Abstract
Obinutuzumab (Gazyva™) is an intravenously administered, humanized and glycoengineered, type II anti-CD20 monoclonal antibody for the treatment of B-cell malignancies. It is approved in the US for use in combination with chlorambucil for the first-line treatment of chronic lymphocytic leukaemia (CLL), and has been filed for approval in the EU in this indication. The antibody is based on GlycArt Biotechnology’s (later Roche Glycart AG) proprietary GlycoMAb® technology, which uses glycoengineered antibodies that specifically increase antibody-dependent cellular cytotoxicity and thereby increase immune-mediated target cell death. Obinutuzumab is a type II anti-CD20 antibody that induces enhanced direct cell death. The monoclonal antibody is in worldwide phase III development with Roche and its subsidiaries, Genentech and Chugai Pharmaceutical, as well as Biogen Idec, for diffuse large B-cell lymphoma and non-Hodgkin’s lymphoma generally, and is also in phase III development in countries outside of the US and EU for CLL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ever since the introduction of rituximab, CD20-targeted monoclonal antibodies, usually in combination with chemotherapeutic agents, have played a key role in the treatment of B-cell malignancies, such as chronic lymphocytic leukaemia (CLL) and other B-cell non-Hodgkin’s lymphomas (NHL). The CD20 antigen is an ideal target, being present on 95 % of all B lymphocytes, but absent on the haematopoietic stem cell, and is highly expressed on malignant B cells and not readily shed from the cell surface [1–5]. Anti-CD20 antibodies invoke antibody-dependent cellular cytotoxicity (ADCC) or phagocytosis (ADCP), complement-dependent cytotoxicity (CDC) and direct cell death [6].
Obinutuzumab (Gazyva™) is a glycoengineered, humanized, type II, anti-CD20 monoclonal antibody of the IgG1 class that was approved in early November 2013 by the US FDA for use in combination with chlorambucil as a first-line therapy for CLL [7]. Obinutuzumab is the first treatment approved with the FDA’s breakthrough therapy designation as a result of the significant activity demonstrated in the pivotal trial (CLL11) in patients with CLL [7]. Obinutuzumab was launched in the US shortly after approval. A marketing application for obinutuzumab in the first-line treatment of CLL has also been submitted to the European Medicines Agency.
Obinutuzumab is administered as an intravenous infusion for six 28-day cycles in the first-line treatment of CLL [8]. In cycle 1, 100 mg is infused on day 1, 900 mg is infused on day 2, then 1,000 mg is infused on days 8 and 15. In cycles 2–6, obinutuzumab 1,000 mg is infused on day 1. For the standard 1,000 mg infusion, it is recommended that the infusion be started at a rate of 100 mg/h and increased by 100 mg/h every 30 min up to a maximum rate of 400 mg/h [8]. Premedication with intravenous glucocorticoids, paracetamol (acetaminophen) and antihistamine before each infusion is recommended to reduce the risk of infusion reactions [8]. As is the case for other anti-CD20 monoclonal antibodies, the US prescribing information carries a boxed warning regarding potential reactivation of hepatitis B virus and the development of progressive multifocal leukoencephalopathy (PML).
Obinutuzumab is in phase III development in many countries worldwide for the first-line combination treatment of CLL, the first-line combination treatment of diffuse large B-cell lymphoma (DLBCL) and the first-line, or second-line or greater, combination treatment of NHL. It is in phase II development in many countries worldwide for second-line or greater monotherapy of NHL. Biogen Idec was assessing the potential therapeutic effect of anti-CD20 monoclonal antibodies for the treatment of early primary biliary cirrhosis [9]. However, it appears that development for this indication was discontinued. Preclinical research had indicated the potential benefit of anti-CD20 antibodies in early primary biliary cirrhosis resistant to other modalities, but had raised caution regarding the use of anti-CD20 antibodies in inflammatory bowel disease [9].
1.1 Commercial Agreements
In November 2012, Chugai Pharmaceuticals (a subsidiary of Roche) and Nippon Shinyaku entered into a co-development and co-marketing agreement for obinutuzumab in Japan. Under the terms of the agreement, Chugai received an undisclosed upfront fee, and milestone payments from Nippon Shinyaku [10].
GlycArt and Roche entered into a collaboration agreement in October 2008, for the joint development and commercialization of obinutuzumab for the potential treatment of haematological malignancies and other oncology-related B-cell disorders. In association with this agreement, Genentech, Roche and GlycArt were to share certain development costs, and Genentech recorded $US105 million in research and development expense in its third quarter 2008 results. Genentech was granted commercialization rights in the US [11].
Biogen Idec elected to participate in the development of obinutuzumab, pursuant to the agreement with Genentech. The agreement included an upfront payment of $US31.5 million to Genentech. The two companies were to share certain development costs and share operating profits and losses in the US. Roche retains commercialization rights outside the US [12]. In October 2010, both companies agreed that Biogen Idec would increase its share of the losses and profits related to the development and commercialization of obinutuzumab in the US to 35 from 30 %. Biogen Idec paid Genentech approximately $US10 million as a catch-up payment for expenses incurred to that date [13].
In July 2005, Roche acquired GlycArt Biotechnology and subsequently renamed it as Roche Glycart [14].
Features and properties of obinutuzumab
Alternative names | Afutuzumab; GA 101; GA101; Gazyva™; R 7159; R7159; RG 7159; RG7159; RO 5072759; RO5072759 |
Class | Monoclonal antibodies |
Mechanism of Action | CD20 antigen inhibitors, immunomodulators |
Route of Administration | Intravenous infusion |
Pharmacodynamics | Demonstrates higher efficacy in chronic lymphocytic leukaemia and mantle cell lymphoma cell lines than rituximab; significantly enhances targeted cell death induction; anti-tumour effects in models of lymphoma; improves autoimmune cholangitis, but exacerbates colitis in a mouse model; rapid and sustained depletion of B cells in patients; increases plasma cytokine levels |
Pharmacokinetics | Both linear and time-dependent saturable clearance components |
Adverse events | |
Most frequent | Infusion reactions, neutropenia |
Occasional | Thrombocytopenia, anaemia, pyrexia, cough, musculoskeletal disorders, infection |
Rare | Tumour lysis syndrome, progressive multifocal leukoencephalopathy, hepatitis B virus reactivation |
ATC codes | |
WHO ATC code | A05B (Liver Therapy, Lipotropics), L01X-C15a (Monoclonal antibodies) |
EphMRA ATC code | A5B (Hepatic Protectors, Lipotropics), L1X3 (Antineoplastic monoclonal antibodies) |
Chemical name | Immunoglobulin G1, anti-(human CD20 (antigen)) (human-mouse monoclonal GA101 heavy chain), disulfide with human-mouse monoclonal GA101 κ-chain, dimer |
2 Scientific Summary
2.1 Pharmacodynamics
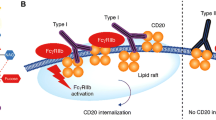
Obinutuzumab is a type II anti-CD20 monoclonal antibody and therefore displays enhanced induction of direct cell death. Anti-CD20 monoclonal antibodies are categorized as type I or II depending upon their mode of binding and their cytolytic activities [5, 6]. Type I anti-CD20 antibodies (e.g. rituximab, veltuzumab, ocrelizumab and ofatumumab) cause translocation of CD20 into large insoluble lipid microdomains (lipid rafts) within the cell membrane, which enhances complement binding, and so type I antibodies exert potent CDC activity. In contrast, type II antibodies (e.g. tositumomab and obinutuzumab) do not cause accumulation of CD20 in lipid rafts and show weak CDC activity. Type I and II antibodies bind to different epitopes on CD20 and B lymphocytes bind twice as many type I antibodies as type II antibodies, which presumably relates to the differences in binding geometry [15]. Type II antibodies are more potent than type I antibodies at inducing intercellular homotypic adhesion. As a result, type II anti-CD20 antibodies more potently evoke direct cell death than type I antibodies by a mechanism involving actin rearrangement, rupture of lysosomes and the generation of reactive oxygen species, but which differs from apoptosis [5, 6]. The Fc region of obinutuzumab was glycoengineered by reduction in fucose content to produce enhanced affinity for FcγRIIIa, resulting in an increased ability to bind and recruit effector cells, and therefore greater ADCC activity [5, 16].
Obinutuzumab was more effective than rituximab or ofatumumab as either first- or second-line therapy in mouse xenograft models of human lymphoma [16, 17]. Complete tumour remission was achieved with obinutuzumab in SU-DHL4 DLBCL xenografts [16]. While both obinutuzumab and rituximab efficiently depleted B cells from peripheral blood in cynomolgus monkeys, obinutuzumab produced greater depletion of B cells in spleen and lymph nodes than rituximab [16]. In in vitro assays using cell lines or cells from patients with CLL, obinutuzumab was more effective than rituximab and ofatumumab at inducing direct cell death or ADCC, and whole-blood B-cell depletion, but was 10–1,000-fold less potent in mediating CDC [17, 18].
In the phase II part of the phase I/II GAUGUIN trial (BO20999) of obinutuzumab monotherapy in heavily pretreated patients (n = 20) with relapsed/refractory CLL, peripheral B cell depletion for all patients was rapid following the first infusion of obinutuzumab, with all patients achieving levels of <4,000/μL within 1 week of treatment [19]. Reduced B-cell levels were sustained during the treatment period.
In the phase I part (n = 13) of the GAUGUIN trial, increases in plasma cytokine levels [interleukin (IL) 6 and IL8, IL10, interferon (IFN)-γ and tumour necrosis factor (TNF)-α] were observed during and after the first infusion with obinutuzumab (400–2,000 mg) [20]. The increases in cytokine levels correlated with a decrease in T-cell subsets and natural killer (NK) cell counts, but no complement activation (C3a, C4a and C5a) was observed. By the end of treatment, CD3+ and CD8+ cells had recovered to normal levels, while CD4+ and CD16/56+ counts remained below the normal limit. Although full T-cell recovery had not occurred, no clinical manifestations were observed [20].
Results from a phase I study in 21 patients with relapsed/refractory CD20-positive NHL showed no significant change in complement fractions (C3, C3a, C4a, C5, C5a, Bb) [21]. Measurement of plasma cytokines during and immediately after the first infusion showed an increase in IL6 and IL8 levels with a smaller increase in IL10, TNF-α and IFN-γ, with recovery by day 8. Median T-cell (CD3+, CD4+ and CD8+) subsets and NK cell counts were low in all patients prior to therapy. Concurrent with the increase in cytokine levels, a further decrease in these lymphocyte subsets was observed after the first infusion, which recovered thereafter, with median values returning to baseline values by end of treatment. In contrast, B-cell (CD19+) depletion was rapid and sustained in 19 of 21 patients. End-of-treatment B-cell recovery was observed in three of the responding patients, with recovery to baseline levels by days 410–532 after the first dose. No significant changes in immunoglobulin levels from baseline were observed [21].
Data from a separate phase I trial also showed increases in IL6, IL8, IL10 and TNF-α levels after the first obinutuzumab infusion. This trial included 22 heavily-pretreated patients with CD20-positive malignancies. Obinutuzumab was administered on days 1, 8 and 22, then every 21 days for a total of 9 doses [22].
2.2 Pharmacokinetics
Obinutuzumab pharmacokinetics were characterized by two clearance components (one linear and one time-dependent saturable component consistent with target-mediated disposition), as demonstrated in the phase I/II GAUGUIN (BO20999) trial in patients with heavily pretreated indolent NHL [23]. Responding patients appeared to eliminate obinutuzumab slower than non-responding patients [23].
Obinutuzumab pharmacokinetic assessments also showed time-dependent and saturable clearance components in patients with CLL in the phase I part of the GAUGUIN trial [20]. Overall clearance at the same dose was faster in patients with CLL than with NHL [20].
Population pharmacokinetic studies indicate that the steady-state mean volume of distribution of obinutuzumab is ≈3.8 L, the mean terminal clearance is 0.09 L/day and the elimination half-life is 28 days [8]. Dose modification of obinutuzumab according to patient age, body weight or renal impairment is not necessary [8].
2.3 Therapeutic Trials
2.3.1 Chronic Lymphocytic Leukaemia
2.3.1.1 Phase III Trial
CLL11 was a randomized, controlled, open-label, international, phase III trial conducted by Roche/Genentech in collaboration with the German CLL Study Group (BO21004) [24]. A total of 781 treatment-naive patients with CLL were included in the three-arm, two-stage trial. Stage 1 of the trial compared the efficacy of obinutuzumab plus chlorambucil, rituximab plus chlorambucil, and chlorambucil alone in 589 patients; an additional 192 patients were enrolled into stage 2, which was a head-to-head comparison of obinutuzumab plus chlorambucil and rituximab plus chlorambucil [24]. Results from stage 1a demonstrated that patients who received obinutuzumab plus chlorambucil had a significantly reduced risk of disease progression or death [hazard ratio (HR) 0.14, 95 % CI 0.09–0.21; p < 0.0001], and had a significantly longer PFS than those who received chlorambucil alone (median 23.0 vs. 10.9 months) [24, 25]. Obinutuzumab was approved by the FDA based on these data. In the stage 2 analysis, obinutuzumab plus chlorambucil demonstrated superiority over rituximab plus chlorambucil (HR 0.39, 95 % CI 0.31–0.49; p < 0.0001) with a significantly longer duration of progression free survival (PFS) (median 26.7 vs. 15.2 months) [24]. The trial met the endpoint ahead of schedule following an independent interim analysis.
2.3.1.2 Phase I/II Trials
Obinutuzumab had promising activity when administered as monotherapy in the phase II part of a phase I/II study in heavily pretreated patients with relapsed/refractory CLL (GAUGUIN) [19]. Twenty patients received obinutuzumab at a dosage of 1,000 mg on days 1, 8, 15 and 22, and then every 21 days for a total of 10 infusions. Among 16 evaluable patients, the end-of-treatment response was 25 % (four partial responses); five patients had stable disease and seven had progressive disease.
Treatment with obinutuzumab resulted in a 62 % response rate in the phase I part of the GAUGUIN trial, which involved 13 patients with CLL [20]. One patient had a complete response, while seven had partial responses and five had stable disease. The duration of response ranged from 3.5 to 8+ months, and was ongoing at time of publication. No dose-response relationship was established. Minimal residual disease was detected in six of seven evaluable patients.
2.3.2 Non-Hodgkin’s Lymphoma
2.3.2.1 Phase II Trials
Treatment with obinutuzumab (1,000 mg; n = 74) once-weekly for 4 weeks resulted in an overall response rate of 44.6 versus 33.3 % for rituximab (375 mg/m2; n = 75) based on the preliminary investigators’ assessment in the subset of patients with follicular lymphoma (primary endpoint) in the phase II, open-label GAUSS (BO21003) trial, which enrolled patients with relapsed CD20-positive indolent NHL [26]. The complete remission rate, including unconfirmed complete remission, was 35.1 % in the obinutuzumab group versus 18.7 % in the rituximab group.
In the phase II part of the phase I/II GAUGUIN (BO20999) trial, end-of-treatment response rates of 24 and 32 % were achieved in patients with heavily pretreated DLBCL or mantle-cell lymphoma (MCL) receiving the obinutuzumab 400/400 mg (n = 21) or 1,600/800 mg (n = 19) regimens, respectively, for eight 21-day cycles [27]. Patients in the 400/400 mg cohort received 400 mg for all doses, while those in the 1,600/800 received 1,600 mg on days 1 and 8 of cycle 1 and 800 mg on day 1 of subsequent cycles. The best overall response was 32 % (4 complete or unconfirmed complete responses) in DLBCL patients (n = 25) and 27 % (2 complete or unconfirmed complete responses) in MCL patients (n = 15) [27]. The median overall response duration was 9.8 months and the median PFS times were 2.6 and 2.7 months in the 400/400 and 1,600/800 mg groups, respectively, after a median observation time of 14.2 months [27].
An additional report on the phase II part of the GAUGUIN study, showed end-of-treatment overall response rates of 17 % for the 400/400 mg regimen (n = 18) and 55 % for the 1,600/800 mg regimens (n = 22), and respective best overall response rates of 33 and 64 % [28]. After a median observation time of 33.7 months, the median duration of response was 17.2 months [28].
2.3.2.2 Phase I Trials
In the phase I, dose-escalation part of the GAUGUIN study, responses to obinutuzumab were observed at all obinutuzumab dose levels, and across all FcγRIIIa genotypes [29]. The trial included 21 patients with relapsed/refractory CD20-positive NHL. Best overall responses were five confirmed and unconfirmed complete responses and four partial responses for an overall response rate of 43 %. Five patients had stable disease, six had progressive disease, and one patient was not evaluable. Of the nine responses (all with follicular lymphoma), eight occurred during the treatment phase. Two of nine rituximab-refractory patients responded [29].
The GAUDI trial (BO21000) enrolled 56 patients with relapsed or refractory follicular lymphoma and 81 patients with previously untreated follicular lymphoma [30, 31]. Previously untreated patients received obinutuzumab in combination with CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone; n = 40) for 6–8 28-day cycles or bendamustine (n = 41) for 4–6 cycles [30]. At the end of the induction period, the overall response rate was 95 % in the obinutuzumab + CHOP arm and 93 % in the obinutuzumab + bendamustine arm, with respective complete response rates of 35 and 39 % [30]. In patients with relapsed or refractory follicular lymphoma, the response rates were 96 % (39 % complete response) and 93 % (50 % complete response) in patients who received obinutuzumab in combination with either CHOP (6–8 cycles) or fludarabine plus cyclophosphamide (4–6 cycles), respectively [31]. Patients in each treatment arm were randomized to one of two dosages of obinutuzumab (400 mg on days 1 and 8 of cycle 1 and 400 mg for subsequent cycles, or 1,600 mg on days 1 and 8 of cycle 1 and 800 mg for subsequent cycles).
In the phase Ib Galton study (GAO4779g), patients with previously untreated CLL were treated with obinutuzumab plus fludarabine/cyclophosphamide (n = 21) or obinutuzumab plus bendamustine (n = 20) for eight 28-day cycles [32]. The overall response rate with obinutuzumab plus fludarabine/cyclophosphamide was 62 % (2 complete responses, 3 complete responses with incomplete blood count recovery and 8 partial responses) and that with obinutuzumab plus bendamustine was 90 % (4 complete responses, 5 complete responses with incomplete blood count recovery and 9 partial responses). None of the patients progressed during the study.
In phase I of the GAUSS trial, patients with relapsed or refractory NHL (n = 17) or CLL (n = 5) received escalating-dose induction therapy with obinutuzumab monotherapy for 28 days (four weekly infusions), after which responders and patients with stable disease and clinical benefit continued maintenance therapy with obinutuzumab [33]. At the end of the induction period, 5 of 21 evaluable patients had a partial response, 12 had stable disease and 4 had disease progression. Eight patients continued with maintenance therapy and four completed 2 years of maintenance treatment without disease progression, with three of these patients improving their response [33].
In a phase I Japanese study, twelve patients with relapsed or refractory NHL received one of four doses of obinutuzumab (200/400, 400/800, 800/1,200 or 1,200/2,000 mg) for 8 cycles, and seven patients responded (2 complete response, 5 partial response and 5 with stable disease) [34]. Ten patients showed a reduction in tumour mass. The responses occurred across doses, with no obvious dose-response relationship.
Key clinical trials of obinutuzumab
Drugs | Indication | Phase | Status | Location | Trial Identifier(s) | Company |
|---|---|---|---|---|---|---|
Obinutuzumab + chlorambucil vs. rituximab + chlorambucil vs. chlorambucil | CLL (previously untreated) | III | Completed | Global | CLL11; NCT01010061 | Roche, Genentech, Biogen Idec |
Obinutuzumab + bendamustine or fludarabine/cyclophosphamide vs. bendamustine or fludarabine/cyclophosphamide | CLL (previously untreated or relapsed/refractory) | III | Recruiting | Global ex-USA | NCT01905943 | Roche |
Obinutuzumab (single agent) | CLL (previously untreated) | II | Enrolment completed | USA | NCT01414205 (GAGE) | Genentech |
Obinutuzumab + CHOP vs. rituximab + CHOP | DLBCL (previously untreated) | III | Recruiting | Global | NCT01287741 (GOYA) | Roche |
Obinutuzumab + CHOP | DLBCL (previously untreated) | II | Enrolment completed | USA | NCT01414855 (GATHER) | Genentech |
Obinutuzumab + bendamustine vs. bendamustine | Indolent NHL (rituximab refractory) | III | Recruiting | USA, Canada, Europe | NCT01059630 (GADOLIN) | Genentech, Roche |
Obinutuzumab + CHOP or CVP or bendamustine vs. rituximab + CHOP or CVP or bendamustine | Indolent NHL (previously untreated) | III | Recruiting | Global | NCT01332968 (GALLIUM) | Roche |
Obinutuzumab vs. rituximab | Indolent NHL (relapsed) | II | Completed | USA, Canada, Europe, Latin America | NCT00576758 (GAUSS) | Roche |
Obinutuzumab + CHOP vs. bendamustine or FC | Follicular lymphoma (relapsed/refractory and previously untreated) | I | Enrolment completed | Global | NCT00825149 (GAUDI) | Roche |
Obinutuzumab | CD20-positive malignancies | I/II | Enrolment completed | France, Germany | NCT00517530 (GAUGUIN) | Roche |
Obinutuzumab + FC or bendamustine | CLL (previously untreated) | I | Completed | USA | NCT01300247 (Galton) | Genentech |
Obinutuzumab | CD20-positive malignancies (relapsed/refractory) | I | Enrolment completed | China | NCT01680991 | Roche |
2.4 Adverse Events
2.4.1 Chronic Lymphocytic Leukaemia
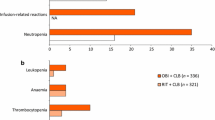
The most common grade 3/4 adverse events with obinutuzumab plus chlorambucil in the CLL11 trial, compared with those who received chlorambucil alone, were neutropenia (34 vs. 16 %), infusion-related reactions (21 vs. 0 %), thrombocytopenia (11 vs. 3 %), leukopenia (5 vs. 0 %) and anaemia (4 vs. 5 %) [8]. The overall incidence of infusion-related reactions of any grade was 69 % in obinutuzumab recipients compared with zero in those who received chlorambucil alone; hence, premedication is recommended prior to administration [8]. The incidence and severity of infusion-related events decreased markedly after the first infusion and no serious infusion-related events were reported beyond the first infusion. Results from stage two of the trial, which compared obinutuzumab in combination with chlorambucil, and rituximab in combination with chlorambucil, were similar to those seen in stage one [24]. Grade 3/4 infusion-related adverse events occurred in 20 % of patients in the obinutuzumab plus chlorambucil group versus 4 % of those in the rituximab plus chlorambucil group [24]. Rare, but potentially serious reactions that may occur with obinutuzumab include hepatitis B virus reactivation, progressive multifocal leukoencephalopathy (PML) and tumour lysis syndrome [8]. Serum antibodies to obinutuzumab have been detected in 13 % of obinutuzumab recipients, but their clinical significance is not known [8].
2.4.2 Non-Hodgkin’s Lymphoma
The tolerability of obinutuzumab in patients with NHL was similar to that in patients with CLL; the most common grade 3/4 adverse events were infusion-related reactions or haematological toxicities [27–29, 31, 33, 34]. The most common infusion-related reactions were hypotension, pyrexia, nausea, vomiting, chills, asthenia, flushing, headache and larynx irritation [29].
2.5 Ongoing Clinical Trials
A phase IIIb trial is underway to assess the safety and efficacy of obinutuzumab, alone or in combination with chemotherapy (bendamustine or chlorambucil), in patients with previously untreated or relapsed/refractory CLL (MO28543; NCT01905943; EudraCT 2013-000087-29). Enrolment of the planned 800 patients has begun in Germany, Finland, Estonia and Slovenia, and is also expected to take place in other countries worldwide except the US.
Recruitment of approximately 1,400 patients for a phase III trial to investigate the efficacy and tolerability of obinutuzumab plus CHOP, compared with rituximab plus CHOP, in patients with previously untreated CD20-positive DLBCL (GOYA; NCT01287741; EudraCT 2010-024194-39) is underway in the US, the EU, Argentina, Colombia, Australia, Canada, China, Hong Kong, Japan, Mexico, Russia, Serbia, South Korea, South Africa, Switzerland, Taiwan and Thailand.
Another large, phase III study (estimated enrolment of 670 patients) in newly diagnosed CD20-positive DLBCL (NCT01659099; GAINED) being conducted in France, Belgium and Portugal is actively recruiting and will compare obinutuzumab + chemotherapy (CHOP or ACVBP) with rituximab + chemotherapy using PET scans to identify early responders and adapt consolidation treatment.
A phase II trial to assess the efficacy and safety of obinutuzumab in combination with CHOP chemotherapy as first-line therapy in patients with advanced CD20-positive DLBCL (GATHER; NCT01414855) is also underway. Enrolment of 101 patients was completed in the US in August 2013.
A phase III trial was initiated in 2010, to evaluate the safety and efficacy of obinutuzumab plus bendamustine, compared with bendamustine monotherapy, in patients with rituximab-refractory, indolent NHL (GADOLIN; NCT01059630). The trial is expected to enrol approximately 360 patients in the US, Canada and Europe. Data from this study are expected in 2015.
Another phase III trial in patients with previously-untreated indolent NHL (GALLIUM; BO21223; NCT01332968) is underway. This trial is comparing obinutuzumab in combination with chemotherapy followed by obinutuzumab maintenance therapy in responders (arm A), and rituximab plus chemotherapy followed by rituximab maintenance therapy in responders (arm B). Approximately 1,400 patients are expected to be enrolled in the US, EU, Australia, Canada and Japan.
Phase I or II studies planned or underway in patients with NHL include NCT01889797 (BO25454), NCT01680991 (YP25623) and NCT01582776 (Galen).
3 Current Status
Obinutuzumab received its first global approval on the 1st of November 2013 for use in combination with chlorambucil to treat patients with previously untreated CLL in the USA.
References
Seiler TM, Hiddemann W. Advances in the management of follicular lymphoma. Curr Opin Oncol. 2012;24(6):742–7.
Goede V, Hallek M. Optimal pharmacotherapeutic management of chronic lymphocytic leukaemia: considerations in the elderly. Drugs Aging. 2011;28:163–76.
Chang JE, Kahl BS. Current status of targeted therapies for mantle cell lymphoma. Drugs. 2011;71:2307–26.
Merli M, Ferrario A, Basilico C, et al. Novel agents in indolent lymphomas. Ther Adv Hematol. 2013;4(2):133–48.
Klein C, Lammens A, Schafer W, et al. Epitope interactions of monoclonal antibodies targeting CD20 and their relationship to functional properties. mAbs. 2013;5(1):22–33.
Honeychurch J, Alduaij W, Azizyan M, et al. Antibody-induced nonapoptotic cell death in human lymphoma and leukemia cells is mediated through a novel reactive oxygen species-dependent pathway. Blood. 2012;119(15):3523–33.
US FDA. FDA approves Gazyva for chronic lymphocytic leukemia [media release]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm373209.htm. Accessed 1 Nov 2013.
US FDA. Gazyva (obinutuzumab): US prescribing information. 2013. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125486s000lbl.pdf. Accessed 14 Nov 2013.
Moritoki YB, Lian ZX, Lindor K, et al. Cell depletion with anti-CD20 ameliorates autoimmune cholangitis but exacerbates colitis in transforming growth factor-beta receptor II dominant negative mice. Hepatology. 2009;50(6):1893–903.
Chugai Pharmaceutical Co Ltd, Nippon Shinyaku Co Ltd. Co-development and co-marketing agreement for the glycoengineered type II anti-CD20 monoclonal antibody, “GA101 (obinutuzumab)” [media release]. http://www.chugai-pharm.co.jp. Accessed 27 Nov 2012.
Genentech, Roche. Genentech, GlycArt and Roche enter into collaboration agreement for potential cancer therapy [media release]. http://www.gene.com. Accessed 3 Oct 2008.
Biogen Idec. Biogen Idec elects to participate with Genentech in the development and commercialization of a next generation anti-CD20 molecule [media release]. http://www.biogenidec.com. Accessed 31 Oct 2008.
Biogen Idec, Genentech Inc. Biogen Idec and Genentech announce restructuring of anti-CD20 collaboration agreement [media release]. http://www.gene.com. Accessed 22 Oct 2010.
GlycArt Biotechnology AG. Acquisition of GlycArt Biotechnology completed [media release]. http://www.glycart.com. Accessed 26 Jul 2005.
Niederfellner G, Lammens A, Mundigl O, et al. Epitope characterization and crystal structure of GA101 provide insights into the molecular basis for type I/II distinction of CD20 antibodies. Blood. 2011;118(2):358–67.
Mossner E, Brunker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115(22):4393–402.
Herter S, Herting F, Mundigl O, et al. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther. 2013;12(10):2031–42.
Rafiq S, Butchar JP, Cheney C, et al. Comparative assessment of clinically utilized CD20-directed antibodies in chronic lymphocytic leukemia cells reveals divergent NK cell, monocyte, and macrophage properties. J Immunol. 2013;190(6):2702–11.
Cartron G, Morschhauser F, Thieblemont C, et al. Results from a phase II study of obinutuzumab (GA101) monotherapy in relapsed/refractory chronic lymphocytic leukemia (CLL) [abstract no. 0101]. Haematologica. 2011;96(Suppl 2):39–40.
Morschhauser F, Cartron G, Lamy T, et al. Phase I study of RO5072759 (GA101) in relapsed/refractory chronic lymphocytic leukemia [abstract no. 884]. Blood. 2009;114 Suppl.
Salles G, Morschhauser F, Lamy T, et al. Phase I study of RO5072759 (GA101) in patients with relapsed/refractory CD20+ non-Hodgkin lymphoma [abstract no. 1704]. Blood. 2009;114 Suppl.
Sehn LH, Assouline SE, Stewart DA, et al. A phase I study of GA101 (RO5072759) monotherapy followed by maintenance in patients with multiply relapsed/refractory CD20+ malignant disease [abstract no. 934]. Blood. 2009;114 Suppl.
Meneses-Lorente G, Carlile D, Birkett J, et al. Pharmacokinetics of RO5072759 (GA101) in patients with relapsed/refractory CD20+ malignant disease (phase I/II study BO20999) [abstract no. 1833]. Blood. 2010;116.
Goede V, Fischer K, Busch R, et al. Head-to-head comparison of obinutuzumab (GA101) plus chlorambucil (Clb) versus rituximab plus Clb in patients with chronic lymphocytic leukemia (CLL) and co-existing medical conditions (comorbidities): final stage 2 results of the CLL11 trial. 55th American Society of Hematology Annual Meeting and Exposition, 7–10 Dec, New Orleans. 2013. https://ash.confex.com/ash/2013/webprogram/Paper60276.html.
Hallek M, Fischer K, Humphrey K, et al. Obinutuzumab (GA101) + chlorambucil (CLB) or rituximab (r) + Clb versus Clb alone in patients with chronic lymphocytic leukaemia (CLL) and pre-existing medical conditions (comorbidities): final stage 1 results of the CLL11 (BO21004) phase 3 trial [abstract no. 056]. Hematol Oncol. 2013;31(Suppl 1):114–5.
Goy A, Offner F, Martinelli G, et al. Randomized phase II trial comparing obinutuzumab (GA101) with rituximab in patients with relapsed CD20+ indolent B-cell non-Hodgkin lymphoma: preliminary analysis of the GAUSS study [abstract no. 0790]. Haematologica. 2012;97(Suppl 1):322–33.
Morschhauser FA, Cartron G, Thieblemont C, et al. Obinutuzumab (GA101) monotherapy in relapsed/refractory diffuse large b-cell lymphoma or mantle-cell lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013;31(23):2912–9.
Salles GA, Morschhauser F, Solal-Celigny P, et al. Obinutuzumab (GA101) in patients with relapsed/refractory indolent non-Hodgkin lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013;31(23):2920–6.
Salles G, Morschhauser F, Lamy T, et al. Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood. 2012;119(22):5126–32.
Dyer MJS, Grigg A, Gonzalez M, et al. Obinutuzumab (GA101) in combination with cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) or bendamustine in patients with previously untreated follicular lymphoma (FL): results of the phase Ib GAUDI study (BO21000) [abstract no. 3686]. Blood. 2012;120(21).
Radford J, Davies A, Cartron G, et al. Obinutuzumab (GA101) plus CHOP or FC in relapsed/refractory follicular lymphoma: results of the GAUDI study (BO21000). Blood. 2013;122(7):1137–43.
Brown JR, O’Brien S, Kingsley CD, et al. Safety and efficacy of obinutuzumab (GA101) with fludarabine/cyclophosphamide (G-FC) or bendamustine (G-B) in the initial therapy of patients with chronic lymphocytic leukemia (CLL): results from the phase 1b Galton trial (GAO4779g). 55th American Society of Hematology Annual Meeting and Exposition, 7–10 Dec, New Orleans. 2013. https://ash.confex.com/ash/2013/webprogram/Paper63449.html.
Sehn LH, Assouline SE, Stewart DA, et al. A phase 1 study of obinutuzumab induction followed by 2 years of maintenance in patients with relapsed CD20-positive B-cell malignancies. Blood. 2012;119(22):5118–25.
Ogura M, Tobinai K, Hatake K, et al. Phase I study of obinutuzumab (GA101) in Japanese patients with relapsed or refractory B-cell non-Hodgkin lymphoma. Cancer Sci. 2013;104(1):105–10.
Author information
Authors and Affiliations
Corresponding author
Additional information
This profile has been extracted and modified from the Adis R&D Insight drug pipeline database. Adis R&D Insight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch.
Rights and permissions
About this article
Cite this article
Cameron, F., McCormack, P.L. Obinutuzumab: First Global Approval. Drugs 74, 147–154 (2014). https://doi.org/10.1007/s40265-013-0167-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-013-0167-3