1 % in the subgroup of patients receiving albiglutide plus metformin and a sulfonylurea compared with 1.1 % in the subgroup of patients receiving albiglutide plus metformin [26].
Add-on therapy with once-weekly albiglutide was associated with a lower risk of hypoglycaemia than add-on therapy with insulin in patients inadequately controlled with metformin with or without a sulfonylurea [26, 36] or insulin glargine with or without metformin and/or a thiazolidinedione [27]. In HARMONY 4, the model-adjusted incidence rate of documented pre-rescue symptomatic hypoglycaemia up to week 52 was significantly lower with albiglutide than with insulin glargine (61.4 vs. 108.8 events per 100 person-years; p = 0.0377) [26]. Corresponding incidence rates for any hypoglycaemia at week 156 were 70.2 and 145.2 events per 100 person-years, respectively [36]. Of note, severe hypoglycaemia occurred only in patients receiving concomitant metformin and a sulfonylurea [26, 36]. In HARMONY 6, the incidence rate for documented pre-rescue symptomatic hypoglycaemia through week 26 was 0.9 events per patient-year in the albiglutide group and 2.3 events per patient-year in the insulin lispro group [27].
5.3 Other Adverse Events of Special Interest
No cases of thyroid cancer were reported in albiglutide recipients in HARMONY 1, 4, 5, 7 and 8 trials [23, 26, 28–30]. Thyroid cancer was reported in one albiglutide recipient and two sitagliptin recipients in HARMONY 3 (none of which were considered study drug-related) [25] and in one albiglutide recipient in HARMONY 6 (albiglutide was discontinued after one dose) [27].
Acute pancreatitis has been observed in patients treated with albiglutide. Across all eight HARMONY trials, 0.3 % of albiglutide recipients developed pancreatitis considered to be likely related to the study drug, compared with 0.1 and 0 % of patients receiving active comparators and placebo [7, 8].
In a pooled analysis of the HARMONY 1–7 trials (2,116 albiglutide recipients), pneumonia occurred in 1.8 % of albiglutide recipients, compared with 0.8 % of all comparators [8]. Corresponding rates of serious pneumonia were 0.4 and 0.1 %, respectively [8].
Atrial fibrillation and atrial flutter were seen in 1.0 and 0.2 % of patients receiving albiglutide versus 0.5 and 0 % of patients receiving placebo or active comparators [8]. According to a meta-analysis (available as an abstract and poster) [42] of data from the HARMONY 1–8 trials and a 16-week Japanese phase IIb study [13] (n = 5,107), there was no excess hazard for first major adverse cardiovascular event plus [MACE+; non-fatal acute myocardial infarction (MI), stroke, cardiovascular (CV) death and hospitalization for unstable angina pectoris] for albiglutide versus all comparators (hazard ratio 1.00; 95 % CI 0.68–1.49). Incidence rates for first MACE+ were 1.2 events per 100 person-years with albiglutide and 1.1 events per 100 person-years for all comparators. Non-MACE+ events (silent MI, hospitalization for other chest pain or heart failure, subdural/extradural haematoma and non-CV death) occurred in 2.3 % of patients in the albiglutide and all comparators groups [42].
5.4 Immunogenicity
Integrated immunogenicity results from the HARMONY 1–7 trials (n = 2,098; available as an abstract and poster) [43] found that 5.5 % of patients tested positive for anti-albiglutide antibodies on at least one occasion. Of these, 64 % developed antibodies during the first 6 months of treatment. Most patients (63 %) had a positive result at only one or two time points, with nine patients developing anti-drug antibodies that lasted for >52 weeks. The antibodies were non-neutralizing and had no effect on the efficacy or benefit-risk profile of albiglutide [43].
6 Dosage and Administration
In the US, albiglutide is indicated as an adjunct to diet and exercise, but is not recommended as first-line therapy in patients with inadequate glycaemic control on diet and exercise [8]. In the EU, albiglutide is indicated as monotherapy in patients with inadequate glycaemic control on diet and exercise alone or in patients for whom the use of metformin is considered inappropriate, or as add-on therapy in combination with other glucose-lowering medications when these, together with diet and exercise, do not provide adequate glycaemic control [7]. The recommended dosage of albiglutide is 30 mg once weekly; this may be increased to 50 mg once weekly to improve glycaemic control [7, 8]. Albiglutide should be administered as a subcutaneous injection on the same day each week, without regard to meals. When albiglutide is given in combination with insulin or a sulfonylurea, the dose of insulin or sulfonylurea may need to be reduced to lower the risk of hypoglycaemia [7, 8]. When albiglutide is added to metformin, the metformin dose may be continued unchanged [7].
Albiglutide should not be used for the treatment of type 1 diabetes or diabetic ketoacidosis, and its use in paediatric patients has not been investigated [7, 8].
The US prescribing information contains a boxed warning stating that the risk of thyroid C-cell tumours was increased in rodents receiving GLP-1 receptor agonists [8]. Albiglutide is contraindicated in patients with a personal or family history of medullary thyroid tumours or in patients with multiple endocrine neoplasia [8].
Local prescribing information should be consulted for further information regarding dosage and administration, contraindications, warnings and precautions related to the use of albiglutide.
7 Place of Albiglutide in the Management of Type 2 Diabetes Mellitus
Metformin is the preferred and most widely used first-line pharmacological agent for type 2 diabetes [1, 2]. If the target HbA1c level is not achieved with metformin monotherapy after ≈3 months, add-on therapy with sulfonylureas, thiazolidinediones, DPP-4 inhibitors, GLP-1 receptor agonists or insulin (usually basal insulin) is recommended [1]. In addition to improving glycaemic control, the choice of pharmacological agent should take into account cost, potential adverse events, hypoglycaemia risk and effects on bodyweight [1]. While most classes of OADs are weight neutral (e.g. metformin, DPP-4 inhibitors) or associated with weight gain (e.g. sulfonylureas, thiazolidinediones, basal insulin), GLP-1 receptor agonists are associated with weight loss [1, 2].
Albiglutide is one of the newer GLP-1 receptor agonists; other such agents currently available include exenatide, exenatide long-acting release, lixisenatide, liraglutide and dulaglutide [2, 11]. Like other drugs of its drug-class, albiglutide activates the GLP-1 receptor, stimulating glucose-dependent insulin secretion, suppressing inappropriate glucagon secretion and delaying gastric emptying (Sect. 2).
A comprehensive phase III trial programme comprising eight individual studies (HARMONY 1–8) has demonstrated the efficacy of subcutaneous albiglutide as monotherapy and as add-on therapy in patients with inadequately controlled type 2 diabetes (Sect. 4). One unique feature of the HARMONY trials was the use of hyperglycaemic rescue for patients with persistent hyperglycaemia. These patients remained in the study after rescue and continued to receive randomized study medication. Efficacy assessments were based on pre-rescue results, while safety assessments also included post-rescue results. Although this study design was intended to reflect a more ‘real world’ scenario typically seen in diabetes clinical practice, it may have complicated interpretation of the study results [25]. While most studies in the HARMONY programme were double-blind, the insulin studies (HARMONY 4 and 6) and the liraglutide study (HARMONY 7) utilised an open-label design to avoid the use of sham injections; this may have biased the reporting of events [27]. It is worth noting that insulin dosing in the HARMONY 4 and 6 trials was not strictly treat-to-target, and the dose of basal insulin glargine in HARMONY 6 may not have been optimally titrated [27]. In several studies (HARMONY 3–6, 8), albiglutide was titrated from 30 to 50 mg as needed, meaning that efficacy and safety data comprised a mixture of these doses.
As monotherapy, albiglutide 30 or 50 mg once weekly was more effective than placebo in terms of improvements in glycaemic control at week 52 [24] (Sect. 4.1). Add-on therapy with albiglutide did not demonstrate noninferiority to pioglitazone in the HARMONY 5 trial [30] or to liraglutide in the HARMONY 7 trial [28] (Sect. 4.2.1). However, in the other HARMONY trials, add-on therapy with albiglutide was either noninferior to or significantly more effective than other comparators, including placebo (HARMONY 1 [23], HARMONY 2 [24], HARMONY 3 [25] and HARMONY 5 [30]), sitagliptin (HARMONY 3 [25] and HARMONY 8 [29]), glimepiride (HARMONY 3 [25]), insulin glargine (HARMONY 4 [26]) and insulin lispro (HARMONY 6 [27]), in terms of improving glycaemic control, in clinical trials of 26–104 weeks’ duration (Sect. 4.2). The improvements in glycaemic control were maintained during treatment with albiglutide for up to 3 years in the HARMONY 1–5 trials (Sects. 4.1, 4.2.1.1).
The term ‘diabesity’ is now used to characterize diabetes associated with obesity [11]. Studies have demonstrated that each 0.4-kg increase in bodyweight is associated with a 2 % increase in the risk of diabetes progression (HbA1c level of ≥7 % or initiation of treatment with antidiabetic drugs) [44]. Relatively small reductions in bodyweight have been shown to reduce the risk of cardiovascular disease and improve glycaemic control in overweight or obese patients with type 2 diabetes [2]. Indeed, evidence suggests that even a 1-kg reduction in bodyweight may be associated with beneficial effects on glycaemic control, as well as reduced morbidity and mortality [44]. With regard to reduction in bodyweight, once-weekly albiglutide was significantly more effective than insulin glargine, insulin lispro, glimepiride and pioglitazone, producing mean weight reductions of 0.4–1.2 kg from baseline compared with weight gains with these comparators in most studies (mean change of +0.8 to +4.4 kg) (Sect. 4). In patients completing up to 3 years of treatment with albiglutide in the HARMONY 1–5 trials, bodyweight decreased or remained stable over the course of treatment (Sects. 4.1, 4.2.1.1). Studies have shown that GLP-1 receptor agonists such as albiglutide and liraglutide reduce food intake by direct activation of GLP-1 receptors in the CNS [45]. Albiglutide is a large protein that cannot cross the blood-brain barrier or diffuse into the hypothalamus and area postrema as easily as liraglutide, a smaller peptide [28]. Therefore, albiglutide may have less impact on food intake and bodyweight. Indeed, weight loss was significantly lower with albiglutide than liraglutide in the HARMONY 7 trial (Sect. 4.2.1). This difference in bodyweight reduction may be partly due to liraglutide’s increased access to specific brain areas involved in appetite regulation [46].
Once-weekly albiglutide was generally well tolerated when administered as monotherapy or as add-on therapy to other antidiabetic agents (Sect. 5). As a drug class, GLP-1 receptor agonists are known to be associated with GI adverse events [2, 47], which may have a negative impact on treatment adherence [48]. GI adverse events such as nausea and diarrhoea were among the most commonly reported adverse events in the HARMONY trials (Sect. 5.1). However, according to pooled analyses of several studies, most GI events were transient and mild or moderate in severity (Sect. 5.1).
A possible link between GLP-1-derived medications and medullary thyroid cancer has been identified [49], but based on currently available results, firm evidence supporting or ruling out this hypothesis is lacking [50]. Across all eight HARMONY trials, only two albiglutide recipients developed thyroid cancer (Sect. 5.3). Nevertheless, albiglutide, similar to all other long-acting GLP-1 receptor agonists, carries an FDA boxed warning stating that it should not be used in patients with a multiple endocrine neoplasia or in patients with a personal or family history of medullary thyroid carcinoma (Sect. 6).
Although safety concerns have been raised in observational studies regarding the potential risk of acute pancreatitis associated with GLP-1 receptor agonists [51], the incidence of acute pancreatitis among albiglutide recipients was low (0.3 %) across all eight HARMONY trials (Sect. 5.3). In addition, it should be noted that type 2 diabetes itself is associated with a heightened risk of pancreatitis [52]. Recent assessments conducted by the US FDA and the European Medicines Agency (EMA) concluded that claims in the scientific literature of a causal relationship between incretin-based therapies (i.e. GLP-1 receptor agonists and DPP-4 inhibitors) and pancreatitis or pancreatic cancer were inconsistent with the available data [53]. Until more data are available, the FDA and the EMA will continue to consider pancreatitis a risk associated with incretin-based therapies [53].
The risk of hypoglycaemia is lower with GLP-1 receptor agonists, DPP-4 inhibitors, metformin and thiazolidinediones than with sulfonylureas, insulins and meglitinides [2, 54]. Indeed, in the HARMONY trials, albiglutide was generally associated with low rates of hypoglycaemia when administered once weekly as monotherapy or add-on therapy (Sect. 5.2). As expected, the incidence of hypoglycaemia was higher when albiglutide was administered in combination with a sulfonylurea or insulin (Sect. 5.2). Cross-talk between sulfonylureas and incretin signalling via an exchange protein directly activated by cyclic adenosine monophosphate (cAMP) 2A (EPAC2A) may be responsible for the severe hypoglycaemia that is sometimes observed in patients receiving DPP-4 inhibitors in combination with sulfonylureas [55]. However, because GLP-1 receptor agonists appear to increase cAMP levels in pancreatic β-cells more strongly than DPP-4 inhibitors, cross-talk between sulfonylureas and incretin signalling may not occur with GLP-1 receptor agonists [55].
Like other GLP-1 receptor agonists, albiglutide is self-administered by subcutaneous injection. Adherence to injectable therapies may be adversely affected by patients’ fear of needles or painful injections [48]. However, evidence suggests that longer-acting drugs such as once-weekly albiglutide may be preferred over injectable agents requiring more frequent administration (e.g. twice-daily exenatide) [47, 48, 56]. Nearly 50 % of patients with type 2 diabetes from a Chronic Illness Panel who completed an anonymous online survey (n = 1,516) indicated that they would be ‘extremely likely’ or ‘very likely’ to take an injectable once-weekly antidiabetic medication if it was recommended by their physician [56]. In the survey, the most commonly endorsed beneficial attributes of once-weekly medications were improved quality of life, greater convenience and better medication adherence [56].
Poor adherence to treatment is a widespread problem among patients with type 2 diabetes [56], and ‘real world’ data indicate that discontinuation rates for injectable non-insulin therapies, including some GLP-1 receptor agonists (e.g. exenatide, liraglutide), are high [57]. According to an analysis of data from the HARMONY 3, 5 and 8 trials (available as an abstract plus poster), once-weekly albiglutide may improve patient acceptability and ease of use relative to once-daily oral medications [57]. Overall compliance rates were high in all treatment groups, but were numerically higher with once-weekly albiglutide than with once-daily oral sitagliptin, glimepiride or pioglitazone. Low compliance rates of <80 % were observed in 7.5–14.9 % of patients receiving active comparators, compared with 1.6–2.3 % of albiglutide recipients [57].
In conclusion, subcutaneous albiglutide is effective and generally well tolerated in patients with inadequately controlled type 2 diabetes. In randomized controlled trials, albiglutide improved glycaemic control when administered as monotherapy or add-on therapy to other antidiabetic agents (including metformin, sulfonylureas, thiazolidinediones and insulins). In addition to improving glycaemic control, albiglutide had beneficial effects on bodyweight, with these benefits maintained during long-term treatment. Albiglutide has a convenient once-weekly administration regimen and a low risk of hypoglycaemia (except when used in combination with agents that may be associated with hypoglycaemia, such as sulfonylureas or insulin). Therefore, once-weekly albiglutide is a useful therapeutic option for the management of type 2 diabetes.
Data selection sources:
Relevant medical literature (including published and unpublished data) on albiglutide was identified by searching databases including MEDLINE (from 1946), PubMed (from 1946) and EMBASE (from 1996) [searches last updated 26 February 2015], bibliographies from published literature, clinical trial registries/databases and websites. Additional information was also requested from the company developing the drug.
Search terms: Albiglutide, Eperzan, Tanzeum, GSK 716155
Study selection: Studies in patients with type 2 diabetes mellitus who received albiglutide. When available, large, well designed, comparative trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
References
American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(Suppl. 1):S14–80.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012;55(6):1577–96.
Baggio LL, Huang Q, Brown TJ, et al. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes. 2004;53(9):2492–500.
Bush MA, Matthews JE, De Boever EH, et al. Safety, tolerability, pharmacodynamics and pharmacokinetics of albiglutide, a long-acting glucagon-like peptide-1 mimetic, in healthy subjects. Diabetes Obes Metab. 2009;11(5):498–505.
Seino Y, Yabe D. Glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1: incretin actions beyond the pancreas. J Diabetes Investig. 2013;4(2):108–30.
Deacon CF, Nauck MA, Toft-Nielsen M, et al. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44(9):1126–31.
European Medicines Agency. Eperzan (albiglutide): summary of product characteristics. 2014. http://www.ema.europa.eu. Accessed 26 Feb 2015.
GlaxoSmithKline. Tanzeum (albiglutide): US prescribing information. 2014. http://www.accessdata.fda.gov. Accessed 26 Feb 2015.
Young MA, Wald JA, Matthews JE, et al. Clinical pharmacology of albiglutide, a GLP-1 receptor agonist. Postgrad Med. 2014;126(7):84–97.
Trujillo JM, Nuffer W. Albiglutide: a new GLP-1 receptor agonist for the treatment of type 2 diabetes. Ann Pharmacother. 2014;48(11):1494–501.
Lorenz M, Evers A, Wagner M. Recent progress and future options in the development of GLP-1 receptor agonists for the treatment of diabesity. Bioorg Med Chem Lett. 2013;23(14):4011–8.
Hompesch M, Jones-Leone A, Carr MC, et al. Albiglutide does not impair the counter-regulatory hormone response to hypoglycemia: a randomized, double-blind, placebo-controlled, stepped glucose clamp study in subjects with type 2 diabetes mellitus. Diabetes Obes Metab. 2014.
Seino Y, Inagaki N, Miyahara H, et al. A randomized dose-finding study demonstrating the efficacy and tolerability of albiglutide in Japanese patients with type 2 diabetes mellitus. Curr Med Res Opin. 2014;30(6):1095–106.
Rosenstock J, Reusch J, Bush M, et al. Potential of albiglutide, a long-acting GLP-1 receptor agonist, in type 2 diabetes: a randomized controlled trial exploring weekly, biweekly, and monthly dosing. Diabetes Care. 2009;32(10):1880–6.
Seino Y, Nakajima H, Miyahara H, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of albiglutide, a long-acting GLP-1-receptor agonist, in Japanese subjects with type 2 diabetes mellitus. Curr Med Res Opin. 2009;25(12):3049–57.
European Medicines Agency. Eperzan assessment report. 2014. http://www.ema.europa.eu. Accessed 26 Feb 2015.
Rosenstock J, Stewart MW. Albiglutide. Drugs Future. 2010;35(9):701–12.
Darpo B, Zhou M, Matthews J, et al. Albiglutide does not prolong QTc interval in healthy subjects: a thorough ECG study. Diabetes Ther. 2014;5(1):141–53.
Bao W, Aravindhan K, Alsaid H, et al. Albiglutide, a long lasting glucagon-like peptide-1 analog, protects the rat heart against ischemia/reperfusion injury: evidence for improving cardiac metabolic efficiency. PLoS One. 2011;6(8):e23570.
Matthews JE, Stewart MW, De Boever EH, et al. Pharmacodynamics, pharmacokinetics, safety, and tolerability of albiglutide, a long-acting glucagon-like peptide-1 mimetic, in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93(12):4810–7.
Bush MA, Smith C, Watanalumlerd P, et al. Population pharmacokinetic (PK) analysis of albiglutide in a phase IIb study in japanese patients with type 2 diabetes [abstract no. 1020-P]. Diabetes. 2012;61(Suppl. 1):A261–2.
Bush M, Scott R, Watanalumlerd P, et al. Effects of multiple doses of albiglutide on the pharmacokinetics, pharmacodynamics, and safety of digoxin, warfarin, or a low-dose oral contraceptive. Postgrad Med. 2012;124(6):55–72.
Reusch J, Stewart MW, Perkins CM, et al. Efficacy and safety of once-weekly glucagon-like peptide 1 receptor agonist albiglutide (HARMONY 1 trial): 52-week primary endpoint results from a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes mellitus not controlled on pioglitazone, with or without metformin. Diabetes Obes Metab. 2014;16(12):1257–64.
Reinhardt R, Nauck MA, Stewart M, et al. HARMONY 2 results at week 52 primary endpoint: once-weekly albiglutide monotherapy for patients with type 2 diabetes mellitus inadequately controlled with diet and exercise [abstract no. 903]. In: 49th Annual Meeting of the European Association for the Study of Diabetes; 2013.
Ahren B, Johnson SL, Stewart M, et al. HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care. 2014;37(8):2141–8.
Weissman PN, Carr MC, Ye J, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57(12):2475–84.
Rosenstock J, Fonseca VA, Gross JL, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice-daily prandial insulin lispro. Diabetes Care. 2014;37(8):2317–25.
Pratley RE, Nauck MA, Barnett AH, et al. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014;2(4):289–97.
Leiter LA, Carr MC, Stewart M, et al. Efficacy and safety of the once-weekly GLP-1 receptor agonist albiglutide versus sitagliptin in patients with type 2 diabetes and renal impairment: a randomized phase III study. Diabetes Care. 2014;37(10):2723–30.
Home PD, Shamanna P, Stewart M, et al. Efficacy and tolerability of albiglutide versus placebo or pioglitazone over 1 year in people with type 2 diabetes currently taking metformin and glimepiride: HARMONY 5. Diabetes Obes Metab. 2014.
Carr MC, Rendell M, Scott RA, et al. Harmony 2 year 3 results: albiglutide monotherapy in drug naive patients with type 2 diabetes mellitus [abstract no. 830 plus poster]. In: 50th Annual Meeting of the European Association for the Study of Diabetes; 2014.
Martin AA, Johnson SL, Ye J. Treatment satisfaction with albiglutide and liraglutide in patients with type 2 diabetes uncontrolled with oral therapy [abstract no. 1010-P]. In: 73rd Annual Scientific Sessions of the American Diabetes Association; 2013.
Bode BW, Stewart M, Cirkel D, et al. Harmony 1 year 3 results: albiglutide vs. placebo in patients with type 2 diabetes mellitus not controlled on pioglitazone (Pio) + metformin (Met) [abstract no. 960-P plus poster]. In: 74th Scientific Sessions of the American Diabetes Association; 2014.
Matthews JE, Ahren B, Ye J, et al. Harmony 3 year 3 results: albiglutide vs sitagliptin and glimepiride in patients with type 2 diabetes mellitus on metformin [abstract no. 831 plus poster]. In: 50th Annual Meeting of the European Association for the Study of Diabetes; 2014.
Shamanna P, Home P, Stewart MW, et al. Harmony 5-year 3 results: albiglutide vs placebo and vs pioglitazone in triple therapy (background metformin and glimepiride) in people with type 2 diabetes [abstract no. 838 plus poster]. In: 50th Annual Meeting of the European Association for the Study of Diabetes; 2014.
Weissman PN, Stewart MW, Cirkel DT, et al. Harmony 4: 3 year efficacy of albiglutide (ALBI) vs insulin glargine (GLAR) in patients with type 2 diabetes mellitus [abstract no. 837 plus poster]. In: 50th Annual Meeting of the European Association for the Study of Diabetes; 2014.
Martin AA, Johnson SL, Ye J, et al. Improved treatment satisfaction with weekly abiglutide vs. thrice daily prandial insulin added to insulin glargine in type 2 diabetes [abstract no. 2590-PO]. In: 73rd Scientific Sessions of the American Diabetes Association; 2013.
Fonseca VL, Ahren B, Chow F, et al. Once weekly GLP-1 receptor agonist albiglutide vs prandial lispro added to basal glargine in type 2 diabetes: similar glycaemic control with weight loss and less hypoglycaemia [abstract no. 581]. In: 48th Annual Meeting of the European Association for the Study of Diabetes 2012.
Nino AJ, Davidson JA, Scott RA, et al. Albiglutide efficacy and safety in the African/African American subpopulation from the integrated phase III program [abstract no. 1003-P plus poster]. In: 74th Scientific Sessions of the American Diabetes Association; 2014.
Davidson JA, Jones-Leone A, Wilson TH, et al. Albiglutide efficacy and safety in the Hispanic/Latino subpopulation from the integrated phase III program [abstract no. 1004-P plus poster]. In: 74th Scientific Sessions of the American Diabetes Association; 2014.
Mallory JM, Leiter LA, Wilson TH, et al. Gastrointestinal safety across the albiglutide development program [abstract no. 1002-P plus poster]. Diabetes. 2014;63(Suppl. 1):A257–8.
Ambery PD, Donaldson J, Ye J, et al. Results of the albiglutide HARMONY program prospective major adverse cardiovascular event (MI, stroke, cardiovascular death, and unstable angina) meta-analysis [abstract no. 908 plus poster]. Diabetologia. 2014;57(Suppl. 1):S370.
Johnson S, Nauck MA, Zhi H, et al. Integrated phase 3 immunogenicity results for albiglutide [abstract no. 1640-P plus poster]. Diabetes. 2014;63(Suppl. 1):A427.
Ross SA, Dzida G, Vora J, et al. Impact of weight gain on outcomes in type 2 diabetes. Curr Med Res Opin. 2011;27(7):1431–8.
Sisley S, Gutierrez-Aguilar R, Scott M, et al. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J Clin Invest. 2014;124(6):2456–63.
Secher A, Jelsing J, Baquero AF, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124(10):4473–88.
Tzefos M, Harris K, Brackett A. Clinical efficacy and safety of once-weekly glucagon-like peptide-1 agonists in development for treatment of type 2 diabetes mellitus in adults. Ann Pharmacother. 2012;46(1):68–78.
Ross SA. Breaking down patient and physician barriers to optimize glycemic control in type 2 diabetes. Am J Med. 2013;126(9 Suppl 1):S38–48.
Gier B, Butler PC, Lai CK, et al. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab. 2012;97(1):121–31.
Nauck MA, Friedrich N. Do GLP-1-based therapies increase cancer risk? Diabetes Care. 2013;36(Suppl 2):S245–52.
Singh S, Chang HY, Richards TM, et al. Glucagonlike peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMA Intern Med. 2013;173(7):534–9.
Brodovicz KG, Kou TD, Alexander CM, et al. Impact of diabetes duration and chronic pancreatitis on the association between type 2 diabetes and pancreatic cancer risk. Diabetes Obes Metab. 2012;14(12):1123–8.
Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs: FDA and EMA assessment. N Engl J Med. 2014;370(9):794–7.
Amiel SA, Dixon T, Mann R, et al. Hypoglycaemia in type 2 diabetes. Diabet Med. 2008;25(3):245–54.
Yabe D, Seino Y. Dipeptidyl peptidase-4 inhibitors and sulfonylureas for type 2 diabetes: friend or foe? J Diabetes Investig. 2014;5(5):475–7.
Polonsky WH, Fisher L, Hessler D, et al. Patient perspectives on once-weekly medications for diabetes. Diabetes Obes Metab. 2011;13(2):144–9.
Leiter LA, Scott RA, Ye J, et al. Medication compliance rates of weekly albiglutide vs. daily oral comparators in phase III trials [abstract no. 994-P plus poster]. In: 74th Scientific Sessions of the American Diabetes Association; 2014.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the authors on the basis of scientific and editorial merit. Hannah Blair and Gillian Keating are salaried employees of Adis/Springer.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: D. S. H. Bell, Southside Endocrinology, University of Alabama, Birmingham, AL, USA; K. Kaku, Department of Internal Medicine, Kawasaki Medical School, Kurashiki, Japan; Y. Seino, Kansai Electric Power Hospital, Osaka, Japan.
Rights and permissions
About this article
Cite this article
Blair, H.A., Keating, G.M. Albiglutide: A Review of Its Use in Patients with Type 2 Diabetes Mellitus. Drugs 75, 651–663 (2015). https://doi.org/10.1007/s40265-015-0370-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0370-5