Abstract
Asthma is a common disease with a complex pathophysiology. It can present in various clinical forms and with different levels of severity. Unbiased cluster analytic methods have unravelled several phenotypes in cohorts representative of the whole spectrum of severity. Clusters of severe asthma include those on high-dose corticosteroid treatment, often with both inhaled and oral treatment, usually associated with severe airflow obstruction. Phenotypes with concordance between symptoms and sputum eosinophilia have been reported, including an eosinophilic inflammation-predominant group with few symptoms and late-onset disease who have a high prevalence of rhinosinusitis, aspirin sensitivity, and exacerbations. Sputum eosinophilia is also a biomarker that can predict therapeutic responses to antibody-based treatments to block the effects of the T-helper (Th)-2 cytokine, interleukin (IL)-5. Low Th2-expression has been predictive of poor therapeutic response to inhaled corticosteroid therapy. Current asthma schedules emphasise a step-up approach to treating asthma in relation to increasing severity, but, in more severe disease, phenotyping or endotyping of asthma will be necessary to determine new treatment strategies as severe asthma is recognized as being a particularly heterogeneous disease. Much less is known about ‘non-eosinophilic’ asthma. Phenotypic characterisation of corticosteroid insensitivity and chronic airflow obstruction of severe asthma is also needed. Phenotype-driven treatment of asthma will be further boosted by the advent of transcriptomic and proteomic technologies, with the application of systems biology or medicine approaches to defining phenotypes and biomarkers of disease and therapeutic response. This will pave the way towards personalized medicine and healthcare for asthma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Unbiased approaches to classifying asthma will continue to lead to the identification of distinct phenotypes linked to known mechanisms. A ‘Th2-high’ identifies patients with high eosinophilia and good therapeutic response to corticosteroids. |
Other characteristic traits of severe asthma include non-eosinophilic asthma, corticosteroid insensitivity, obesity-associated and exacerbation-prone, and these need to be linked to mechanisms. |
Newer treatments for asthma will emerge from better endotyping, and will be targeted to specific phenotypes. This will lead to a future world of personalized medicine in asthma. |
1 Introduction
Asthma is a complex disease. Although the basis of asthma remains uncertain, it should be considered as a disease with multifactorial components that can present in different ways. Clinicians have long been aware of the varied presentation of asthma and of the differences in responsiveness to currently available treatments. However, despite this recognition, the treatment approaches for asthma have been uniformly applied irrespective of ‘type’ of asthma. First, the guidelines for asthma management that were first widely disseminated in the 1990s have focused on a uniform step-wise escalation of treatments (mostly inhaled corticosteroids and β-adrenergic bronchodilators) that were deemed to be mostly effective in all patients with asthma irrespective of type or etiology, and the stepwise approach was mainly aimed at controlling severity of disease with the lowest amount of medications. Second, attempts at phenotyping asthma have been limited to descriptive impressions of groups of patients into categories that only relate to a small proportion of asthmatic patients [1].
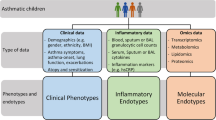
The realization that there was a core of patients with asthma that did not respond to existing therapies, classified as severe asthma [2, 3] with diverse presentations, has driven interest in using unbiased approaches to phenotype the disease, initially in terms of essential clinical and physiologic features. Increasingly, inflammatory markers are being used with the aim of defining and understanding the pathophysiological mechanisms underlying each phenotype (with the hypothesis that each phenotype is different in terms of its underlying pathophysiological mechanisms). This approach of defining mechanistic phenotypes has been termed endotyping [4, 5], and it will be most useful at defining targets for the development of new therapies and treatments for well-defined phenotypes or endotypes of asthma.
This article reviews recent published work in terms of unbiased approaches to phenotyping asthma and emphasizes how the phenotyping exercise is an important step to formalizing new treatment paradigms for asthma, particularly severe asthma where a recognized unmet need is the discovery and use of new effective treatments.
2 Defining Phenotypes of Asthma by Cluster Analysis
The application of unbiased statistical approaches has led to the definition of several phenotypes of asthma and represents a major advance of the last few years. Cluster analysis is a statistical approach in which data objects based only on information found in the data that describe the objects and their relationships are grouped in such a way that objects in the same group are more similar to each other than to those in other groups. Models that have been most commonly used for asthma phenotyping have included the hierarchical clustering builds models based on distance connectivity, and the k-means algorithm representing each cluster by a single mean vector. A recent study warned that the use of different unsupervised statistical methods and different variable sets and encoding can lead to multiple and inconsistent subtypes of asthma [6], and has advocated that a more careful selection of markers should be used that would be consistent across all cohorts analyzed. However, there has now been some degree of concordance between the results of several cluster analyses that have been published so far.
The Severe Asthma Research Program (SARP) adult and pediatric cohorts [7, 8], and the UK Leicester adult cohort [9] have used hierarchical cluster analysis while the European Community Respiratory Health Survey (ECRHS) and Epidemiological Study of the Genetics and Environment of Asthma (EGEA) European cohorts [10] have chosen a model-based clustering analysis to define clusters of asthma using cohorts that express a range of asthma severities from mild to severe. Despite differences in clinical variables used for analysis, these studies report phenotypes that have common, although not entirely similar, features (Table 1). Such analyses have identified patients with little airflow obstruction and activity of disease, patients with early age of onset of disease with an atopic background, and a more severe group of asthma patients associated with adult-onset disease and active disease. Thus, age of onset of disease, lung function, and atopic state featured highly in these clusters or phenotypes. Such clusters have been also reported from cohorts in Korea and Japan [11, 12]. An analysis of adult asthmatics attending a hospital-based asthma clinic in New York reported clusters that were qualitatively similar to those described for SARP [13], as did an analysis of childhood asthma clusters in the US Childhood Asthma Research and Education (CARE) network clinical trials [14]. In the Outcomes and Treatment Regimens (TENOR) cluster analysis, five clusters distinguished by sex, atopic status, and non-White race were reported in an adolescent and adult cohort and in a pediatric cohort, but, while passive smoke exposure was a distinguishing feature in children, it was aspirin sensitivity in the adolescent and adult cohort [15].
Clearly, there were clusters that related to more severe disease. For example, clusters 4 and 5 of the adult SARP cohort described patients on high-dose inhaled corticosteroid therapy, often taken together with oral corticosteroid treatment, usually associated with severe airflow obstruction. In a small analysis of refractory asthma in Korean patients [16], four clusters were described, with three of the four closely resembling clusters 4 and 5 of SARP. The Korean cluster 4 consisted predominantly of male cigarette smokers, representing the influence of cigarette smoking on increasing asthma severity. In the TENOR study, the fifth cluster, described in adolescents and adults, was associated with aspirin sensitivity, in primarily White, female, and atopic patients with late-onset asthma, and these patients were more likely than any patient in the other four clusters to experience exacerbations [15]. The CARE Network reported replication of asthma clusters reported by the SARP study in children [8] and found that one cluster characterized by early-onset asthma with severe lung function was associated with the best response to the combination therapy of fluticasone and salmeterol [14].
New clinical groups, such as those associated with obesity, have also been defined in both the SARP and Leicester cohorts. This has now been confirmed in other analyses that have specifically examined the contribution of obesity [17, 18]. Two clusters of obese individuals were described: obese uncontrolled and obese well-controlled, and these asthma clusters differed from one another with regard to age of asthma onset, measures of asthma symptoms and control, exhaled nitric oxide concentration, and airway hyper-responsiveness, but were similar with regard to measures of lung function, airway eosinophilia, and serum immunoglobulin (Ig)-E [17]. A group of obese women with late-onset asthma and frequent symptoms with high healthcare use, but with low sputum eosinophil counts, has also been described [19].
The stability of phenotypes with time remains unclear and has yet to be extensively studied. One study has looked at clinical clusters of a cohort of asthmatics over a 10-year period and concluded that, overall, the clusters were stable, since phenotypes observed 10 years apart showed strong similarities, with the probability of remaining in the same asthma phenotype at both times varying between 54 and 88 % [20].
3 Current Treatments for Asthma
Combined inhaled therapy with a bronchodilator, a long-acting β-adrenergic agonist (LABA), plus an anti-inflammatory agent, a corticosteroid, has become the most effective mainstay treatment of asthma. This forms the backbone of the Global Initiative for Asthma (GINA) guidelines, where inhaled combination LABA and corticosteroids are used at steps 3 and above for control of asthma (http://www.ginasthma.org/documents/1/Pocket-Guide-for-Asthma-Management-and-Prevention). The efficacy of such treatments has been examined in studies of adult asthmatic patients who are on low to high doses of inhaled corticosteroid (ICS), where the addition of a LABA reduced the exacerbation rates requiring oral corticosteroids, improved lung function (forced expiratory volume in 1 sec [FEV1]) and decreased the need for rescue short-acting β-agonists [1]. With combination LABA and ICS, asthma control can be reasonably achieved in ~68 % of patients, with the least effectiveness in the most severe group [21], indicating that such treatment, even at the maximal doses allowable, is not effective in all asthmatic patients. With increasing severity and poor control of asthma, other controller medications are added to the combination LABA and ICS therapy, such as slow-release theophylline and leukotriene inhibitors [22]. Finally, at the highest step (5), the addition of oral therapy with corticosteroids is advocated, and a new class of therapy, an anti-IgE humanized monoclonal antibody, is now also used as an additive treatment for severe allergic asthma.
Even with maximization of such therapies, between 5 and 10 % of patients with asthma are refractory to these treatments and such patients have been labelled as severe asthma or refractory-resistant asthma [2, 3]. It is in this group of patients that more efficacious treatments are needed. It is now becoming evident that asthma, particularly severe asthma, cannot be considered as one disease but as a heterogeneous collection of several different phenotypes that could be determined by different pathway mechanisms. The idea that all asthma treatments are beneficial to all asthmatics is seen in most current guidelines, but this is less applicable in the severe asthma patient. The recently published joint European Respiratory Society and American Thoracic Society severe asthma guidelines have recognized the heterogeneity of severe asthma and proposes targeted therapies for defined phenotypes of asthma [23]. Recommendations regarding the determination of sputum eosinophilia to help in directing treatments for severe asthma have been made; use of sputum eosinophilia may be useful in pinpointing the patients who may benefit from treatment with T-helper (Th)-2 cytokine-targeted therapies.
4 Phenotyping/Endotyping and Responsiveness to Corticosteroid Therapies
Phenotyping for predicting responsiveness to therapies has been driven by the established observation that asthma characterized by eosinophilia is usually responsive to ICS. Increased sputum eosinophilia has been associated with exacerbations and decreased asthma control in some asthmatic subjects [24]. ICS use usually decreases sputum eosinophilia and improves asthma control [25–27].
Cluster analyses that have included sputum eosinophilia and more recently concomitant sputum neutrophilia have been useful in defining corticosteroid-sensitive and -insensitive groups. The Leicester cohort of patients with refractory asthma showed discordance between symptoms and the presence of sputum eosinophilia [9]. One cluster was that of an early-onset, symptom-predominant group with minimal eosinophilic disease, with a high prevalence of obesity and female gender, while the other cluster consisted of an eosinophilic inflammation-predominant group with few symptoms, late-onset disease, and a greater proportion of males, with a high prevalence of rhinosinusitis, aspirin sensitivity, and exacerbations. In the Amsterdam analysis of adult-onset asthma, the cluster associated with severe eosinophilic inflammation had persistent airflow obstruction with low symptom scores, while the low sputum eosinophil scores were seen in obese women with frequent symptoms and high healthcare utilization [19]. The more recent SARP cluster analysis, which included both measures of sputum eosinophils and neutrophils, found that mild-to-moderate allergic asthma was associated with minimal or eosinophil-predominant sputum inflammation, while moderate-to-severe asthma was linked to neutrophil-predominant or mixed granulocytic inflammation, indicating the potential importance of neutrophils in more severe disease. This study also found that the presence of neutrophilic inflammation could be an indicator of poor responsiveness to corticosteroid therapy [28].
An important proportion of mild-to-moderate asthma subjects do not respond, or respond poorly, to a middle dose of ICS in terms of improvement in FEV1 [29]. Increased sputum neutrophilia may indicate corticosteroid insensitivity [30], and smoking asthmatics and obese asthmatics are more likely to develop corticosteroid insensitivity [31, 32]. In the SARP cohort, the group of patients with severe asthma on systemic corticosteroids that could be considered to have corticosteroid-insensitivity were more likely to report a diagnosis of recurrent bronchitis, to have an FEV1 of <60 % of their predicted value, a higher fractional exhaled nitric oxide (FeNO) and a lower forced vital capacity (FVC)% predicted [33]. Work on cells from patients with corticosteroid-dependent asthma has highlighted some of the mechanisms. Activation of p38 mitogen-activated protein kinase [34, 35], inability to recruit the histone deacetylase 2 to the glucocorticoid receptor (GR) transcriptional complex [36], reduced effectiveness of the ligand for GR binding [37], and an increase in the expression of the spliced variant of GR, GR-β, [38] have been proposed. Further characterization of this phenotype is required.
5 Eosinophilic and Non-Eosinophilic (Neutrophilic) Asthma
The prevalence of sputum eosinophilia in asthma has been examined, and the eosinophilic asthma subphenotype constitutes 36 % of subjects with asthma not taking an ICS and 17 % of ICS-treated subjects with asthma in a recent series [39]. Anti-inflammatory therapy with corticosteroids caused significant improvements in airflow obstruction in eosinophilic asthma, but not in persistently noneosinophilic asthma. Non-eosinophilic asthma was more predominant in mild-to-moderate asthma, just as neutrophilic asthma is also predominant in severe refractory asthma [30, 40].
Eosinophil and neutrophil sputum numbers show wide variability in severe asthma, with patients demonstrating none to very high levels of either cell type [30, 40]. The investigation by Baines et al. [41] of asthma phenotypes, using gene expression profiling of induced sputum and unsupervised hierarchical clustering of these expression profiles, has led to the description of three phenotypes: (1) chronic airflow obstruction and less well controlled asthma, increased exhaled nitric oxide, and sputum eosinophils; (2) airflow obstruction and higher sputum neutrophils; and (3) higher sputum macrophages and lower eosinophils and neutrophils, and lung function in normal range. Genes in the interleukin (IL)-1 and tumor necrosis factor (TNF)-α/nuclear factor-κB pathways were also overexpressed and correlated with clinical parameters and neutrophilic airway inflammation. In severe asthma patients, mixed neutrophilia and eosinophilia have been shown to be linked with lower lung function and higher frequency of daily wheeze and healthcare utilization [42]. The mechanisms behind these diverse inflammatory profiles are likely to be complex, but a neutrophilic response may signify a non-Th2-driven mechanism and, most likely, non-steroid-responsive asthma. Bacterial colonization in the airways of patients with severe asthma could contribute to neutrophilic asthma [43, 44]. Defective phagocytosis of bacteria or of apoptotic cells by macrophages has also been reported in severe asthma [45, 46]. Corticosteroids themselves can contribute to the neutrophilia to some extent and even Th1 factors could play a role [47, 48]. Th17 cells have also been implicated as a cause of neutrophilia in severe asthma, perhaps even contributing to corticosteroid insensitivity [49]. Therefore, there may be many underlying causes of a neutrophilic asthma, which may also indicate a more severe asthma.
Woodruff and colleagues [50], by examining the gene signature of airway epithelial brushings divided mild-moderate asthmatics into Th2-high and Th2-low groups, according to the degree of expression of IL-13-inducible genes, periostin, chloride channel regulator 1, and serpin peptidase inhibitor. The Th2-high asthmatic patients had a greater degree of bronchial hyper-responsiveness; higher serum IgE levels; greater blood and airway eosinophilia, subepithelial fibrosis, and airway mucin gene expression [51], and responded well to ICS. Those with a low Th2 signature showed little or no response to ICS treatment. Th2-gene signatures can also be obtained by performing reverse transcription polymerase chain reaction (RT-PCR) of sputum cells and can be used to denote Th-2-high patients with asthma [52]. The extent to which the presence of eosinophils indicates a high-Th2 signature is not known.
6 Biologic Treatments Targeted at Specific Phenotypes
Advances in our understanding of the pathophysiology of asthma has led to new treatments based on targeting eosinophil and immune/inflammatory pathways initiated through Th2 CD4+ T-cell activation with the production of IL-4, IL-5, and IL-13 [53, 54]. Th2 cytokines are expressed in bronchial submucosa of patients with asthma and contribute to airway inflammation, triggering the activation and recruitment of IgE antibody-producing B cells, mast cells, and eosinophils. Expression profiles of airway epithelial cells from asthma subjects indicated that those with a Th2 signature have characteristics of an allergic inflammatory response [21]. Targets of Th2 pathway have included IgE, IL-5, IL-4, IL-13, and IL-4R. For non-eosinophilic targets, the main focus has been on neutrophils. Experience with the specific blocking antibodies has emphasized the importance of targeting the right patient for maximal therapeutic effects [55].
6.1 Targeting Eosinophilic Inflammation
6.1.1 Anti-IgE Antibody, Omalizumab
Omalizumab is a humanized monoclonal antibody that binds to the high-affinity IgE receptor present on mast cells and basophils, leading to a reduction in circulating IgE and preventing mast cells and basophils from releasing mediators when in contact with allergens. In allergic patients defined by raised serum IgE levels and evidence of allergy to one or more aeroallergens with inadequately controlled severe persistent allergic asthma, despite high-dose ICS and LABA therapy, and often additional therapy, omalizumab significantly reduced the rate of severe exacerbations and emergency visits, together with an improvement in asthma quality-of-life scores, with improved symptoms [56–58]. Asthmatics with high levels of exhaled nitric oxide levels, peripheral blood eosinophils, and serum periostin showed greatest reduction in exacerbations in response to omalizumab [59].
6.1.2 Anti-Interleukin (IL)-5 Antibody
IL-5 is a Th2 cytokine that is essential for the terminal differentiation, maturation, and survival of eosinophils. The anti-IL5 antibody, mepolizumab, was not effective in an unselected cohort of adult asthma patients [60], but in severe asthma patients with persistent sputum eosinophilia, two anti-IL-5 antibodies, mepolizumab and reslizumab, decreased exacerbations, oral corticosteroid use, and improved symptoms and lung function [61–63]. A larger study with mepolizumab showed efficacy in patients with recurrent severe asthma exacerbations and eosinophilic inflammation in reducing exacerbation rates, without improvement in FEV1 and quality of life [64].
6.1.3 Anti-IL-4Rα Antibody
IL-4 activates Th2 cells, causes isotype class switching of B cells towards IgE synthesis, and is involved in mast cell recruitment. IL-4 binds to IL-4Rα within two different types of receptor (type I and type II) that leads to the signalling of both IL-4 and IL-13 [65]. AMG 317, a human monoclonal antibody to IL-4Rα that blocks both IL-4 and IL-13 pathways, did not demonstrate clinical efficacy in moderate to severe asthma [66]. However, in patients with persistent, moderate-to-severe asthma and elevated eosinophil levels (either in blood or sputum) who used ICS and LABAs, dupilumab (a human monoclonal antibody to IL4-Rα) was associated with fewer asthma exacerbations when LABAs and ICS were withdrawn, and with improved lung function and reduced levels of Th2-associated inflammatory markers [67].
6.1.4 Anti-IL-13 Antibody
IL-13 together with IL-4 can regulate IgE synthesis and has an important role in mucus hyperplasia and airway hyper-responsiveness. A monoclonal antibody to IL-13, lebrikizumab, improved FEV1 in moderately severe asthmatic adults stratified according to a Th2-low and Th2-high status, without affecting exacerbations and asthma symptoms [68]; those who responded had elevated serum periostin levels, a proposed surrogate marker of Th2 activity, or had raised levels of nitric oxide in the exhaled breath. Another anti-IL-13 antibody, tralokinumab, did not improve symptoms but resulted in a non-significant increase in FEV1 when compared with placebo, with better effects in patients with detectable sputum IL-13 levels [69].
6.2 Non-Eosinophilic Inflammation
6.2.1 CXCR2 Antagonist
CXCL8 (IL-8) is a chemokine involved in the chemoattraction and activation of neutrophils through the CXCR2 receptor, particularly in severe asthma. A CXCR2 antagonist, SCH527123, reduced sputum neutrophilia in severe adult asthma, and modestly lowered the number of mild exacerbations, but without improving asthma control [70].
6.2.2 Macrolide Antibiotic Therapy
Macrolide antibiotics have been used in severe asthma on the basis that there may be bacteria underlying the increase in severity [43, 44] and that these antibiotics possess anti-neutrophilic effects. Clarithromycin, as an add-on treatment to ICS in a group of patients with severe asthma that were unstratified, reduced sputum neutrophils and IL-8 levels, and delivered an improvement in quality-of-life measures without changes in FEV1 [71]. In an exacerbation-prone severe asthma cohort where patients were beforehand stratified into eosinophilic and non-eosinophilic groups, azithromycin was associated with a lower rate of severe exacerbations and lower respiratory tract infections than placebo in subjects with non-eosinophilic severe asthma defined by a blood eosinophilia of ≤200/µl [72].
6.2.3 Anti-IL-17R Antibody
Brodalumab, a human anti-IL-17 receptor A monoclonal antibody, had no effect in subjects with inadequately controlled moderate to severe asthma taking ICS [73]. These patients were not otherwise stratified, but a post hoc examination of subgroups did not reveal any group that responded particularly well to this treatment.
7 Phenotype-Driven Treatment, Systems Biology, and Personalized Medicine
Phenotype-driven treatment will gradually become a reality. The question is how refined or deep do we need to proceed in terms of phenotyping. For example, it should be important to refine the different biomarkers that will determine the best responders to each targeted therapy developed for the Th2-high patient. On the other hand, much less work has been done to define non-Th2 asthma and non-eosinophilic asthma. However, this area is gathering pace with the advent of high throughput -omics technologies, which generate molecular profiles from bio-specimens that can be translated into clinical tests that may be useful for guiding management decisions. This will no doubt increase the reality of phenotype-driven treatment and ultimately lead towards personalized care in asthma. This journey is just beginning for asthma.
Demonstration of efficacy of new therapies will depend in part on the precision by which patients can be endotyped for specific therapies [74]. Endotyping has been confined to measurements such as sputum eosinophils, exhaled breath markers such as nitric oxide, and mediators in blood such as serum periostin or blood eosinophils [75]. Sputum eosinophils and serum periostin could define a particular subset of patients who may respond well to certain therapies such as the anti-Th2 approaches using anti-IL5 or anti-IL-13 antibodies. Use of the Th2 signature derived from airway epithelial cells could be used to choose patients who would respond to ICS therapy; exhaled breath levels of nitric oxide could be used as a surrogate marker for therapeutic responsiveness to corticosteroid therapy [76]. Serum periostin is a biomarker that could replace the use of epithelial cell expression of Th-2 cytokines, and it has been shown to correlate with airway eosinophilia [77]. On the other hand, serum periostin has also been shown in a Japanese asthma cohort on ICS treatment to denote those with chronic airflow obstruction [78]. This indicates that biomarker studies need to be confirmed in independent cohorts. More validated markers are needed for non-eosinophilic asthma. Using the percentage of neutrophils in induced sputum may not be the best biomarker for neutrophilic asthma. Recently, a raised level of hydrogen sulphide in induced sputum has been proposed as a potential marker for neutrophilic asthma, associated with chronic airflow obstruction [79].
The availability of high-throughput biological data has now opened up an important avenue for the discovery of biomarkers useful to delineate phenotypes and to predict therapeutic response. Biologic processes involved in inflammation, immunity, cell cycle, apoptosis, or metabolism will need to be linked to the clinical and phenotypic expression of asthma. Analysis of clinical, physiologic and genomic, transcriptomic, lipidomic and proteomic data will provide a more complex but more definitive phenotypic representation of the patient’s disease. In addition, epigenetic mechanisms may modulate environmental effects, such as road traffic pollution and cigarette smoking, which can influence the development and course of asthma [80]. We have reported recently that severe asthma is associated with the activation of blood CD8+ T-cells but not CD4+ T-cells, and that this was correlated with the down-regulation of the micro-RNAs miR-146a/b and miR-28-5p, as well as changes in the expression of lncRNA species [81]. In a proteomic analysis of bronchial biopsies from subjects with asthma, more than 1,800 proteins were identified, linked to acute phase response signalling, cell-to-cell signaling, and tissue development associations [82]. Furthermore, protein–protein interactions involved in inflammation and cellular proliferation signalling have been modelled mathematically and used to predict new drug targets in asthma [83]. The Innovative Medicines Initiative (IMI)-funded project on Unbiased Biomarkers of Respiratory Diseases (UBIOPRED) is using a systems biology approach to phenotype severe asthma and find new targets for therapy [84].
Systems biology is a strategy to obtain information from complex quantitative biological data, and systems medicine is the similar counterpart applied to information from quantitative data related to complex diseases such as asthma. Collection and analysis of clinical and physiologic parameters, and of high-throughput data from genomics, transcriptomic, lipidomic and proteomic analyses using complex statistical and computational methods form the basis of systems biology and medicine [85]. This approach has been used to demonstrate that different combinations of genomic and proteomic signatures can be used to define subphenotypes of breast cancer and chronic lymphocytic leukemia and determine whether these phenotypes are linked to the development or progression of disease or indicate responsiveness to specific interventions [86].
Much more work is needed in terms of more precise and relevant endotyping of the asthma patient that could be delivered through the new -omics science. More targetted specific treatments are also needed, which could also come from -omics technology and analysis. Being able to endotype the patient with severe asthma will allow for a more precise and rationale way of getting these specific treatments to the individual patient, and this will be the first step towards personalized medicine [85]. The challenge of delivering the benefits of personalized medicine to the patient remains high [87], but this is the roadmap by which the right medications will be delivered to the right patient.
8 Conclusion
Clinicians have always placed a high value on individualized treatments, and the time has come for more personalized medicine in a disease as complex as asthma. In the field of asthma, there has been a conventional ‘one size fits all’ approach in terms of the treatment of asthma. However, we are now getting more tools to approach personalized medicine for asthma such as the validated measures of activity and severity of asthma [88], and with the definition of severe asthma using control measures of asthma [23]. The recent addition of biomarkers that characterize the eosinophilic phenotype and that predicts response to specific therapies will add to the increasing confidence of delivering more personalized management for asthma, particularly for severe asthma.
References
Bel EH. Clinical phenotypes of asthma. Curr Opin Pulmonary Med. 2004;10(1):44–50.
Chung KF, Godard P, Adelroth E, Ayres J, Barnes N, Barnes P, et al. Difficult/therapy-resistant asthma: the need for an integrated approach to define clinical phenotypes, evaluate risk factors, understand pathophysiology and find novel therapies. ERS Task Force on Difficult/Therapy-Resistant Asthma European Respiratory Society. Eur Respir J. 1999;13(5):1198–208.
Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society. Am J Respir Crit Care Med. 2000;162(6):2341–51.
Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372(9643):1107–19.
Lotvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127(2):355–60.
Prosperi MC, Sahiner UM, Belgrave D, Sackesen C, Buchan IE, Simpson A, et al. Challenges in identifying asthma subgroups using unsupervised statistical learning techniques. Am J Respir Crit Care Med. 2013;188(11):1303–12.
Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315–23.
Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, et al. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol. 2011;127(2):382–389.e1-13.
Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218–24.
Siroux V, Basagana X, Boudier A, Pin I, Garcia-Aymerich J, Vesin A, et al. Identifying adult asthma phenotypes using a clustering approach. Eur Respir J. 2011;38(2):310–7.
Kim TB, Jang AS, Kwon HS, Park JS, Chang YS, Cho SH, et al. Identification of asthma clusters in two independent Korean adult asthma cohorts. Eur Respir J. 2013;41(6):1308–14.
Kaneko Y, Masuko H, Sakamoto T, Iijima H, Naito T, Yatagai Y, et al. Asthma phenotypes in Japanese adults—their associations with the CCL5 and ADRB2 genotypes. Allergol Int. 2013;62(1):113–21.
Patrawalla P, Kazeros A, Rogers L, Shao Y, Liu M, Fernandez-Beros ME, Shang S, Reibman J. Application of the asthma phenotype algorithm from the Severe Asthma Research Program to an urban population. PLoS ONE. 2012;7(9):e44540.
Chang TS, Lemanske RF Jr, Mauger DT, Fitzpatrick AM, Sorkness CA, Szefler SJ, et al. Childhood asthma clusters and response to therapy in clinical trials. J Allergy Clin Immunol. 2014;133:363–9.
Schatz M, Hsu JW, Zeiger RS, Chen W, Dorenbaum A, Chipps BE, et al. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2013 Dec 3 [Epub ahead of print].
Jang AS, Kwon HS, Cho YS, Bae YJ, Kim TB, Park JS, et al. Identification of subtypes of refractory asthma in Korean patients by cluster analysis. Lung. 2013;191(1):87–93.
Sutherland ER, Goleva E, King TS, Lehman E, Stevens AD, Jackson LP, et al. Cluster analysis of obesity and asthma phenotypes. PLoS ONE. 2012;7(5):e36631.
Gibeon D, Batuwita K, Osmond M, Heaney LG, Brightling CE, Niven R, et al. Obesity-associated severe asthma represents a distinct clinical phenotype: analysis of the British Thoracic Society Difficult Asthma Registry Patient cohort according to BMI. Chest. 2013;143(2):406–14.
Amelink M, de Nijs SB, de Groot JC, van Tilburg PM, van Spiegel PI, Krouwels FH, et al. Three phenotypes of adult-onset asthma. Allergy. 2013;68(5):674–80.
Boudier A, Curjuric I, Basagana X, Hazgui H, Anto JM, Bousquet J, et al. Ten-year follow-up of cluster-based asthma phenotypes in adults. A pooled analysis of three cohorts. Am J Respir Crit Care Med. 2013;188(5):550–60.
Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170(8):836–44.
Chung KF, Caramori G, Adcock IM. Inhaled corticosteroids as combination therapy with beta-adrenergic agonists in airways disease: present and future. Eur J Clin Pharmacol. 2009;65(9):853–71.
Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–73.
Deykin A, Lazarus SC, Fahy JV, Wechsler ME, Boushey HA, Chinchilli VM, et al. Sputum eosinophil counts predict asthma control after discontinuation of inhaled corticosteroids. J Allergy Clin Immunol. 2005;115(4):720–7.
Cowan DC, Cowan JO, Palmay R, Williamson A, Taylor DR. Effects of steroid therapy on inflammatory cell subtypes in asthma. Thorax. 2010;65(5):384–90.
Dente FL, Bacci E, Bartoli ML, Cianchetti S, Costa F, Di Franco A, et al. Effects of oral prednisone on sputum eosinophils and cytokines in patients with severe refractory asthma. Ann Allergy Asthma Immunol. 2010;104(6):464–70.
Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360(9347):1715–21.
Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2013 Dec 8 [Epub ahead of print].
Israel E, Chervinsky PS, Friedman B, van Bavel J, Skalky CS, Ghannam AF, et al. Effects of montelukast and beclomethasone on airway function and asthma control. J Allergy Clin Immunol. 2002;110(6):847–54.
Jatakanon A, Uasuf C, Maziak W, Lim S, Chung KF, Barnes PJ. Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1532–9.
Chaudhuri R, Livingston E, McMahon AD, Thomson L, Borland W, Thomson NC. Cigarette smoking impairs the therapeutic response to oral corticosteroids in chronic asthma. Am J Respir Crit Care Med. 2003;168(11):1308–11.
Sutherland ER, Goleva E, Strand M, Beuther DA, Leung DY. Body mass and glucocorticoid response in asthma. Am J Respir Crit Care Med. 2008;178(7):682–7.
Wysocki K, Park SY, Bleecker E, Busse W, Castro M, Chung KF, et al. Characterization of factors associated with systemic corticosteroid use in severe asthma: data from the Severe Asthma Research Program. J Allergy Clin Immunol. 2014;133:915–8.
Chang PJ, Bhavsar PK, Michaeloudes C, Khorasani N, Chung KF. Corticosteroid insensitivity of chemokine expression in airway smooth muscle of patients with severe asthma. J Allergy Clin Immunol. 2012;130(4):877–885.e5.
Bhavsar P, Khorasani N, Hew M, Johnson M, Chung KF. Effect of p38 MAPK inhibition on corticosteroid suppression of cytokine release in severe asthma. Eur Respir J. 2010;35(4):750–6.
Ito K, Lim S, Caramori G, Chung KF, Barnes PJ, Adcock IM. Cigarette smoking reduces histone deacetylase 2 expression, enhances cytokine expression, and inhibits glucocorticoid actions in alveolar macrophages. FASEB J. 2001;15(6):1110–2.
Irusen E, Matthews JG, Takahashi A, Barnes PJ, Chung KF, Adcock IM. p38 Mitogen-activated protein kinase-induced glucocorticoid receptor phosphorylation reduces its activity: role in steroid-insensitive asthma. J Allergy Clin Immunol. 2002;109(4):649–57.
Leung DYM, Hamid Q, Vottero A, Szefler SJ, Surs W, Minshall E, et al. Association of glucocorticoid insensitivity with increased expression of glucocorticoid receptor beta. J Exp Med. 1997;186(9):1567–74.
McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chinchilli VM, et al. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am J Respir Crit Care Med. 2012;185(6):612–9.
Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160(3):1001–8.
Baines KJ, Simpson JL, Wood LG, Scott RJ, Gibson PG. Transcriptional phenotypes of asthma defined by gene expression profiling of induced sputum samples. J Allergy Clin Immunol. 2011;127(1):153–160.e1-9.
Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol. 2010;125(5):1028–1036.e13.
Zhang Q, Illing R, Hui CK, Downey K, Carr D, Stearn M, et al. Bacteria in sputum of stable severe asthma and increased airway wall thickness. Respir Res. 2012;13:35.
Wood LG, Simpson JL, Hansbro PM, Gibson PG. Potentially pathogenic bacteria cultured from the sputum of stable asthmatics are associated with increased 8-isoprostane and airway neutrophilia. Free Radic Res. 2010;44(2):146–54.
Fitzpatrick AM, Holguin F, Teague WG, Brown LA. Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J Allergy Clin Immunol. 2008;121(6):1372–8.
Huynh ML, Malcolm KC, Kotaru C, Tilstra JA, Westcott JY, Fadok VA, et al. Defective apoptotic cell phagocytosis attenuates PGE2 and 15-HETE in severe asthma alveolar macrophages. Am J Respir Crit Care Med. 2005;172:972–9.
Nguyen LT, Lim S, Oates T, Chung KF. Increase in airway neutrophils after oral but not inhaled corticosteroid therapy in mild asthma. Respir Med. 2005;99(2):200–7.
Shannon J, Ernst P, Yamauchi Y, Olivenstein R, Lemiere C, Foley S, et al. Differences in airway cytokine profile in severe asthma compared to moderate asthma. Chest. 2008;133(2):420–6.
Al-Ramli W, Prefontaine D, Chouiali F, Martin JG, Olivenstein R, Lemiere C, et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123(5):1185–7.
Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci USA. 2007;104(40):15858–63.
Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–95.
Peters MC, Mekonnen ZK, Yuan S, Bhakta NR, Woodruff PG, Fahy JV. Measures of gene expression in sputum cells can identify T2-high and T2-low subtypes of asthma. J Allergy Clin Immunol. 2014;133:388–94.
Adcock IM, Caramori G, Chung KF. New targets for drug development in asthma. Lancet. 2008;372(9643):1073–87.
Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012;18(5):673–83.
Chung KF. New treatments for severe treatment-resistant asthma: targeting the right patient. Lancet Respir Med. 2013;1:639–52.
Humbert M, Beasley R, Ayres J, Slavin R, Hebert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309–16.
Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364(11):1005–15.
Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes-Rivera I, Zhu J, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154(9):573–82.
Hanania NA, Wenzel S, Rosen K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187(8):804–11.
Flood-Page P, Swenson C, Faiferman I, Matthews J, Williams M, Brannick L, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. 2007;176(11):1062–71.
Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973–84.
Castro M, Mathur S, Hargreave F, Boulet LP, Xie F, Young J, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184(10):1125–32.
Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360(10):985–93.
Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–9.
Maes T, Joos GF, Brusselle GG. Targeting interleukin-4 in asthma: lost in translation? Am J Respir Cell Mol Biol. 2012;47(3):261–70.
Corren J, Busse W, Meltzer EO, Mansfield L, Bensch G, Fahrenholz J, et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. Am J Respir Crit Care Med. 2010;181(8):788–96.
Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368:2455–66.
Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365(12):1088–98.
Piper E, Brightling C, Niven R, Oh C, Faggioni R, Poon K, et al. A phase II placebo-controlled study of tralokinumab in moderate-to-severe asthma. Eur Respir J. 2013;41(2):330–8.
Nair P, Gaga M, Zervas E, Alagha K, Hargreave FE, O’Byrne PM, et al. Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: a randomized, placebo-controlled clinical trial. Clin Exp Allergy. 2012;42(7):1097–103.
Simpson JL, Powell H, Boyle MJ, Scott RJ, Gibson PG. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respir Crit Care Med. 2008;177(2):148–55.
Brusselle GG, Vanderstichele C, Jordens P, Deman R, Slabbynck H, Ringoet V, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68(4):322–9.
Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188(11):1294–302.
Gibeon D, Chung KF. The investigation of severe asthma to define phenotypes. Clin Exp Allergy. 2012;42(5):678–92.
Chung KF. Inflammatory biomarkers in severe asthma. Curr Opin Pulmonary Med. 2012;18(1):35–41.
Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–15.
Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol. 2012;130(3):647–654.e10.
Kanemitsu Y, Matsumoto H, Izuhara K, Tohda Y, Kita H, Horiguchi T, et al. Increased periostin associates with greater airflow limitation in patients receiving inhaled corticosteroids. J Allergy Clin Immunol. 2013;132(2):305–312.e3.
Saito J, Zhang Q, Hui C, Macedo P, Gibeon D, Menzies-Gow A, et al. Sputum hydrogen sulfide as a novel biomarker of obstructive neutrophilic asthma. J Allergy Clin Immunol. 2013;131(1):232–234.e1-3.
Kabesch M, Adcock IM. Epigenetics in asthma and COPD. Biochimie. 2012;94(11):2231–41.
Tsitsiou E, Williams AE, Moschos SA, Patel K, Rossios C, Jiang X, et al. Transcriptome analysis shows activation of circulating CD8+ T cells in patients with severe asthma. J Allergy Clin Immunol. 2012;129(1):95–103.
O’Neil SE, Sitkauskiene B, Babusyte A, Krisiukeniene A, Stravinskaite-Bieksiene K, Sakalauskas R, et al. Network analysis of quantitative proteomics on asthmatic bronchi: effects of inhaled glucocorticoid treatment. Respir Res. 2011;12:124.
Hwang S, Son SW, Kim SC, Kim YJ, Jeong H, Lee D. A protein interaction network associated with asthma. J Theor Biol. 2008;252(4):722–31.
Auffray C, Adcock IM, Chung KF, Djukanovic R, Pison C, Sterk PJ. An integrative systems biology approach to understanding pulmonary diseases. Chest. 2010;137(6):1410–6.
Chen R, Snyder M. Systems biology: personalized medicine for the future? Curr Opin Pharmacol. 2012;12(5):623–8.
Landau DA, Carter SL, Stojanov P, McKenna A, Stevenson K, Lawrence MS, et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell. 2013;152(4):714–26.
Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med. 2010;363(4):301–4.
Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99.
Acknowledgments
The author has no potential conflicts of interest to declare in relation to the contents of this review. The author is funded through the Innovative Medicines Initiative in terms of the UBIOPRED project, which is looking into a systems biology approach to severe asthma. No funding was obtained for the writing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chung, K.F. Defining Phenotypes in Asthma: A Step Towards Personalized Medicine. Drugs 74, 719–728 (2014). https://doi.org/10.1007/s40265-014-0213-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-014-0213-9