Abstract
Pembrolizumab [Keytruda® (US)], a humanized monoclonal antibody against the programmed death receptor-1 (PD-1) protein, has been developed by Merck & Co for the treatment of cancer. Pembrolizumab has received its first global approval for the treatment of advanced, unresectable or metastatic malignant melanoma in the US, for use in patients with disease progression after prior treatment with ipilimumab and, for BRAF V600 mutation-positive patients, a BRAF inhibitor. It is the first anti-PD-1 therapy to receive regulatory approval in the US, and is currently under regulatory review in the EU. This article summarizes the milestones in the development of pembrolizumab leading to this first approval for the treatment of malignant melanoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The programmed death receptor-1 (PD-1) is a surface molecule expressed on antigen-stimulated T-cells [1–4], as well as monocytes, B cells, natural killer T cells, and dendritic cells [5]. In normal tissues PD-1 acts as an immune checkpoint receptor, enabling self-tolerance by T-cells and thus preventing autoimmune reactions [2, 5]. When unbound, PD-1 allows the normal immune response by T cells to occur [2]. However, binding of PD-1 to its ligands, PD-L1 and PD-L2, suppresses the immune response by inducing downstream signalling that inhibits the proliferation of T-cells, cytokine release and cytotoxicity [1–3].
Abnormal PD-L1 expression on the surface of tumour cells, including melanoma cells, activates PD-1 and suppresses cytotoxic T cell activity; this T cell tolerance allows the tumour cells to avoid recognition and attack by the immune system [1–4, 6]. Anti-PD-1 therapies represent a novel therapeutic approach to the treatment of cancer, reactivating the immune response by blocking the interaction between PD-1 and its ligands [7].
Features and properties of pembrolizumab
Alternative names | Anti-PD-1 monoclonal antibody—Merck; humanised monoclonal IgG4 antibody against PD-1—Merck; Keytruda®; Lambrolizumab; MK 3475; MK-3475; MK3475; SCH 900475; SCH-900475; SCH900475 |
Class | Monoclonal antibodies |
Mechanism of action | PDCD 1 protein inhibitors |
Route of administration | Intravenous |
Pharmacodynamics | Inhibition of PD-1 activity reduces tumour growth in mice |
Immunogenicity | No treatment-emergent anti-pembrolizumab antibody formation detected to date |
Pharmacokinetics | Dose-dependent pharmacokinetics; t1/2 26 days, CL 0.22 L/day. AUCss for 2 mg/kg q3w dose of 0.643 g·day/L |
Adverse events | |
Most frequent | Fatigue, cough, pruritus, nausea, rash, decreased appetite, constipation, arthralgia and diarrhoea, anaemia, hyperglycaemia, hyponatraemia, hypoalbuminaemia, hypertriglyceridaemia, increased AST levels, hypocalcaemia |
Occasional | Pneumonitis; thyroid disorders |
Rare | Immune-mediated reactions |
ATC codes | |
WHO ATC code | L01X-C (monoclonal antibodies) |
EphMRA ATC code | L1X3 (antineoplastic monoclonal antibodies) |
Chemical name | Immunoglobulin G4, anti-[human protein PDCD1 (programmed cell death 1)] (human-Mus musculus monoclonal heavy chain), disulfide with human-Mus musculus monoclonal light chain, dimer |
Pembrolizumab [Keytruda® (US)] is a humanized monoclonal immunoglobulin G4 (IgG4) kappa antibody against PD-1 [8]. It received its first global approval in the US on 4 September 2014 for the treatment of unresectable or metastatic melanoma in patients with disease progression following treatment with ipilimumab and, for patients with the BRAF V600 mutation, a BRAF inhibitor [9]. Pembrolizumab is the first anti-PD-1 therapy to receive regulatory approval in the US. The US Food and Drug Administration (FDA) granted accelerated approval for pembrolizumab based on the surrogate endpoints of tumour response rate and response duration [8, 9]. The benefits of pembrolizumab with respect to disease-related symptoms and survival have yet to be established, and continued approval may depend on clinical benefit being verified and described in confirmatory clinical trials [8]. The FDA granted a breakthrough therapy designation for pembrolizumab in January 2013 after initial clinical data indicated that it could offer substantial clinical benefit compared with the treatments that were available at the time [10]. Pembrolizumab also received priority review status and an orphan drug designation in the US [9].
The approved dosage for pembrolizumab is a 2 mg/kg intravenous (IV) infusion, to be administered over 30 min every 3 weeks [8]. Prescribing information includes warnings regarding the risk of immune-mediated adverse reactions, for which treatment interruption or discontinuation may be required, and the potential risk of foetal harm if used during pregnancy.
The approval of pembrolizumab is based on the KEYNOTE-001 study (NCT01295827), an open-label phase Ib clinical trial of pembrolizumab in solid tumours in which a cohort of 173 patients with advanced melanoma refractory to ipilimumab received pembrolizumab at either the approved 2 mg/kg dose or at a higher dose of 10 mg/kg, every 3 weeks [9]. This trial included a total of 411 ipilimumab-refractory or ipilimumab-naïve patients with advanced melanoma who received pembrolizumab 2 mg/kg every 3 weeks or 10 mg/kg every 2 weeks or every 3 weeks.
Pembrolizumab is currently under review in the EU and in phase III development in other countries worldwide, with other regulatory filings expected in 2014 [11–13]. It is available through an expanded access programme in certain countries as second-line or greater therapy for patients with malignant melanoma who have unresectable or metastatic disease and limited or no treatment options.
Pembrolizumab is in phase III and phase II/III trials for non-small cell lung cancer (NSCLC) in the first-line and second-line or greater settings, respectively. It is also being investigated for the treatment of other cancers, including renal cell carcinoma (RCC), triple-negative breast cancer and other solid tumours, and haematological malignancies.
1.1 Company Agreements
Pembrolizumab is being developed by Merck & Co, which has entered into collaboration agreements with a number of other pharmaceutical companies to conduct clinical trials of pembrolizumab in combination with other anti-cancer agents.
Merck has signed an agreement with Incyte to collaborate on a phase I/II trial of pembrolizumab plus INCB 24360 in patients with previously treated recurrent and metastatic NSCLC (KEYNOTE-037; NCT02178722) [14–16]; this trial began in June 2014. Merck has entered an agreement with Advaxis for a phase I/II trial of pembrolizumab in combination with Advaxis’ ADXS PSA; the trial will be sponsored and conducted by Advaxis [17]. Merck is collaborating with Pfizer for a phase Ib trial of pembrolizumab in combination with Pfizer’s crizotinib in patients with NSCLC; this trial will be conducted by Pfizer [18]. Merck and Pfizer have also agreed to collaborate on a phase I/II trial of pembrolizumab in combination with axitinib, and a separate trial of pembrolizumab and Pfizer’s 4-1BB agonist antibody, PF-05082566 (PF2566), in patients with RCC [15, 18, 19].
Merck is collaborating with GlaxoSmithKline (GSK) on a phase I/II trial of pembrolizumab plus trametinib and dabrafenib in patients with advanced melanoma (KEYNOTE-022; NCT02130466) and a phase I/II trial of pembrolizumab plus pazopanib in treatment-naïve patients with advanced RCC (NCT02014636).
Merck also has a collaborative agreement with Amgen to conduct a multicentre open-label phase Ib/II trial of pembrolizumab plus talimogene laherparepvec in patients with mid-to-late-stage melanoma [15, 20].
2 Scientific Summary
2.1 Pharmacodynamics
Binding of anti-PD-1 monoclonal antibodies with PD-1 prevents it from binding with its ligands, thus allowing the normal immune response by T cells to tumour cells to occur [2]. PD-1 blockade may also increase the activity of natural killer cells and production of antibodies by B cells [5].
Pembrolizumab acts by blocking the binding of the PD-1 receptor to the ligands PD-L1 and PD-L2, preventing PD-1-mediated inhibition of active T-cell immune surveillance of tumours [8]. This has been shown to reduce tumour growth in mouse models.
2.2 Immunogenicity
Pembrolizumab, like other monoclonal antibody treatments, has the potential to cause immunogenicity. However, an analysis of samples from 97 patients treated with pembrolizumab 2 mg/kg every 3 weeks who had pembrolizumab concentrations that were lower than the drug tolerance limit of the anti-product antibody assay did not detect the presence of treatment-emergent anti-pembrolizumab antibodies in any patients [8].
2.3 Pharmacokinetics
Pembrolizumab doses of 2–10 mg/kg every 3 weeks demonstrated linear pharmacokinetics, with dose-proportional increases in peak concentration (Cmax), trough concentration (Cmin) and steady state area under the plasma concentration-time curve (AUCss) in a pharmacokinetic study in which 479 patients received pembrolizumab 1–10 mg/kg every 2 weeks or 2–10 mg/kg every 3 weeks [8]. According to a population pharmacokinetic analysis, pembrolizumab had a mean clearance and elimination half-life (t1/2) of 0.22 L/day and 26 days, respectively. Among patients with melanoma in the KEYNOTE-001 trial, pembrolizumab 2 mg/kg every 3 weeks had an AUCss of 0.643 g·day/L [3]. The AUCss for pembrolizumab 10 mg/kg every 3 weeks was 3.77 g·day/L.
2.3.1 Effects of Renal or Hepatic Impairment
A population pharmacokinetic study indicated that dose adjustment is not necessary for patients with mild, moderate or severe renal impairment who are receiving pembrolizumab [8]. This study showed no clinically important differences in pembrolizumab clearance in patients with renal impairment [estimated glomerular filtration rate (eGFR) 15–89 mL/min/1.73 m2; n = 255] compared with those with normal renal function (eGFR ≥90 mL/min/1.73 m2; n = 221).
Dose adjustment is also unnecessary for patients with mild hepatic impairment, defined as either total bilirubin levels at or below the upper limit of normal (ULN) with aspartate aminotransferase (AST) levels higher than ULN or total bilirubin >1–1.5 times ULN and any AST level [8]. In the population pharmacokinetic analysis there were no clinically important differences in pembrolizumab clearance between patients with mild hepatic impairment (n = 59) and those with normal hepatic function (total bilirubin and AST at or below ULN; n = 410). Data are not available for patients with moderate or severe hepatic impairment.
2.4 Therapeutic Trials
2.4.1 Melanoma
Pembrolizumab was associated with a high rate of tumour regression in patients with advanced melanoma who were enrolled in the phase I KEYNOTE-001 trial (NCT01295827) [3, 21]. In this open-label trial, which enrolled a total of 1,137 patients with melanoma, NSCLC or other carcinomas, patients received IV pembrolizumab 10 mg/kg every 2 or 3 weeks, or 2 mg/kg every 3 weeks and were assessed for tumour response every 12 weeks.
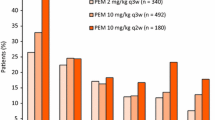
Among patients with melanoma who were enrolled in the non-randomized cohorts (n = 135), the confirmed objective response rate (ORR) according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 was 38 % in the full analysis set (FAS) [21]. Response durations ranged from 1.9 to 10.8 months at the time of the analysis, and the median response duration had not yet been reached after a median follow-up period of 11 months. The ORR was highest among patients receiving pembrolizumab 10 mg/kg every 2 weeks (52 %), although this cohort also had the highest incidence of treatment-related adverse events. Among patients receiving pembrolizumab 2 mg/kg every 3 weeks who had no prior ipilimumab treatment (n = 20), the confirmed ORR was 25 %. Response rates were similar for patients who had experienced disease progression during prior ipilimumab therapy and those who had not (38 vs. 37 %). The overall median progression-free survival (PFS) duration was >7 months and at the time of analysis 41 of 52 (81 %) patients who had responses continued to receive pembrolizumab. Although the majority of responses occurred within the first 12 weeks of treatment, some patients had delayed responses that appeared to be consistent with immune-related responses. When assessed according to immune-related response criteria (irRC), ORRs were 37 % for the whole cohort, and 56, 27 and 14 %, respectively, for the groups receiving 10 mg/kg every 2 weeks, 10 mg/kg every 3 weeks and 2 mg/kg every 3 weeks.
Key clinical trials of pembrolizumab
Drugs(s) | Indication | Phase | Status | Location(s) | Identifier | Company |
|---|---|---|---|---|---|---|
Pembrolizumab, ipilimumab | Melanoma | III | Enrolment completed | Multinational | NCT01866319; EudraCT2012-004907-10; MK-3475-006; KEYNOTE-006 | Merck |
Pembrolizumab, carboplatin, paclitaxel, dacarbazine, temozolomide | Melanoma | II | Enrolment completed | US? | NCT01704287; P08719; KEYNOTE-002 | Merck |
Pembrolizumab | Melanoma, NSCLC, other carcinomas | I | Enrolment completed | Multinational | NCT01295827; EudraCT2011-002371-42; P07990; MK-3475-001; KEYNOTE-001 | Merck |
Pembrolizumab, carboplatin, paclitaxel, cisplatin, pemetrexed, gemcitabine | NSCLC | III | Recruiting | Multinational | NCT02142738; EudraCT2014-000323-25; MK-3475-024; KEYNOTE-024 | Merck |
Pembrolizumab, docetaxel | NSCLC | II/III | Recruiting | Multinational | NCT01905657; EudraCT2012-0021391-19; MK3475-010; KEYNOTE-010 | Merck |
Pembrolizumab | Melanoma, NSLC (brain metastases) | II | Recruiting | US | NCT02085070; 1401013290; IND 121 564 | Yale University |
Pembrolizumab | MSI + tumours (including colorectal cancer) and MSI– colorectal cancer | II | Recruiting | US | NCT01876511; J1365; MK3475-016; NA_00085756 | Sidney Kimmel Comprehensive Cancer Center; Merck |
Pembrolizumab, paclitaxel, carboplatin, pemetrexed, bevacizumab, ipilimumab, erlotinib, gefitinib | NSCLC | I/II | Recruiting | US, Taiwan | NCT02039674; MK3475-021; KEYNOTE-021 | Merck |
Pembrolizumab, trametinib, dabrafenib | Melanoma | I/II | Recruiting | US | NCT02130466; MK-3475-022; KEYNOTE-022 | Merck, Glaxo Wellcome |
Pembrolizumab | Haematological cancers | Ib | Recruiting | Multinational | NCT01953692; EudraCT2013-001603-37; MK-3475-013; KEYNOTE-013 | Merck |
Pembrolizumab, cisplatin, pemetrexed, carboplatin, paclitaxel | NSCLC, solid tumours | I | Recruiting | Japan | NCT01840568; MK-3475-011; KEYNOTE 011 | Merck |
Pembrolizumab | Solid tumours | Ib | Recruiting | Multinational | NCT02054806; EudraCT2013-004507-39; MK-3475-927; KEYNOTE-028 | Merck |
Pembrolizumab | Solid tumours | I | Recruiting | Multinational | NCT01848834; EudraCT2012-005771-14; MK-3475-012; KEYNOTE-012 | Merck |
Pembrolizumab, ipilimumab, PegIFN = 2b | Melanoma, RCC | I/II | Recruiting | Multinational | NCT02220894; EudraCT2013-004072-36; MK-3475-029; KEYNOTE-29 | Merck |
Pembrolizumab, lenalidomide, dexamethasone | Multiple myeloma | I | Recruiting | Multinational | NCT02036502; EudraCT2014-003512-44; MK3475-023; KEYNOTE-023 | Merck, Celgene |
Pembrolizumab | NSCLC | I | Recruiting | US | NCT02007070; MK-3475-025; KEYNOTE-025 | Merck |
Pembrolizumab | Melanoma | I | Recruiting | Japan | NCT02180061; MK3475-041) | Merck |
Pembrolizumab, PF-05082566 | Solid tumours | I | Recruiting | US | NCT02179918; B1641003; KEYNOTE-0036 | Pfizer, Merck |
Pembrolizumab, INCB 24360 | NSCLC, solid tumours | I/II | Recruiting | US | NCT02178722; INCB 24360-202; KEYNOTE-037 | Merck, Incyte |
Pembrolizumab, pazopanib | RCC | I/II | Recruiting | US, UK | NCT02014636; 200249 | Merck, GlaxoSmithKline |
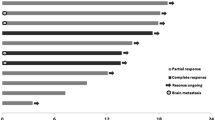
Updated data from the non-randomized cohorts (n = 135) after ≥13 months of follow-up showed that pembrolizumab had durable effects [22]. Among patients with measurable disease who were included in the efficacy analysis according central review, the ORR by RECIST was 41 %. Some late responses were observed, with objective responses and conversions to complete response occurring as late as 64 and 72 weeks, respectively. The median response duration had not been reached at the time of the analysis, but 87 % of responses were ongoing. The median overall survival duration was also not reached, but median PFS was 31 weeks. The 1-year overall survival rate was 81 %. Among patients with evaluable tumour PD-L1 expression (n = 71), expression of PD-L1 was associated with improved RECIST ORR (51 vs. 6 %; p = 0.0012) and median PFS duration (12 vs. 3 months; p = 0.0004) compared with non-expression; however, there was no difference in 1-year overall survival (84 vs. 69 %).
In a randomized expansion cohort of KEYNOTE-001, 178 patients who had progressive, measurable unresectable advanced melanoma and had received two or more doses of ipilimumab ≥3 mg/kg every 3 weeks were randomized to receive pembrolizumab 2 mg/kg (n = 91) or 10 mg/kg (n = 87) every 3 weeks [3]. The as-treated population (n = 173) included 89 patients receiving pembrolizumab 2 mg/kg and 84 receiving the 10 mg/kg dose, while the FAS included 81 and 76 patients receiving pembrolizumab 2 and 10 mg/kg, respectively. One complete response and 20 partial responses were observed among the 89 patients in the as-treated population who received pembrolizumab 2 mg/kg, giving an ORR of 24 % [8]. At the time of the analysis, responses were ongoing in 18 patients (86 %); response durations ranged from 1.4+ to 8.5+ months (≥6 months for eight patients). Three patients had disease progression after initial responses lasting 2.8, 2.9 and 8.2 months, respectively. Another patient had two new lesions at the first assessment but a 75 % decrease in overall tumour burden that was maintained for 5+ months with continued pembrolizumab treatment.
An analysis of FAS for this cohort (median follow-up 8 months, minimum 6 months) showed that, among the 157 patients assessed by RECIST, objective responses occurred in 41 patients (26 %) and disease stabilization in 38 patients (24 %) [3]. ORRs for the pembrolizumab 2 mg/kg and 10 mg/kg groups were both 26 %; disease control rates in the two cohorts were 51 and 50 %, respectively. The median time to RECIST response in the 2 mg/kg and 10 mg/kg groups was 12 weeks. While most responses occurred by week 12, responses were seen after as long as 36 weeks of treatment. The median PFS durations for the pembrolizumab 2 and 10 mg/kg groups were 22 and 14 weeks, respectively; estimated Kaplan–Meier PFS rates at 24 weeks were 45 and 37 %. There was no significant difference between cohorts for either the ORR or disease control rate when assessed according to irRC, with ORRs for pembrolizumab 2 and 10 mg/kg of 27 and 32 %, respectively; disease control rates in these groups were 62 and 64 %. Median times to response for the pembrolizumab 2 and 10 mg/kg groups were both 12 weeks. Median PFS durations were 31 and 35 weeks, respectively; for both doses the Kaplan–Meier estimate of PFS at 24 weeks was 57 %. Kaplan-Meier estimates of overall survival at 1 year were 58 % for pembrolizumab 2 mg/kg and 63 % for pembrolizumab 10 mg/kg.
Comparison of ipilimumab-naïve (n = 103) and ipilimumab-resistant (n = 173) cohorts in KEYNOTE-001 showed that pembrolizumab had efficacy in both cohorts, with slightly higher response rates in ipilimumab-naïve patients [23]. After a minimum of 9 months’ follow-up, ORRs for pembrolizumab 2 and 10 mg/kg were 33 and 40 % for ipilimumab-naïve patients and 26 and 26 %, respectively, for ipilimumab-resistant patients. Response durations ranged from 6+ weeks to 39+ weeks at the time of follow-up (median durations not yet reached), and most patients were continuing to respond (~90 %). PFS rates for pembrolizumab 2 and 10 mg/kg at week 24 were 51 and 48 % for ipilimumab-naïve patients and 45 and 37 %, respectively, for ipilimumab-resistant patients.
According to exploratory analyses in KEYNOTE-001, response rates were generally similar across major subgroups apart from the comparison between the BRAF-mutant and BRAF wild-type subgroups [3]. The ORR was numerically lower in the BRAF-mutant subgroup, all of whom had received previous BRAF or MEK inhibitor therapy (n = 26), compared with those in the BRAF wild-type subgroup (n = 131), although the 95% CIs for these subgroups overlapped [19 % (95 % CI 7–39) vs. 28 % (95 % CI 20–36)].
A pooled analysis of 411 patients treated in KEYNOTE-001 found that among patients with measurable disease at baseline (n = 365) ORRs for pembrolizumab in ipilimumab-naïve and ipilimumab-experienced patients were 40 and 28 %, respectively [24]. The median PFS durations according to RECIST were 24 and 23 weeks for ipilimumab-naïve and -experienced patients, respectively. The median overall survival duration had not been reached in either subgroup at the time of the analysis. One-year overall survival rates for the overall population and patients with measurable disease at baseline were 71 and 69 %, respectively [25]. Univariate analysis showed that factors associated with worse 1-year survival rates were elevated lactate dehydrogenase level, baseline tumour size >90 mm, M-stage 1c, and ECOG performance status of 1 (vs. 0). Although patients with baseline tumour size >90 mm had a 1-year overall survival rate of 54.8 % (vs 84.3 % for patients with smaller tumours), they had median overall survival duration with pembrolizumab of 14 months, suggesting that treatment had beneficial effects.
Another analysis of the 411 patients in the three melanoma cohorts showed unique response patterns in patients treated with pembrolizumab [26]. Of the 192 patients who received pembrolizumab for >28 weeks at the time of the analysis, seven (3.6 %) had tumour flare. Best overall responses in these seven patients, according to immune-related response criteria, were complete response in one patient, partial response in four and stable disease in two. Six patients had an atypical delayed response. Overall survival for 51 patients with progressive disease according to RECIST, but complete or partial response or stable disease when assessed by irRC, was favourable when compared with the 145 patients who had disease progression according to both RECIST and irRC.
2.4.2 NSCLC
Confirmed or unconfirmed responses occurred in more than one-third of patients with locally advanced or metastatic NSCLC treated with pembrolizumab as first-line therapy in the KEYNOTE-001 study [27]. A cohort of 45 patients who had tumours expressing PD-1 and had not received systemic treatment for metastatic disease received pembrolizumab 10 mg/kg every 2 weeks (n = 16), 10 mg/kg every 3 weeks (n = 23) or 2 mg/kg every 3 weeks (n = 6). The ORRs (confirmed and unconfirmed) according to irRC were 36 % in the overall cohort, 35 % for patients receiving pembrolizumab 10 mg/kg every 2 weeks, 27 % for the 10 mg/kg every 3 weeks group, and 67 % for the group receiving 2 mg/kg every 3 weeks. At the time of this preliminary analysis, 25 patients remained on treatment, with treatment durations ranging from 12+ to 48+ weeks.
Pembrolizumab also demonstrated activity in the cohort of 221 patients with previously treated, progressive, advanced or metastatic NSCLC enrolled in KEYNOTE-001 [28]. Patients with detectable PD-L1 expression were randomized to receive pembrolizumab 10 mg/kg every 2 weeks (n = 59) or every 3 weeks (n = 119), while patients whose tumours did not express PD-L1 but who had received ≥2 previous treatments (n = 43) all received pembrolizumab 10 mg/kg every 2 weeks. Preliminary data showed the irRC and RECIST ORRs (both confirmed and unconfirmed) were 15 and 21 %, respectively in the overall cohort, 15 and 24 % for patients with PD-L1-positive tumours and 10 and 8 % for patients with PD-L1-negative tumours; ORRs (irRC/RECIST) for 2-weekly and 3-weekly pembrolizumab were 19/31 and 15/22 %, respectively.
2.5 Adverse Events
Prescribing information for pembrolizumab contains warnings and precautions regarding the risk of immune-mediated adverse events [8]. Treatment with pembrolizumab should either be withheld or discontinued permanently, depending on the severity of these events, and corticosteroids should be administered according to the severity of the reaction.
Immune-mediated adverse events that have been reported in patients treated with pembrolizumab include pneumonitis, colitis, hepatitis, hypophysitis, nephritis, hyperthyroidism and hypothyroidism [8]. Among the 411 patients with melanoma enrolled in the KEYNOTE-001 trial, these events occurred in <10 % of patients: pneumonitis (2.9 %; grade 3 in 0.2 %), colitis (1 %; grade 3 in 0.5 %), hepatitis (0.5 %; grade 4 in 0.2 %), hypophysitis (0.5 %; grade 4 in 0.2 %), nephritis (0.7 %; including grade 3 or 4 interstitial nephritis with renal failure in 0.5 %), hyperthyroidism (1.2 %; grade 3 in 0.2 %) and hypothyroidism (8.3 %; grade 3 in 0.2 %).
The most common adverse events among 89 patients with unresectable or metastatic melanoma receiving pembrolizumab 2 mg/kg every 3 weeks in the expansion cohort of the KEYNOTE-001 trial were fatigue (47 % of patients, any grade), cough (30 %), pruritus (30 %), nausea (30 %), rash (29 %), decreased appetite (26 %), constipation (21 %), arthralgia (20 %) and diarrhoea (20 %) [8]. The majority of these events were mild or moderate in intensity, with the only grade 3–4 events being fatigue (7 %), anaemia (5 %), dyspnoea (2 %) and peripheral oedema, cough, pain in the extremities, myalgia, back pain and upper respiratory tract infection (1 % each). There were no grade 5 adverse events. Adverse events led to treatment discontinuation in 6 % of patients. Laboratory abnormalities occurring in ≥20 % of patients receiving pembrolizumab 2 mg/kg in the study were anaemia (55 %), hyperglycaemia (40 %), hyponatraemia (35 %), hypoalbuminaemia (34 %), hypertriglyceridaemia (25 %), increased AST levels (24 %) and hypocalcaemia (24 %). In most cases these events were mild-to-moderate in intensity, but grade 4 anaemia, AST elevation and hyperglycaemia occurred in one patient each.
2.6 Ongoing Clinical Trials
Clinical trials of pembrolizumab are ongoing in a number of cancer indications.
Follow-up is ongoing in three studies:
-
KEYNOTE-006, a randomized, open-label phase III trial comparing pembrolizumab with ipilimumab in 645 patients with unresectable or metastatic melanoma (NCT01866319) [29];
-
KEYNOTE-002, a randomized, double-blind phase II trial comparing pembrolizumab with chemotherapy in 510 patients with advanced melanoma (NCT01704287) [30];
-
KEYNOTE-001, a phase I trial of pembrolizumab in 1,137 patients with progressive, locally advanced or metastatic NSCLC, melanoma or other type of carcinoma (NCT01295827) [31]; and
A number of studies in melanoma or NSCLC are currently recruiting patients:
-
KEYNOTE-010, a multinational, randomized, open-label phase II/III trial comparing pembrolizumab with docetaxel as second-line therapy in approximately 920 previously-treated patients with NSCLC (NCT01905657) [32, 33];
-
KEYNOTE-021, a randomized, open-label phase I/II trial of pembrolizumab in combination with chemotherapy or immunotherapy in 320 patients with advanced or metastatic NSCLC (NCT02039674) [34];
-
KEYNOTE-22, a randomized, double-blind phase I/II study of pembrolizumab in combination with trametinib and dabrafenib in 204 patients with advanced melanoma (NCT02130466) [35];
-
KEYNOTE-024, a multinational, randomized, open-label phase III trial of pembrolizumab versus platinum-based chemotherapy as first-line therapy in 300 patients with metastatic NSCLC (NCT012142738) [36];
-
KEYNOTE-025, a single-arm, open-label phase I trial of pembrolizumab in 24 patients with advanced NSCLC (NCT02007070) [37];
-
KEYNOTE-29, a randomised, open-label phase I/II trial comparing pembrolizumab alone or in combination with either Peg-interferon-2b or ipilimumab in 343 patients with melanoma or RCC (NCT02220894) [38];
-
KEYNOTE-037, a phase I/II trial of pembrolizumab plus INCB 24360 in patients with previously treated recurrent and metastatic NSCLC or select solid tumours (NCT02178722) [39];
-
A phase II trial of pembrolizumab in patients with melanoma or NSCLC who have brain metastases is being conducted by Yale University (NCT02085070) [40].
In addition, KEYNOTE-042, an open-label phase III study comparing pembrolizumab with standard platinum-based chemotherapy as first-line treatment for patients with PD-L1-positive advanced or metastatic NSCLC (NCT02220894) [41] has been registered but is yet to begin recruiting.
Numerous phase I and II trials (including KEYNOTE-011, KEYNOTE-012, KEYNOTE-013, KEYNOTE-023, KEYNOTE-28, KEYNOTE-31 and KEYNOTE-0036) evaluating pembrolizumab monotherapy or combination therapy in solid tumours or haematological malignancies are also recruiting or are registered but yet to recruit.
3 Current Status
Pembrolizumab received its first global approval on 4 September 2014 for the treatment of unresectable or metastatic melanoma in patients with disease progression following treatment with ipilimumab and, for BRAF V600 mutation-positive patients, a BRAF inhibitor.
4 Disclosure
The preparation of this report was not supported by any external funding. During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. R.M. Poole is a contracted employee of Adis, Springer SBM.
References
Ascierto PA, Kalos M, Schaer DA, et al. Biomarkers for immunostimulatory monoclonal antibodies in combination strategies for melanoma and other tumor types. Clin Cancer Res. 2013;19(5):1009–20.
Mamalis A, Garcha M, Jagdeo J. Targeting the PD-1 pathway: a promising future for the treatment of melanoma. Archiv Dermatol Res (Archiv fur Dermatologische Forschung). 2014;306(6):511–9.
Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109–17.
Karimkhani C, Gonzalez R, Dellavalle RP. A review of novel therapies for melanoma. Am J Clin Dermatol. 2014;15(4):323–37.
Tang PA, Heng DYC. Programmed death 1 pathway inhibition in metastatic renal cell cancer and prostate cancer. Curr Oncol Rep. 2013;15(2):98–104.
Creelan BC. Update on immune checkpoint inhibitors in lung cancer. Cancer Control. 2014;21(1):80–9.
Johnson DB, Rioth MJ, Horn L. Immune Checkpoint Inhibitors in NSCLC. Curr Treat Options Oncol. 2014;. doi:10.1007/s11864-014-0305-5.
Merck & Co. KEYTRUDA (Pembrolizumab) Prescribing Information. 2014. http://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf. Accessed 17 Sept 2014.
U.S. Food and Drug Administration. FDA approves Keytruda for advanced melanoma [media release]. 4 Sept 2014. www.fda.gov.
Merck & Co. Merck announces breakthrough therapy designation for lambrolizumab an investigational antibody therapy for advanced melanoma [media release]. 24 April 2013. www.merck.com.
Merck & Co. MSDs investigational anti-PD-1 antibody, pembrolizumab, under regulatory review in europe for the treatment of advanced melanoma [media release]. 30 June 2014. www.merck.com.
Merck & Co. Merck announces FDA acceptance for review of MK-3475 biologics license application for advanced melanoma [media release]. 6 May 2014. www.merck.com.
Merck & Co. Merck initiates rolling submission of U.S. biologics license application for MK-3475, an investigational anti-PD-1 immunotherapy, in patients with advanced melanoma [media release]. 12 Jan 2014. www.merck.com.
Incyte Corporation. Incyte announces clinical trial collaboration with merck to evaluate combination of two novel immunotherapies [media release]. 5 Feb 2014. www.incyte.com.
Merck & Co. Merck enters strategic collaborations with Amgen, Incyte and Pfizer to evaluate novel combination anti-cancer regimens with MK-3475 [media release]. 5 Feb 2014. www.merck.com.
Incyte Corporation. Incyte reports 2014 first-quarter financial results and updates shareholders on key clinical programs [media release]. 1 May 2014. www.incyte.com.
Advaxis. Advaxis and Merck form collaboration to evaluate investigational combination of two novel immunotherapy candidates for advanced prostate cancer [media release]. 25 Aug 2014. www.advaxis.com.
Pfizer. Pfizer and Merck to collaborate on study evaluating novel anti-cancer combination regimen [media release]. 26 Aug 2014. www.pfizer.com.
Pfizer. Pfizer and Merck to collaborate on innovative anti-cancer combination studies. [media release]. 5 Feb 2014. www.pfizer.com.
Amgen, Merck. Amgen and Merck announce collaboration to evaluate investigational combination treatment for advanced melanoma. [media release]. 5 Feb 2014. www.amgen.com.
Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–44.
Kefford R, Ribas A, Hamid O, et al. Clinical efficacy and correlation with tumor PD-L1 expression in patients (pts) with melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475 [abstract no. 3005]. In: 50th Annual Meeting of the American Society of Clinical Oncology: 2014; Chicago, IL.
Hamid O, Robert C, Ribas A, et al. Randomized comparison of two doses of the anti-PD-1 monoclonal antibody MK-3475 for ipilimumab-refractory (IPI-R) and IPI-naive (IPI-N) melanoma (MEL) [abstract no. 3000]. In: 50th Annual Meeting of the American Society of Clinical Oncology: 2014; Chicago, IL.
Ribas A, Hodi FS, Kefford R, et al. Efficacy and safety of the anti-PD-1 monoclonal antibody mk-3475 in 411 patients (PTS) with melanoma (MEL). J Clin Oncol Conf. 2014;32(18 Suppl 1):LBA9000.
Joseph RW, Elassaiss-Schaap J, Wolchok JD, et al. Baseline tumor size as an independent prognostic factor for overall survival in patients with metastatic melanoma treated with the anti-PD-1 monoclonal antibody MK-3475. J Clin Oncol Conf. 2014;32(15 Suppl 1):3015.
Hodi FS, Ribas A, Daud A, et al. Evaluation of immune-related response criteria (irRC) in patients (pts) with advanced melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475. J Clin Oncol Conf. 2014;32(15 Suppl 1):3006.
Rizvi NA, Garon EB, Patnaik A, et al. Safety and clinical activity of MK-3475 as initial therapy in patients with advanced non-small cell lung cancer (NSCLC) [abstract no. 8007]. In: 50th Annual Meeting of the American Society of Clinical Oncology: 2014; Chicago, IL.
Garon EB, Leighl NB, Rizvi NA, et al. Safety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer (NSCLC) [abstract no. 8020]. In: 50th Annual Meeting of the American Society of Clinical Oncology: 2014; Chicago, IL.
Merck Sharp & Dohme Corp. Study to Evaluate the Safety and Efficacy of Two Different Dosing Schedules of Pembrolizumab (MK-3475) Compared to Ipilimumab in Participants With Advanced Melanoma (MK-3475-006/KEYNOTE-006) [ClinicalTrials.gov Identifier: NCT01866319]; US National Institutes of Health, ClinicalTrials.gov. 2013. http://clinicaltrials.gov/ct2/show/NCT01866319?term=pembrolizumab&rank=11. Accessed 17 Sept 2014.
Merck Sharp & Dohme Corp. Study of Pembrolizumab (MK-3475) Versus Chemotherapy in Participants With Advanced Melanoma (P08719/KEYNOTE-002) [ClinicalTrials.gov Identifier: NCT01704287] US National Institutes of Health, ClinicalTrials.gov. 2012. http://clinicaltrials.gov/ct2/show/NCT01704287?term=pembrolizumab&rank=9. Accessed 17 Sept 2014.
Merck Sharp & Dohme Corp. Study of Pembrolizumab (MK-3475) in Participants With Progressive Locally Advanced or Metastatic Carcinoma, Melanoma, or Non-small Cell Lung Carcinoma (P07990/MK-3475-001/KEYNOTE-001) [ClinicalTrials.gov Identifier: NCT01295827] US National Institutes of Health, ClinicalTrials.gov. 2011. http://clinicaltrials.gov/ct2/show/NCT01295827?term=pembrolizumab&rank=2. Accessed 17 Sept 2014.
Merck Sharp & Dohme Corp. Study of Two Doses of MK-3475 (Pembrolizumab) Versus Docetaxel in Previously-Treated Participants With Non-Small Cell Lung Cancer (MK-3475-010/KEYNOTE-010) [ClinicalTrials.gov Identifier: NCT01905657] US National Institutes of Health, ClinicalTrials.gov. 2013. http://clinicaltrials.gov/ct2/show/NCT01905657?term=pembrolizumab&rank=8. Accessed 17 Sept 2014.
Herbst RS, Gurpide A, Surmont V, et al. A phase II/III randomized trial of two doses of MK-3475 versus docetaxel in previously treated subjects with non-small cell lung cancer [abstract no. TPS8124]. In: 50th Annual Meeting of the American Society of Clinical Oncology: 2014; Chicago, IL.
Merck Sharp & Dohme Corp. A Study of Pembrolizumab (MK-3475) in Combination With Chemotherapy or Immunotherapy in Participants With Lung Cancer (MK-3475-021/KEYNOTE-021) [ClinicalTrials.gov Identifier: NCT02039674] US National Institutes of Health, ClinicalTrials.gov. 2014. http://clinicaltrials.gov/ct2/show/NCT02039674?term=pembrolizumab&rank=7. Accessed 17 Sept 2014.
Merck Sharp & Dohme Corp. A Study of the Safety and Efficacy of Pembrolizumab (MK-3475) in Combination With Trametinib and Dabrafenib in Participants With Advanced Melanoma (MK-3475-022) [ClinicalTrials.gov Identifier: NCT02130466] US National Institutes of Health, ClinicalTrials.gov. 2014. http://clinicaltrials.gov/ct2/show/NCT02130466?term=pembrolizumab&rank=16. Accessed 17 Sept 2014.
Merck Sharp & Dohme Corp. Study of Pembrolizumab (MK-3475) Compared to Platinum-Based Chemotherapies in Participants With Metastatic Non-Small Cell Lung Cancer (MK-3475-024/KEYNOTE-024) [ClinicalTrials.gov Identifier: NCT02142738] US National Intitutes of Health, ClinicalTrials.gov. 2014. http://clinicaltrials.gov/ct2/show/NCT02142738?term=pembrolizumab&rank=4. Accessed 17 Sept 2014.
Merck Sharp & Dohme Corp. Study of Pembrolizumab (MK-3475) in Participants With Advanced Non-small Cell Lung Cancer (MK-3475-025/KEYNOTE-025) [ClinicalTrials.gov Identifier: NCT02007070] US National Institutes of Health, ClinicalTrials.gov. 2013. http://clinicaltrials.gov/ct2/show/NCT02007070?term=pembrolizumab&rank=17. Accessed 17 Sept 2014.
Merck Sharp & Dohme Corp. Safety and Tolerability of Pembrolizumab (MK-3475) + Pegylated Interferon Alfa-2b and Pembrolizumab + Ipilimumab in Participants With Advanced Melanoma or Renal Cell Carcinoma (MK-3475-029/KEYNOTE-29) [ClinicalTrials.gov identifier NCT02220894]; US National Institutes of Health, ClinicalTrials.gov. 2014. http://clinicaltrials.gov/ct2/show/NCT02089685?term=pembrolizumab&rank=10. Accessed 17 Sept 2014.
Incyte Corporation. A Phase 1/2 Study Exploring the Safety, Tolerability, and Efficacy of MK-3475 in Combination With INCB024360 in Subjects With Selected Solid Tumors and Advanced NSCLC (INCB 24360-202 / MK-3475-037 / KEYNOTE-037) [ClinicalTrials.gov Identifier: NCT02178722] US National Institutes of Health, ClinicalTrials.gov. 2014. http://clinicaltrials.gov/ct2/show/NCT02178722?term=NCT02178722&rank=1. Accessed 18 Sept 2014.
Yale University. MK-3475 in Melanoma and NSCLC Patients With Brain Metastases [ClinicalTrials.gov Identifier: NCT02085070] US National Institutes of Health, ClinicalTrials.gov. 2014. http://clinicaltrials.gov/ct2/show/NCT02085070?term=mk-3475&rank=4. Accessed 18 Sept 2014.
Merck Sharp & Dohme Corp. Study of MK-3475 (Pembrolizumab) Versus Platinum-based Chemotherapy for Participants With PD-L1-positive Advanced or Metastatic Non-small Cell Lung Cancer (MK-3475-042/KEYNOTE-042) [ClinicalTrials.gov Identifier: NCT02220894] US National Institutes of Health, ClinicalTrials.gov. 2014. http://clinicaltrials.gov/ct2/show/NCT02220894?term=pembrolizumab&rank=3. Accessed 17 Sept 2014.
Author information
Authors and Affiliations
Corresponding author
Additional information
This profile has been extracted and modified from the Adis R&D Insight drug pipeline database. Adis R&D Insight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch.
Rights and permissions
About this article
Cite this article
Poole, R.M. Pembrolizumab: First Global Approval. Drugs 74, 1973–1981 (2014). https://doi.org/10.1007/s40265-014-0314-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-014-0314-5