Abstract
Management of renal anemia in the large and at-risk population of non-dialysis chronic kidney disease (CKD) patients is a critical issue. In particular, definition of the optimal hemoglobin (Hb) target for therapy is controversial but highly warranted by physicians and patients worldwide. Recently, international clinical practice guidelines have recommended delayed initiation of erythropoiesis-stimulating agents (ESA) and lower Hb target levels during maintenance therapy. However, geographical differences in terms of ESA dose needed to achieve a given Hb value can be evidenced, with US patients showing higher prevalence of ESA resistance. On the other hand, non-US patients are often maintained in a higher Hb range by means of low ESA doses. This critical point has never been addressed. Nevertheless, outside of the US, translating the restrictive recommendations of new guidelines, which are essentially based on trials in US patients, can lead to negative effects, such as an increased need for a blood transfusion, and worsening of quality of life. In this article we provide a reappraisal of current recommendations on anemia management in non-dialysis CKD in light of the geographical differences in individual responsiveness to ESA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Care of non-dialysis chronic kidney disease patients (CKD-ND) is aimed at slowing progression to end-stage renal disease (ESRD) and limiting the associated worsening of cardiovascular (CV) damage. At this stage, the intervention of a nephrologist is a complex task because it often represents tertiary prevention in patients with advanced cardiorenal damage and several comorbidities. Identifying optimal care, including the definition of therapeutic goals, is therefore highly warranted.
Among the different complications, renal anemia represents a paradigmatic case of the complexity of identifying the ideal goal of therapy in CKD-ND. Indeed, observational studies have demonstrated consistent advantages of higher hemoglobin (Hb) levels [1–5], while key randomized controlled trials (RCTs) have disclosed either harm or no benefit of higher Hb target [6, 7]. Although the main RCTs have been predominantly conducted in the US, where higher doses of erythropoiesis-stimulating agents (ESA) are used compared with other countries, critical evaluation of RCTs specifically addressing more complex issues such as dose and responsiveness has not been systematically collated. On the other hand, clinical practice guidelines (CPG) that are mainly based on US trials use literature as published, and while recommendations of individualization have been made, the main interpretation has been lowering of Hb target levels.
We argue here that recommendations should take into account geographical differences in individual responsiveness to ESA.
2 New Recommendations on Hemoglobin Target in Chronic Kidney Disease
The Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) study and the Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) have largely contributed to the concerns on safety and usefulness of increasing Hb to near-normal levels in CKD-ND [6, 7] (Table 1). The Normal Hematocrit Cardiac Trial is another negative RCT, albeit in hemodialysis (HD) patients with a history of CV disease, demonstrating that complete correction does not improve outcomes [8].
The CHOIR study showed that patients randomized to an Hb target of 13.5 g/dL were at higher risk of death and CV complications than controls (target Hb 11.3). In the more recent TREAT study, composite CV risk in the active group (Hb target of approximately 13.0 g/dL) was not different compared with the control group. The study also reported, as a secondary finding, a significant increased incidence of stroke (5.0 vs. 2.6 %; p < 0.001) in the active arm; however, the incidence rate of stroke in all TREAT patients was markedly lower (3.8 %) than observed in a pooled analysis of a US community-based study (7.5 %) [9]. Moreover, it was not possible to identify predictors of the risk of stroke in a deeper sub-analysis of TREAT data [10].
Mainly on the basis of these RCTs, the US FDA mandated the change in the product label for ESAs in June 2012 [11]. According to the US agency, CKD-ND patients should initiate ESA treatment only when Hb is less than 10 g/dL, and reduce or stop it when Hb exceeds 10 g/dL. The FDA also points out that treatment should be individualized to use the lowest possible dose of ESA to prevent blood transfusion. The newly released Kidney Disease Improving Global Outcomes (KDIGO) guidelines for renal anemia endorsed these recommendations [12] (Table 2). Noteworthy, when examining the KDIGO statements, the highest scores in the grading for quality of evidence are reached by recommendations concerning overcorrection and ESA use in patients with cancer or history of stroke (Table 2). This advice is very useful in clinical practice related to frail patients. Unfortunately, the indications more frequently required by clinical nephrologists (Hb range in the CKD population at large) do not reach adequate scores, being based on low or very low quality of evidence (Table 2).
Less restrictive recommendations have been generated in the 2011 update of the guidelines issued by the National Institute for Health and Clinical Excellence (NICE) in the UK recommending an aspirational Hb target range of 10–12 g/dL for all patients, which should be maintained by promptly adjusting treatment in the presence of minor Hb changes, i.e. within the range limits [13]. The most recent position statement on KDIGO 2012 granted by the European Renal Best Practice panel confirms the NICE target range (10–12 g/dL) [14]. Furthermore, they recommend an early treatment start, i.e. when Hb is 10 g/dL or even higher in younger patients with very few comorbidities, in those with worsening ischemic symptoms associated with anemia, or when the benefit on quality of life (QoL) can be foreseen. This latter issue is not trivial. Indeed, the impact of anemia management on QoL in chronic diseases, such as CKD, should be considered together with hard endpoints, especially from the patient’s point of view [15]. Of note, improvement of QoL is an anticipated benefit of ESA therapy; however, results on this specific topic are controversial. In the CHOIR study there were no differences in QoL in the high- vs. low-Hb group [6], while a small improvement in fatigue and overall QoL was reported in the high-Hb group in the TREAT study [16]. This issue is also more complex than expected because patients may not perceive QoL changes as the improvement after anemia treatment is gradual in onset [17].
Definitely, the limited quality of evidence, the heterogeneous recommendations of CPGs, and the limitations inherent to QoL analyses, leave on the ground more doubts than certainties. Moreover, not unrealistic is the concern that the new policies on Hb target will increase the use of blood transfusions, which may not be a safe approach in potential candidates for a kidney transplant [18].
3 Generalizability of Recommendations
As mentioned, the more restrictive recommendations on target Hb are mainly driven by CHOIR and TREAT, which are the largest trials carried out so far in CKD-ND and, consequently, those that have more weight in meta-analyses. However, these trials may not be easily translatable to non-US patients. Investigators have had to titrate ESA doses to reach the assigned target in the high-Hb arm, and it was not always possible to achieve the high targets without over-aggressive dosing. In fact in both trials, where US patients were predominant (TREAT) or exclusive (CHOIR), high doses of ESA were used to target complete correction of anemia. The scenario changes remarkably when examining the RCTs carried outside the US that included a large number of European patients (Table 1) [19–27]. These studies show that much lower doses of ESA (one-third, on average) allow similar Hb target levels to be reached in the Hb normalization arm (Fig. 1). From the RCTs reported in Table 1, we calculated weighted means for Hb levels and ESA dose in the high-Hb subgroups in order to take into account the different population included in each study. We found that compared to US trials, studies outside the US reached similar Hb levels in the Hb normalization arm (weighted means 13.1 ± 0.7 in non-US studies vs. 12.6 ± 0.1 g/dL in US studies) despite much lower doses of ESA (4,911 ± 1,265 in non-US studies vs. 12,958 ± 1,039 IU/week in US studies). Furthermore, no CV risk excess was observed in the high-Hb arm of the one non-US trial to assess this endpoint, while improved QoL was a consistent finding in the high- vs. low-Hb groups across most non-US trials.
Regional differences in dose of ESA and achieved Hb in the high-Hb arm of randomized controlled trials carried out in the last decade in adult non-dialysis CKD patients to test efficacy of complete vs. partial correction of anemia. Weighted mean values are reported in the text. ESA erythropoiesis-stimulating agents, Hb hemoglobin, CKD chronic kidney disease
It is also important to highlight a finding of the TREAT trial reported in the Appendix in the article by Pfeffer et al. [7]. The risk of primary CV endpoints in patients treated to complete anemia correction varied by geographical region of enrollment, and diverged when comparing patients coming from Western Europe and Australia with US patients (Table 3). Indeed, there was a trend, which was not statistically significant, toward a reduced hazard ratio (HR) of reaching the primary composite endpoint in the active group. With the caution imposed by the post hoc nature of the analysis, this finding may suggest a possible protective role of complete anemia correction in West Europe/Australia.
A formal comparison of non-US vs. US trials appears to be difficult because non-US studies generally have lower sample size and length of follow-up. Furthermore, the CREATE trial, which is the largest trial conducted outside the US (mainly in Europe), was underpowered, with the annual rate of CV events being much lower than expected (6 % vs. 15 %). Of note, we did not consider two additional RCTs on the effects of Hb normalization in non-US CKD-ND patients because ESA dosing was not reported; one study from Australia showed no effect on left ventricular hypertrophy [28], while the other one, performed in a small group of Greek patients, evidenced a significant slowing of CKD progression [29].
While it is now difficult to imagine future studies in Europe, or other non-US countries, focusing on the optimal Hb target in CKD-ND, additional evidence on this issue has recently been provided by a French RCT in kidney transplant recipients (KTR) with basal glomerular filtration rate (GFR) 34 mL/min/1.73 m2) [27]. This trial investigated the effects of normalizing Hb levels (mean achieved Hb value 13 g/dL) vs. partial correction (mean achieved Hb value 11.5 g/dL). Complete correction was obtained with low epoetin dose (about 6,200 IU/week), had a positive impact on general health, exercise capacity, and physical scores, and, more importantly, allowed a threefold slower GFR decline in the absence of increased CV risk. These results are in agreement with the small Greek RCT [29], but they are at variance with those obtained in larger trials in CKD-ND, where either a neutral or detrimental effect on CKD progression was detected [6, 7, 22, 30]. Correctly, in fact, the authors conclude that kidney disease in KTR constitutes a particular entity and that the negative results on cardiorenal prognosis obtained in ESA studies conducted in CKD patients should not be extrapolated to KTR. Nevertheless, the country where the study was performed (France), the long history of transplant (8 years on average) and the relatively low dose of ESA in the high-Hb arm may actually make these patients not dissimilar from the CKD-ND patients of trials conducted outside the US.
4 Responsiveness to Erythropoiesis-Stimulating Agents and Prognosis
The higher ESA dosage needed to achieve similar Hb levels in US vs. non-US CKD-ND patients strongly suggests that US patients are characterized by a larger prevalence of hyporesponsiveness to ESA. This assumption is supported by a formal comparison in the HD population; in this setting, the percentage of hyporesponsive patients (use of ESA doses >35,000 IU/week) is threefold higher in the US than in South Europe (about 18 % vs. 6 %) in the presence of similar levels of achieved Hb and iron dosing [31].
Interestingly, the high dose of ESA rather than the high-Hb target may be associated with poor prognosis. A secondary analysis of CHOIR has showed that the use of epoetin at a high dose (>20,000 IU/week) was associated with increased risk of the composite CV endpoint independently of randomization to the higher Hb target [32]. More recently, CHOIR investigators re-analyzed 1,244 subjects with complete data and found that irrespective of achieved Hb, the risk for CV events significantly increased in patients receiving epoetin alpha at doses >10,095 IU/week [33].
The exact mechanism underlying the association between high-dose ESA use and CV risk is unknown. Experimental studies have suggested that erythropoietin receptors (EpoRs) may also be present on human endothelial cells and multiple other sites, and that, in non-erythroid cells, the levels of EpoR expression are generally low [34, 35]. Accordingly, large doses of ESAs, while not reflecting normal erythropoiesis, may have unwanted non-erythropoietic effects, including reduced nitric oxide release, increased endothelial release of vasoconstrictors (endothelin-1, prostaglandin F2α, and thromboxane), and increased platelet number and function and impairment of the coagulation system [36, 37]. Furthermore, higher ESA doses are associated with greater odds of higher levels of tumor necrosis factor-α, interleukin (IL)-6, IL-8, and C-reactive protein (CRP) [38, 39], as well as with increased levels of soluble erythropoietin receptor (sEpoR), which is a factor that limits erythropoiesis by blocking and inactivating circulating epoetin [39]. Two hypotheses can therefore be made to explain the association between ESA dose and CV risk. On the one hand, it is likely that severely inflamed patients, who usually present a greater burden of CV comorbidities [37], are also more severely anemic and consequently require aggressive ESA therapy (‘confounding by indication’). An alternative hypothesis is that uptitrating ESA dosage in poor responders, while not effectively correcting anemia because of the ‘barrier’ created by the high circulating levels of sEpoR, can induce release of inflammatory cytokines by stimulating EpoRs on macrophages or other inflammatory cells. On the basis of the dose-dependent proinflammatory effect of ESA, it is reasonable to hypothesize that in US patients randomized to the Hb normalization group, the greater (or non-decreased) risk of adverse CV outcome may be dependent on the frequent use of ESA doses that are higher, either in absolute terms or relative to the inflammation status.
The prognostic role of ESA dosing has been recently supported by a meta-regression analysis of trials in dialysis and non-dialysis patients [40]. The study identified a significant association between higher ESA dose and increased mortality, with the relationship persisting after adjustment for target or achieved Hb. However, the association was significant only in dialysis trials, while it did not reach statistical significance when only the non-dialysis trials were examined, therefore suggesting that in CKD-ND patients, ESA dosing may not act as a unique player. More insight into this phenomenon has been provided in a post hoc analysis of TREAT evaluating the Hb response to the initial two fixed weight-based doses of darbepoetin in patients in the active arm [41]. Patients in the lowest quartile of response had a 31 % higher CV risk and 41 % higher mortality risk. The authors report that the ability to predict a poor initial Hb response from a model incorporating as many as 92 baseline characteristics was limited. These results therefore suggest that, in CKD-ND, ESA response prevails over ESA dose in predicting CV outcome, and that individual characteristics predominate over the commonly measured determinants of response. Nevertheless, in the secondary analysis of TREAT, darbepoetin doses were maintained in the high range (median dose during follow-up was 232 and 167 µg/month, i.e. 58 and 42 µg/week, in the poor and better response groups, respectively). Therefore, the study left unanswered the critical question of whether the association between responsiveness and adverse outcome also holds true for the lower ESA dosages commonly administered in daily nephrology practice outside the US. An additional, unexplored question was whether responsiveness also modifies the risk of ESRD, i.e. the main outcome of patients regularly seen in nephrology [42]. In this regard, a recent study by our group has suggested that in CKD-ND patients under nephrology care, hyporesponsiveness is associated with a greater risk of ESRD (Fig. 2) [43]. The results of this study obtained in the presence of low ESA dosing (mean darbepoetin-equivalent dose in the first 6 months was 17.6 ± 8.2, 23.9 ± 9.8, and 23.5 ± 11.9 µg/week in the good, intermediate, and poor response groups, respectively) support the need to explore also the usefulness of ESA response as a prognostic tool for renal survival in patients receiving low-dose ESA.
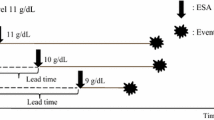
Effects of the ESA-R in the first 6 months of treatment on the subsequent risk of ESRD, as assessed by Kaplan–Meier analysis (a) and multivariable Cox analysis (b). Cox model was adjusted for age, gender, diabetes, systolic blood pressure, glomerular filtration rate, proteinuria, hemoglobin, phosphate, and C-reactive protein (modified from Minutolo et al. [43]). ESA-R response to erythropoiesis-stimulating agents, ESRD end-stage renal disease
5 Why the Difference?
No study has formally evaluated the potential mechanisms underlying the differences between US and European patients in terms of responsiveness to ESA and related outcome. It is well known that the leading cause of ESA hyporesponsiveness is represented by iron deficiency [12, 13]. However, the presence of low iron indices does not seem to be relevant in explaining geographical differences in ESA response because iron deficiency is largely as prevalent in US CKD patients as in European CKD patients [44, 45]. More intriguing is the observation that the inflammatory status of CKD patients significantly differs in the US and Europe. A retrospective cohort study in HD patients receiving care at a Fresenius Medical Care-North America facility has shown a median CRP value of 20.4 mg/L [46], i.e. a value double that observed in European HD patients [47–50]. Similar differences have also been found in the non-dialysis CKD population, where CRP levels in US patients are three to six times higher the value registered in European patients [41, 43, 51–54]. These data support the hypothesis that adverse non-erythropoietic effects of ESA are more evident in the presence of more severe inflammation. Indeed, it is well known that, in CKD, inflammation contributes to the genesis of anemia and that the two conditions concur in worsening patient prognosis [37, 38, 55].
Additional differences, however, should be considered. The higher age-adjusted rates of all-cause mortality and CV mortality in HD patients observed in the US vs. Europe are explained for as much as 50 % by differences existing in the mortality rates of respective general populations [56]. The impact of general population mortality on HD mortality has also been confirmed within Europe, where 26 % of the European north-south mortality difference could be attributed to the variability of general population mortality [57]. Interestingly, this background risk also influences the association between main traditional CV risk factors and mortality due to coronary heart disease (CHD); the Seven Countries Study showed that, at similar degrees of hypercholesterolemia or hypertension, age-adjusted CHD mortality in the general population was more than threefold higher in men from the US and northern Europe than in Mediterranean southern Europe [58, 59]. It is of great interest that remarkable geographical differences in CV mortality still persist nowadays [60], and, moreover, that such differences mimic the discrepant results, by country, of the TREAT trial (Table 3). Notably, habitual diet may contribute to the different background risk; a recent RCT has in fact demonstrated that the Mediterranean diet typical of southern Europe is independently associated with a 30 % lower risk of CV events [61], the effect being possibly due to the significant reduction of systemic vascular inflammation [62].
Finally, among the environmental factors that potentially contribute to the geographical differences in ESA responsiveness and outcome, timing of referral of CKD patients to a nephrologist may also play a significant role. In Europe, early referral (at least 6 months before the first HD session) has been reported in 50–70 % of the CKD population [63–65]. Conversely, late referral is common in the US; a recent retrospective cohort study of the Veterans Health Administration examining 89,585 patients with CKD and anemia has shown that only 16 % were seen by a nephrologist [66]. Indeed, one of the main goals of the Healthy People 2020 program in the US is to increase early referral to a nephrologist from the 2007 value of 27.1 % to 29.8 % [67]. Steady nephrology care is likely to be essential to limit the phenomenon of ESA resistance. There are in fact main nephrologist interventions that improve responsiveness to ESA, namely slowing of GFR decline [68], and prescription of a low-protein diet [51], iron supplements [69], and vitamin D [70]. Whether other factors, such as genetic background or healthcare organization system, may play a role in explaining the geographical differences in ESA responsiveness and outcome remains to be elucidated.
6 Conclusions
ESA responsiveness is emerging as a critical element in the management of anemic CKD patients. This hypothesis is supported by the secondary analyses of CHOIR and TREAT [32, 41], including the most recent analysis showing that higher Hb levels (>11.5 g/dL) are not associated with the penalty of adverse CV outcomes if maintained by means of low doses of ESA (<10,000 IU/week) [33]. Hence, individualizing care is now recommended by current KDIGO guidelines (Table 2). This holds true everywhere in the world. However, US patients require higher ESA doses compared with individuals living outside the US. Therefore, if different targets are to be applied to patients who respond differently, higher Hb targets may possibly be indicated outside the US where patients can more often be maintained in a higher Hb range by means of low ESA doses. These good responders can indeed potentially gain advantages from higher Hb levels because of the reduced need for blood transfusions and better QoL. However, hypotheses must be adequately verified before changing clinical practice. Indeed, as stated by the KDIGO panel of experts [12], ad hoc studies are required to gain insight into the observed ‘differences in anemia treatment outcomes between different geographic regions’.
References
Mohanram A, Zhang Z, Shahinfar S, Keane WF, Brenner BM, Toto RD. Anemia and end stage renal disease in patients with type 2 diabetes and nephropathy. Kidney Int. 2004;66:1131–8.
Levin A, Djurdjev O, Duncan J, Rosenbaum D, Werb R. Haemoglobin at time of referral prior to dialysis predicts survival: an association of haemoglobin with long-term outcomes. Nephrol Dial Transpl. 2006;21:370–7.
Kovesdy CP, Trivedi BK, Kalantar-Zadeh K, Anderson JE. Association of anemia with outcomes in men with moderate and severe chronic kidney disease. Kidney Int. 2006;69:560–4.
De Nicola L, Minutolo R, Chiodini P, et al. SIN-TABLE CDK Study Group. Prevalence and prognosis of mild anemia in non-dialysis chronic kidney disease: a prospective cohort study in outpatient renal clinics. Am J Nephrol 2010;32:533–40.
De Nicola L, Minutolo R, Chiodini P, et al. Italian Society of Nephrology Study Group Target Blood pressure Levels (TABLE) in CKD. The effect of increasing age on the prognosis of non-dialysis patients with chronic kidney disease receiving stable nephrology care. Kidney Int 2012;82:482–8.
Singh AK, Szczech L, Tang KL, et al. CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006;355:2085–98.
Pfeffer MA, Burdmann EA, Chen CY, et al. The TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009;361:2019–32.
Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–90.
Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–15.
Skali H, Parving HH, Parfrey PS, et al. TREAT Investigators. Stroke in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia treated with Darbepoetin Alfa: the trial to reduce cardiovascular events with Aranesp therapy (TREAT) experience. Circulation 2011;124:2903–8.
Manns BJ, Tonelli M. The new FDA labeling for ESA: implications for patients and providers. Clin J Am Soc Nephrol. 2012;7:348–53.
Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int 2012;Suppl. 2:279–335.
National Institute for Health and Clinical Excellence (NICE). Anaemia management in chronic kidney disease. February 2011. http://www.nice.org.uk/nicemedia/live/13329/52851/52851.pdf. Accessed 12 Dec 2013.
Locatelli F, Bárány P, Covic A, et al. on behalf of the ERA-EDTA ERBP Advisory Board. Kidney Disease Improving Global Outcomes (KDIGO) guidelines on anaemia management in chronic kidney disease: a European Renal Best Practice (ERBP) position statement. Nephrol Dial Transpl 2013;28:1346–59.
Prisant A. TREAT versus treatment: a patient’s view of a scientific interpretation. Am J Kidney Dis. 2010;55:A31–2.
Lewis EF, Pfeffer MA, Feng A, et al. TREAT Investigators. Darbepoetin alfa impact on health status in diabetes patients with kidney disease: a randomized trial. Clin J Am Soc Nephrol 2011;6:845–55.
Lefebvre P, Vekeman F, Sarokhan B, Enny C, Provenzano R, Cremieux PY. Relationship between hemoglobin level and quality of life in anemic patients with chronic kidney disease receiving epoetin alfa. Curr Med Res Opin. 2006;22:1929–37.
Macdougall IC, Obrador GT, El Nahas M. How important is transfusion avoidance in 2013? Nephrol Dial Transpl. 2013;28(5):1092–9. doi:10.1093/ndt/gfs575.
Furuland H, Linde T, Ahlmen J, Christensson A, Strombom U, Danielson BG. A randomized controlled trial of haemoglobin normalization with epoetin alfa in pre-dialysis and dialysis patients. Nephrol Dial Transpl. 2003;18(2):353–61.
Levin A, Djurdjev O, Thompson C, et al. Canadian randomized trial of hemoglobin maintenance to prevent or delay left ventricular mass growth in patients with CKD. Am J Kidney Dis. 2005;46:799–811.
Rossert J, Levin A, Roger SD, et al. Effect of early correction of anemia on the progression of CKD. Am J Kidney Dis. 2006;47:738–50.
Drueke TB, Locatelli F, Clyne N, et al. CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006;355:2071–84.
Ritz E, Laville M, Bilous RW, et al. Anemia Correction in Diabetes Study Investigators. Target level for hemoglobin correction in patients with diabetes and CKD: primary results of the Anemia Correction in Diabetes (ACORD) Study. Am J Kidney Dis 2007;49:194–207.
Cianciaruso B, Ravani P, Barrett BJ, Levin A. Italian randomized trial of hemoglobin maintenance to prevent or delay left ventricular hypertrophy in chronic kidney disease. J Nephrol. 2008;21:861–70.
Villar E, Lièvre M, Kessler M, et al. Anemia normalization in patients with type 2 diabetes and chronic kidney disease: results of the NEPHRODIAB2 randomized trial. J Diabetes Complicat. 2011;25:237–43.
Akizawa T, Gejyo F, Nishi S, et al. Positive outcomes of high hemoglobin target in patients with chronic kidney disease not on dialysis: a randomized controlled study. Ther Apher Dial. 2011;15:431–40.
Choukroun G, Kamar N, Dussol B, et al. CAPRIT Study Investigators. Correction of post kidney transplant anemia reduces progression of allograft nephropathy. J Am Soc Nephrol 2012;23:360–8.
Roger SD, McMahon LP, Clarkson A, et al. Effects of early and late intervention with epoetin alpha on left ventricular mass among patients with chronic kidney disease (stage 3 or 4): results of a randomized clinical trial. J Am Soc Nephrol. 2004;15:148–56.
Gouva C, Nikolopoulos P, Ioannidis JP, Siamopoulos KC. Treating anemia early in renal failure patients slows the decline of renal function: a randomized controlled trial. Kidney Int. 2004;66:753–60.
Inrig JK, Barnhart HX, Reddan D, et al. Effect of hemoglobin target on progression of kidney disease: a secondary analysis of the CHOIR (Correction of Hemoglobin and Outcomes in Renal Insufficiency) trial. Am J Kidney Dis. 2012;60:390–401.
McFarlane PA, Pisoni RL, Eichleay MA, Wald R, Port FK, Mendelssohn D. International trends in erythropoietin use and hemoglobin levels in hemodialysis patients. Kidney Int. 2010;78:215–23.
Szczech LA, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int. 2008;74:791–8.
McCullough PA, Barnhart HX, Inrig JK, Reddan D, Sapp S, Patel UD, Singh AK, Szczech LA, Califf RM. Cardiovascular toxicity of epoetin-alfa in patients with chronic kidney disease. Am J Nephrol. 2013;37:549–58.
Anagnostou A, Liu Z, Steiner M, et al. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci USA. 1994;91:3974–8.
Elliott S, Sinclair AM. The effect of erythropoietin on normal and neoplastic cells. Biologics. 2012;6:163–89.
Streja E, Kovesdy CP, Greenland S, et al. Erythropoietin, iron depletion, and relative thrombocytosis: a possible explanation for hemoglobin-survival paradox in hemodialysis. Am J Kidney Dis. 2008;52:727–36.
Vaziri ND. Anemia and anemia correction: surrogate markers or causes of morbidity in chronic kidney disease? Nat Clin Pract Nephrol. 2008;4:436–45.
Keithi-Reddy SR, Addabbo F, Patel TV, Mittal BV, Goligorsky MS, Singh AK. Association of anemia and erythropoiesis stimulating agents with inflammatory biomarkers in chronic kidney disease. Kidney Int. 2008;74:782–90.
Inrig JK, Bryskin SK, Patel UD, Arcasoy M, Szczech LA. Association between high-dose erythropoiesis-stimulating agents, inflammatory biomarkers, and soluble erythropoietin receptors. BMC Nephrol. 2011;12:67–77.
Koulouridis I, Alfayez M, Trikalinos TA, Balk EM, Jaber BL. Dose of erythropoiesis-stimulating agents and adverse outcomes in CKD: a metaregression analysis. Am J Kidney Dis. 2013;61:44–56.
Solomon SD, Uno H, Lewis EF, et al. Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) Investigators. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med 2010;363:1146–55.
De Nicola L, Chiodini P, Zoccali C, et al. SIN-TABLE CKD Study Group. Prognosis of CKD patients receiving outpatient nephrology care in Italy. Clin J Am Soc Nephrol 2011;6:2421–8.
Minutolo R, Conte G, Cianciaruso B, et al. Hyporesponsiveness to erythropoiesis-stimulating agents and renal survival in non-dialysis CKD patients. Nephrol Dial Transpl. 2012;27:2880–6.
Fishbane S, Pollack S, Feldman HI, Joffe MM. Iron indices in chronic kidney disease in the National Health and Nutritional Examination Survey 1988–2004. Clin J Am Soc Nephrol. 2009;4:57–61.
Minutolo R, Locatelli F, Gallieni M, et al. REport of COmorbidities in non-Dialysis Renal Disease Population in Italy (RECORD-IT) Study Group. Anaemia management in non-dialysis chronic kidney disease (CKD) patients: a multicentre prospective study in renal clinics. Nephrol Dial Transpl 2013;28:3035–45.
Bradbury BD, Critchlow CW, Weir MR, Stewart R, Krishnan M, Hakim RH. Impact of elevated C-reactive protein levels on erythropoiesis-stimulating agent (ESA) dose and responsiveness in hemodialysis patients. Nephrol Dial Transpl. 2009;24:919–25.
Locatelli F, Andrulli S, Memoli B, et al. Nutritional-inflammation status and resistance to erythropoietin therapy in haemodialysis patients. Nephrol Dial Transpl. 2006;21:991–8.
Panichi V, Rosati A, Bigazzi R, et al. RISCAVID Study Group. Anaemia and resistance to erythropoiesis-stimulating agents as prognostic factors in haemodialysis patients: results from the RISCAVID study. Nephrol Dial Transpl 2011;26:2641–8.
de Francisco AL, Kim J, Anker SD, et al. An epidemiological study of hemodialysis patients based on the European Fresenius Medical Care hemodialysis network: results of the ARO study. Nephron Clin Pract. 2011;118:143–54.
Locatelli F, Altieri P, Andrulli S, et al. Predictors of haemoglobin levels and resistance to erythropoiesis-stimulating agents in patients treated with low-flux haemodialysis, haemofiltration and haemodiafiltration: results of a multicentre randomized and controlled trial. Nephrol Dial Transpl. 2012;27:3594–600.
Di Iorio BR, Minutolo R, De Nicola L, et al. Supplemented very low protein diet ameliorates responsiveness to erythropoietin in chronic renal failure. Kidney Int. 2003;64:1822–8.
Muntner P, Hamm LL, Kusek JW, Chen J, Whelton PK, He J. The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med. 2004;140:9–17.
Shlipak MG, Fried LF, Crump C, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107:87–92.
Stevens PE, Schernthaner G, Raptis S, Wanner C, Scherhag A, Lameire N. Characteristics, cardiovascular comorbidity and medicines management in patients with type 2 diabetes and CKD: results of the IRIDIEM study. Kidney Blood Press Res. 2010;33:119–28.
Wagner M, Alam A, Zimmermann J, et al. Endogenous erythropoietin and the association with inflammation and mortality in diabetic chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:1573–9.
Yoshino M, Kuhlmann MK, Kotanko P, et al. International differences in dialysis mortality reflect background general population atherosclerotic cardiovascular mortality. J Am Soc Nephrol. 2006;17:3510–9.
van Dijk PC, Zwinderman AH, Dekker FW, et al. Effect of general population mortality on the north-south mortality gradient in patients on replacement therapy in Europe. Kidney Int. 2007;71:53–9.
Verschuren WM, Jacobs DR, Bloemberg BP, et al. Serum total cholesterol and long-term coronary heart disease mortality in different cultures. Twenty-five-year follow-up of the seven countries study. JAMA. 1995;274:131–6.
van den Hoogen PC, Feskens EJ, Nagelkerke NJ, Menotti A, Nissinen A, Kromhout D. The relation between blood pressure and mortality due to coronary heart disease among men in different parts of the world. Seven Countries Study Research Group. N Engl J Med. 2000;342(1):1–8.
Go AS, Mozaffarian D, Roger VL, et al. on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics–2013 Update: A Report From the American Heart Association. Circulation 2013;127:e6–e245.
Estruch R, Ros E, Salas-Salvadó J, et al. PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90.
Galland L. Diet and inflammation. Nutr Clin Pract. 2010;25:634–40.
Locatelli F, Zoccali C, SIR SIN Study Investigators. Clinical policies on the management of chronic kidney disease patients in Italy. Nephrol Dial Transpl 2008;23:621–6.
Thilly N, Boini S, Kessler M, Briançon S, Frimat L. Management and control of hypertension and proteinuria in patients with advanced chronic kidney disease under nephrologist care or not: data from the AVENIR study (AVantagE de la Nephroprotection dans l’Insuffisance Renale). Nephrol Dial Transpl. 2009;24:934–9.
Pérez-García R, Martín-Malo A, Fort J, et al. ANSWER study. Baseline characteristics of an incident haemodialysis population in Spain: results from ANSWER-a multicentre, prospective, observational cohort study. Nephrol Dial Transpl 2009;24:578–88.
Lawler EV, Gagnon DR, Fink J, et al. Initiation of anaemia management in patients with chronic kidney disease not on dialysis in the Veterans Health Administration. Nephrol Dial Transpl. 2010;25:2237–44.
US Department of Health and Human Services. Healthy people 2020: summary of objectives. Chronic kidney disease. http://healthypeople.gov/2020/topicsobjectives2020/pdfs/ChronicKidneyDisease.pdf. Accessed 28 Aug 2013.
Jones C, Roderick P, Harris S, Rogerson M. Decline in kidney function before and after nephrology referral and the effect on survival in moderate to advanced chronic kidney disease. Nephrol Dial Transpl. 2006;21:2133–43.
Rozen-Zvi B, Gafter-Gvili A, Paul M, Leibovici L, Shpilberg O, Gafter U. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: systematic review and meta-analysis. Am J Kidney Dis. 2008;52:897–906.
Icardi A, Paoletti E, De Nicola L, Mazzaferro S, Russo R, Cozzolino M. Renal anaemia and EPO hyporesponsiveness associated with vitamin D deficiency: the potential role of inflammation. Nephrol Dial Transpl. 2013;28:1672–9.
Acknowledgments
No sources of funding were used to assist in the preparation of this article. Dr Luca De Nicola has received consulting fees from Abbott, MSD, and BMS. Dr Francesco Locatelli has served as an advisor for Abbott, Affymax, Amgen, Fresenius, Pharmacosmos, Fibrogen, Hoffmann-La Roche, GSK, Takeda, Janssen, Vifor, and Sandoz. Dr Giuseppe Conte has received honoraria for lectures for Amgen, and Roche, and advisory board membership from Abbott. Dr. Roberto Minutolo has received consulting fees from Roche and honoraria for lectures for Abbott, Amgen, and Roche.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Nicola, L., Locatelli, F., Conte, G. et al. Responsiveness to Erythropoiesis-Stimulating Agents in Chronic Kidney Disease: Does Geography Matter?. Drugs 74, 159–168 (2014). https://doi.org/10.1007/s40265-013-0175-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-013-0175-3