Abstract
Major depressive disorder (MDD) is a leading cause of disability worldwide and less than one-third of patients with MDD achieve stable remission of symptoms, despite currently available treatments. Although MDD represents a serious health problem, a complete understanding of the neurobiological mechanisms underlying this condition continues to be elusive. Accumulating evidence from preclinical and animal studies provides support for the antidepressant potential of modulators of KCNQ voltage-gated potassium (K+) channels. KCNQ K+ channels, through regulation of neuronal excitability and activity, contribute to neurophysiological mechanisms underlying stress resilience, and represent potential targets of drug discovery for depression. The present article focuses on the pharmacology and efficacy of KCNQ2/3 K+ channel openers as novel therapeutic agents for depressive disorders from initial studies conducted on animal models showing depressive-like behaviors to recent work in humans that examines the potential for KCNQ2/3 channel modulators as novel antidepressants. Data from preclinical work suggest that KCNQ-type K+ channels are an active mediator of stress resilience and KCNQ2/3 K+ channel openers show antidepressant efficacy. Similarly, evidence from clinical trials conducted in patients with MDD using the KCNQ2/3 channel opener ezogabine (retigabine) showed significant improvements in depressive symptoms and anhedonia. Overall, KCNQ channel openers appear a promising target for the development of novel therapeutics for the treatment of psychiatric disorders and specifically for MDD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Major depressive disorder (MDD) represents a serious health problem; its neurobiological mechanisms are still being elucidated. |
Preclinical data suggest antidepressant potential for modulators of KCNQ voltage-gated potassium (K+) channels. |
Initial clinical trials of patients with MDD have shown significant efficacy for the KCNQ2/3 K+ channel opener ezogabine. |
Larger randomized controlled trials of KCNQ modulators in depressive disorders are warranted. |
1 Need for Mechanistically Novel Antidepressant Agents
Major depressive disorder (MDD) is a chronic and debilitating condition characterized by social and occupational impairment. Major depressive disorder is among the most common disorders, affecting more than 264 million worldwide [1]. Approximately 20% of adults in the USA may experience a major depressive episode at some point in their lifetime [2], while in Europe, recent data suggest a 6.4% overall prevalence of current depressive disorder [3]. Major depressive disorder is a heterogenous disorder with symptoms that vary considerably from patient to patient. Impeding novel treatment discovery, the field lacks a complete understanding of the essential neurobiological mechanisms that lead to depression [4, 5]. One of the first theories regarding the pathophysiology of MDD was the monoamine hypothesis of depression developed approximately 50 years ago. The monoamine hypothesis suggests that depression is associated with lower levels of serotonin, norepinephrine, and dopamine. First-generation antidepressants, including tricyclic antidepressants and monoamine oxidase inhibitors, and second-generation drugs (selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, atypical antidepressants, and serotonin modulators) target largely the monoamine neurotransmitter system and increase the levels of these signaling molecules in the synaptic space. Subsequent work suggests that an imbalance of the monoaminergic transmission is not a condition sufficient to regulate mood, indicating that monoamines may primarily have a modulatory effect on other neurobiological pathways associated with MDD [6, 7]. Moreover, the latency of response to antidepressant medications (2–4 weeks or longer) and the percentage of patients with MDD who do not respond to treatment (approximately one-third [8]) provide additional support to the notion that the monoaminergic theory of depression is incomplete in describing the neurobiological alterations contributing to the disorder. More recently, clinical studies have identified a rapid-onset antidepressant effect of the glutamate N-methyl-d-aspartate receptor antagonist ketamine, which shows improvement in symptoms of depression within hours [9,10,11]. Since ketamine was first tested as a treatment for depression, scientific investigations have examined the role of the glutamate system in the neurobiology of MDD and its potential as a target for novel therapeutics, paving the way for the possibility of more direct pharmacological targets [12, 13]. In 2019, the US Food and Drug Administration (FDA) approved intranasal esketamine spray as an adjunct to standard antidepressants for treatment-resistant depression. Despite this important advance in treatment, the role of the glutamate system in the pathophysiology of depression is not fully characterized.
In addition to incomplete understanding of the neurobiology of MDD, relatively little is known about the biological or psychological mechanisms of resilience, the ability to maintain a state of mental health well-being in the face of stress or adversity [14,15,16]. A growing body of preclinical evidence suggests that stress-resilient animals develop adaptive regulation of more genes and ion channel functions in the brain as compared with stress-susceptible depressive animals, supporting that resilience is an active stress-coping mechanism, rather than a simple lack of pathological alterations in response to prolonged adverse stimuli [15, 17,18,19,20]. Importantly, it has become clear that enhancement of active resilient mechanisms is able to reverse depressive dysfunctions in the brain and achieve antidepressant efficacy in both rodents and human patients [20,21,22], which opens a conceptually novel therapeutic strategy for depression treatment (see more below).
2 Molecular Mediators of Resilience to Stress
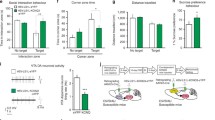
In addition to human studies, preclinical work allows the identification of the detailed mechanisms underlying MDD that can be then translated into humans, leading to the identification of new targets for drug discovery [23]. Numerous animal models have been established to elucidate the neurobiological mechanism underlying MDD and to test novel antidepressant treatments. A well-validated chronic stress animal model of depression is the chronic social defeat stress (CSDS) model, in which the animal is exposed to repeated and chronic social stress [17, 24]. This model aims to translate animals back into the life events (e.g., loss of social status) that are associated with an increased risk of developing MDD in humans. Chronic social defeat stress induces a depression-like phenotype in a subgroup of mice (susceptible) that is reversed by antidepressant treatment [25,26,27,28]. According to this protocol [17, 24], mice are daily attacked by a larger and aggressive mouse (social defeat). Defeated mice that do not develop a depressed phenotype are considered resilient. The resilient mice show an up-regulation of voltage-gated potassium (K+) channel functions within the ventral tegmental area (VTA) compared with the susceptible and stress-naïve mice (Fig. 1) [17, 20]. The VTA dopaminergic neurons, part of a discrete reward circuit together with the ventral striatum (VS)/nucleus accumbens (NAc) and the ventromedial prefrontal cortex (PFC), play an important role also in mediating stress responses [25, 29]. Ventral tegmental area dopaminergic neurons exhibit two patterns of activity in vivo: low-frequency tonic firing and high-frequency phasic firing [30]. The phasic high-frequency firing of the VTA dopaminergic neurons projecting to the NAc is upregulated by CSDS in the susceptible mice, but not in the resilient mice [31, 32]. Consistently, optogenetic induction of phasic firing of the VTA dopaminergic neurons in this circuit is able to induce a susceptible phenotype in the subthreshold-defeated mice and previously resilient mice. This increase in VTA dopaminergic neuron phasic firing in susceptible mice is induced by an up-regulation of excitatory hyperpolarization-activated cyclic nucleotide-gated (HCN) cation channel-mediated current (Ih) that can be attenuated pharmacologically, which leads to an improvement of the depressive behavior [27]. Long-term treatment with the antidepressant fluoxetine normalizes the hyperactivity (and decreases this excitatory Ih current) in these neurons [27]. Interestingly, the resilient mice show a larger Ih current, in parallel with increased inhibitory K+ channel currents, that stabilizes VTA dopaminergic neurons counterbalancing the hyperactivity of these neurons observed in the susceptible mice (Fig. 1) [20]. Among the upregulated channels in the VTA of resilient mice, a prominent role is displayed by KCNQ3 (Kv7.3), a slow voltage-activated K+ channel [17, 20]. The KCNQ (or KV7) family of K+ channels, which includes KCNQ1-5, are important regulators of neuronal excitability and represent targets for drug discovery for central nervous system (CNS) disorders [33,34,35].
Regulatory function of KCNQ potassium (K+) channels in the ventral tegmental area (VTA) dopaminergic neurons innervating nucleus accumbens (NAc) neurons. A There is baseline expression of hyperpolarization-activated cyclic nucleotide-gated cation (HCN) channels and KCNQ potassium K+ channels in VTA-NAc dopamine neurons in stress-naïve control animals, which contribute to the control levels of Ih and Ik currents and the baseline firing activity of these circuit neurons. The baseline level of these channels is indicated by four HCN and KCNQ channels, respectively on the VTA neuron. B Susceptible subgroup shows increased Ih current without alteration in HCN channel expression, possibly through the phosphorylation by PKC as illustrated in the VTA neuron. The increased Ih current drives the hyper firing activity of VTA-NAc dopamine neurons with no alteration in KCNQ expression, thus no changes in Ik current. C The resilient phenotype displays an even bigger Ih current that concurred with an increased Ik current (illustrated by more KCNQ channels), which maintains the control level of firing activity of VTA-NAc dopamine neurons. The bigger Ih current is possibly induced by stronger Protein kinase C (PKC) phosphorylation (thick arrow). These observations demonstrate a regulatory effect of the KCNQ K+ channel on the firing of these dopamine neurons. D In the susceptible animals, which show various depression-related behaviors, over-expression of KCNQ channel (illustrated by more KCNQ channels) or administration of KCNQ channel openers inhibit the pathophysiological hyperactivity of these neurons and has antidepressant efficacy at a behavioral level
Overall, these data provide a rationale for exploring K+ channels, especially the KCNQ subtype, as a target for drug discovery for MDD. Here, we summarize the evidence from animal and human studies on drugs targeting K+ channels as potential novel treatments for MDD. However, as this evidence has been tested primarily in animal models of depression subsequent to CSDS, further studies using other stress-inducing paradigms are warranted to test the antidepressant properties of KCNQ channels.
3 General Structure and Function of KCNQ Potassium Channels
Electrical signaling in the neurons occurs thanks to the generation and propagation of action potentials, a consequence of the diffusion of ions across cell membranes through ion channels. Potassium (K+) channels represent a class of ion channels and KCNQ genes encode members of the Kv7 family of K+ channel subunits [36,37,38]. Although voltage-gated K+ channels (KV) are present throughout multiple systems in the body [39], four members of this family, Kv7.2–Kv7.5, are expressed in the CNS in which they contribute to the regulation of neural excitability and modulation of action potential firing frequency [40]. K+ channels are formed by four transmembrane, pore-forming, voltage-sensing α-subunits each consisting of six segments. Four of these segments have a voltage-sensing component and modify the structure of the channel in response to changes in cell membrane potential (voltage gating). These segments surround two additional segments that form an ionic conductance pore. Based on the characteristic of the α-subunits, voltage-gated K+ channels are described as homotertamers (four identical α-subunits), or heteroteramers (two or more distinct α-subunits; as in the case of KV 7.2/KV 7.3—KCNQ2/3). K+ channels are then completed by additional cytoplasmic and transmembrane β-subunits able to modify α-subunit localization and function [41, 42]. KV channels are divided into 12 subfamilies: KV1–KV12. Among these, only a few serve as drug targets for CNS disorders such as epilepsy (KV7) in which KV7 (KCNQ) channels trigger the voltage-dependent K+ current (M-current) that regulates neuronal excitability and action potential firing frequency [43, 44]. Recent work also supports an additional physiological function of KCNQ channels aiming at compensating excessively excited neurons, which is evidenced in hippocampus cells [45] and VTA dopamine neurons [21].
4 KCNQ Potassium Channel as a Target for Treatments for CNS Disorders
The KCNQ family of K+ channels, and specifically KCNQ2 and/or KCNQ3 has been studied for the treatment of epilepsy. Within the members of the KNCQ family, mainly channels from KCNQ2 to KCNQ5 are located in the brain. Specifically, mutations of the KCNQ2 and KCNQ3 genes have been associated with the development of benign familial neonatal seizures, thus suggesting a role in the pathophysiology of epilepsy that could be used as a drug target for the development of new treatments [46]. Ezogabine, also known as retigabine (N-[2-amino-4-(4-fluorobenzylamino)-phenyl] carbamic acid ethyl ester) is a selective KCNQ2/3 channel opener that exerts its effects by increasing the activity of these channels in the CNS and consequentially leading to an overall decrease in neuronal excitability. Ezogabine was developed and then approved by the FDA (in 2011) as an adjunctive treatment of partial-onset seizures in patients aged 18 years or older. Several trials have shown ezogabine has efficacy for the treatment of partial epilepsy [47,48,49].
Ezogabine has also been shown to reduce membrane excitability in amyotrophic lateral sclerosis [50]. Following ezogabine withdrawal from the market in 2017, owing to limited use and declining numbers of patients initiating drug therapy, new drugs targeting the KCNQ channels have been developed. XEN1101 is a novel positive allosteric modulator of the KCNQ2/3 channels currently under development for the treatment of focal epilepsy [51]. A summary of the drugs targeting the KCNQ channels is included in Table 1.
5 KCNQ Potassium Channel in Animal Models of Depression
As stated above, KCNQ channels play an active role in counterbalancing pathological hyperactivity of VTA dopaminergic neurons in mice resilient to CSDS [20], indicating that KCNQ is able to functionally regulate the firing activity of these neurons. Consistent with this, bath application of the KCNQ opener ezogabine to in vitro VTA slice preparation suppressed the hyper firing activity of VTA dopamine neurons in susceptible VTA of susceptible mice [21]. Moreover, local infusion of ezogabine into the VTA and viral overexpression of KCNQ3, a subunit that is upregulated in the resilient mice, both induce antidepressant effects in susceptible mice (Fig. 1). Further in vivo studies show that systemic administration of ezogabine has similar antidepressant efficacy in susceptible mice, indicating a translational potential of this KCNQ opener [21]. Another study using fasudil as KCNQ channel opener shows that fasudil downregulates VTA dopamine neuron firing and induces antidepressant effect in the CSDS model. Interesting, these efficacies are mainly mediated by KCNQ4 in the VTA because the regulatory effects on the dopamine neuron activity and depressive behaviors are absent in KCNQ knockout mice [52]. A more recent study with a novel KCNQ opener, Lu [53] AA41178, demonstrate an antidepressant-like effect in the forced swim test. These studies suggest that KCNQ openers may have different selectivity on KCNQ subunits, but behaviorally induce consistent antidepressant efficacy in the stress-related models of depression.
6 Evidence of Dysregulation within the Reward System in MDD
Anhedonia (loss of interest or pleasure) is a core symptom of MDD [54], and convergent lines of research link anhedonia to quantitative deficits in reward processing [29]. Results from behavioral studies have shown that MDD is associated with a reduced response to reward [55], which correlates with anhedonia severity [56] and illness chronicity [57]. Notably, pre-treatment reward deficits and anhedonia are related to a poor antidepressant response [57, 58] and a long-term antidepressant response [59] and appear to persist after remission [60]. Interestingly, although conventional antidepressants are known to regulate resilience-related mechanisms [61,62,63], there is no study exploring the effect of FDA-approved antidepressants on KCNQ channels. In humans, the use of neuroimaging with functional MRI (fMRI) has shown the potential to identify the neurobiological underpinnings of MDD, thus potentially expediting the development of novel treatments [64]. Brain functional data from resting-state and task-based fMRI studies suggested that MDD is characterized by a dysfunction within the reward circuitry, in which subjects with MDD show a blunted neural response to reward compared with healthy volunteers at the level of fronto-striatal functioning and connectivity [65]. The reduced response to reward stimuli in MDD appears to involve both the reward anticipation and feedback, and is characterized by striatal hypoactivation [66]. Further, alterations in reward processing have been associated with an increased risk for depression [67] with a recent study suggesting that increased connectivity of the VS predicts MDD [68], in accordance with animal studies that highlight that role of the reward circuit in the pathophysiology of MDD [29].
7 Human Studies on KCNQ Potassium Channel for Depression
The first study evaluating the antidepressant effect of the KCNQ2/3 positive modulator ezogabine in subjects with MDD was an open-label study enrolling 18 patients with depression who received ezogabine up to 900 mg/day orally over the course of 10 weeks [22]. The 900-mg daily dosage was selected based on evidence showing that ezogabine reaches adequate brain concentrations and has demonstrated efficacy for partial-onset seizures at dosages between 600 mg/day and 1200 mg/day [69]. This study assessed both depressive and anhedonic clinical symptoms and reward circuitry change following treatment with ezogabine. After treatment, a significant reduction in depressive (Montgomery–Åsberg Depression Rating Scale mean score change: − 13.7 ± 9.7, p < 0.001) and anhedonic symptom severity (Snaith–Hamilton Pleasure Scale) mean score change: − 6.1 ± 5.3, p < 0.001] was reported. Depressive symptoms decreased significantly from pre-treatment to post-treatment and throughout the study, with a significant improvement starting from week 3 onwards (p < 0.001). On the primary outcome, 44% and 28% of the subjects satisfied criteria for response and remission, respectively. Moreover, the improvement in depression and anhedonia was associated with decreased functional connectivity between the ventral caudate (a key component of the VS that receives dopaminergic innervation from the midbrain, an area affected by KCNQ channel modulation), and clusters within the mid-cingulate cortex and posterior cingulate cortex. In addition, data from a subgroup of these participants (n = 9) from who reward learning data from a computer-based task pre-treatment and post-treatment were available showed increased reward learning following treatment. The drug was overall well tolerated and no increase in suicidal ideation or emergence of suicidal behavior was reported during the trial. The most common adverse events reported were dizziness, confusion, and headache and only three participants did not achieve the targeted dose of 900 mg/day, but were retained in the study at the lower dosages of 750 mg/day (n = 1) and 600 mg/day (n = 2). Despite the encouraging results, the small sample size and the open-label nature of the study limit the generalizability of these findings.
The potential antidepressant effect of the KCNQ2/3 modulator ezogabine has been tested subsequently in a randomized placebo-controlled clinical trial exploring its effect on clinical symptoms and brain response during reward anticipation [70], a translational measure of target engagement that appears to normalize with antidepressant treatment [71, 72]. In this study, 45 patients with depression were randomly assigned to receive ezogabine (up to 900 mg daily) or placebo in a 1:1 approach and underwent fMRI during a reward task prior and following the treatment period; clinical measures of depression, anhedonia, and global symptom severity were also collected at weekly visits. Ezogabine compared with placebo was associated with a large improvement in depression as measured by the Montgomery–Åsberg Depression Rating Scale (p < 0.001) and the Quick Inventory of Depressive Symptomatology-Self-Report (p = 0.002) and equivalent to a medium-large effect size (differences in the change in means for the Montgomery–Åsberg Depression Rating Scale and Quick Inventory of Depressive Symptomatology-Self-Report were d = 0.76 and d = 0.56, respectively). The ezogabine group showed also a significant improvement in hedonic capacity as measured by the Snaith–Hamilton Pleasure Scale (p < 0.001), the anticipatory and consummatory subscales of the Temporal Experience of Pleasure Scale (p < 0.001 and p = 0.05, respectively), and other anhedonia measures. The ezogabine group showed also a trend towards an increase in VS response to reward anticipation compared with placebo post-treatment compared to baseline (p = 0.07), albeit not reaching statistical significance.
Interestingly, this study had a similar design to a recent randomized controlled trial aiming to test the anti-anhedonic effect of the selective kappa opioid receptor antagonist JNJ-67953964 across a range of mood and anxiety disorders [73], including the use of fMRI during a reward task to assess the effect of treatment on the VS. Specifically, both studies used fMRI activation during a reward task to assess the effect of treatment on the VS as a primary endpoint, while measures of clinical improvement were listed as secondary outcomes. This design is particularly suited for early phase trials of novel interventions for mental disorders that aim to translate basic science into clinical hypotheses with the main purpose of gathering knowledge on the relationship between the underlying processes of mental disorders and the mechanisms of action of the proposed intervention. However, despite the similar methodology, while ezogabine was associated with beneficial effects on several secondary clinical outcomes, it did not show a statistically significant effect on the VS, possibly reflecting a false-negative finding due to the limited sample size. Overall, these initial findings encourage larger randomized controlled trials investigating longer term efficacy or tolerability to establish the potential of the KCNQ2/3 K+ channel as a target for the development of novel medications to treat depression and anhedonia. Further, future studies are also needed to compare the effect of ezogabine on depressive and anhedonic symptoms to current FDA-approved antidepressants.
8 Role of KCNQ channel openers in other psychiatric disorders and other potassium channels under study
KCNQ channel openers have been explored as pharmacological targets for CNS disorders, such as epilepsy. Ezogabine showed efficacy in several animal models of epilepsy and has been marketed as an adjunctive treatment of partial-onset seizures in patients aged 18 years and older [9,10,11]. Currently, other KCNQ channel openers are under development. A novel positive allosteric modulator (XEN1101) of the KCNQ2/3 channels is currently under development for the treatment of focal epilepsy [51], with a mechanism of action similar to ezogabine. A recently concluded phase I clinical trial on XEN1101 by Premoli et al. [74] found an effect of XEN1101 on corticospinal excitability using transcranial magnetic stimulation. The authors enrolled 20 healthy volunteers using a cross-over design and completed transcranial magnetic stimulation‐evoked electroencephalogram potentials prior to and at regular intervals following the administration of the drug. Similar to other antiepileptic drugs, XEN1101 was shown to suppress cortical and corticospinal excitability, thus demonstrating its activity in the CNS. A randomized, double-blind, placebo-controlled, multicenter, phase II clinical trial of XEN1101 is currently underway to evaluate the clinical efficacy, safety, and tolerability of the drug as adjunctive treatment for focal epilepsy in adults (ClinicalTrials.gov number: NCT03796962).
Other studies at the preclinical level have explored the potential of KCNQ channels as a target for the treatment of other psychiatric disorders. Preliminary evidence from animal studies suggests a potential effect of ezogabine on mania, thus with possible use in cases of bipolar disorder. Ezogabine reduced hyperactivity induced by amphetamine and chlordiazepoxide without altering the basal levels of locomotor activity [75]. Other authors replicated this finding and showed that the administration of ezogabine was able to reduce neuronal firing in the VTA, and dopamine release in the NAc [76]. KCNQ channels have been studied also in the context of substance use disorder, including alcohol. Recent evidence suggests that ethanol inhibits KCNQ2/3 channels [77], while ezogabine administration into the NAc significantly reduced voluntary alcohol intake especially in high-drinking rats [78] and systemic treatment with ML213 (KCNQ2 and KCNQ4 opener) also reduced ethanol drinking in rodents [79]. Similarly, in rats exposed to cocaine, methylphenidate, or phencyclidine, the administration of ezogabine inhibited drug-induced locomotor activity and reduced c-Fos expression in the NAc and primary motor cortex, and reduced basal extracellular levels of dopamine metabolites, leading to a reduction in striatal and cortical excitability and thus the CNS effects of these drugs on the animal reward circuit [80]. KCNQ channels have also been investigated in an animal model of cocaine relapse [81]. Researchers showed that cues associated with cocaine were able to trigger drug-seeking behavior in mice, which is mediated by PFC neurons. Among the changes observed in these neurons, a reduction of inhibition mediated by KCNQ channels was reported, indicating the hyperactivity of the PFC neurons. In these animals, administration of ezogabine led to normalized PFC neuronal excitability and reduced drug-seeking behavior. Overall, these data suggest that KCNQ could be an important target for drug development for the treatment for drug and alcohol addiction.
Finally, KCNQ channels appear to regulate PFC connectivity, an area involved in higher cognitive function. Specifically, KCNQ2 channels have been found in medial PFC layers II/III and V pyramidal cells, in which increased KCNQ currents induced by chronic stress exposure have been associated with reduced neuronal firing. Similarly to chronic stress exposure, ezogabine has been shown to increase the open state of KCNQ channels and increase the slow relaxation amplitude of KCNQ channels, leading to a reduction in the firing of medial PFC neurons in animals [82]. These data possibly explain the side effects most commonly associated with ezogabine (confusion and memory impairment), and should also be explored in humans to fully understand the potential of KCNQ channel openers for the treatment of mental disorders.
Of note, other members of the KCNQ family of channels may represent interesting targets for drug development. For example, KCNQ1 channels also appear to be expressed in the CNS [83] and recent evidence suggests a role for KCNQ1 polymorphism in the cognitive deficits and decreased white matter integrity associated with schizophrenia [84].
9 Conclusions
K+ channels appear a promising target for the development of novel therapeutics for the treatment of psychiatric disorders and specifically for depression. The targeting of this channel represents a completely different mechanism of action compared with any currently available antidepressant. Both preclinical and clinical studies provide initial evidence on the antidepressant effect of the K+ channel modulators, especially for KCNQ channel openers in which modulation of these channels can enhance stress resilience in animals and improve depressive and anhedonic symptoms in patients with MDD. Based on these findings, larger randomized controlled trials of KCNQ modulators in depressive disorders are warranted to confirm their potential as a viable treatment for depressive disorders and other stress-related disorders, ultimately leading to the development of a different class of antidepressant medications.
References
World Health Organization. WHO fact sheet. 2017. https://www.who.int/news-room/fact-sheets/detail/depression.
Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiat. 2018;75:336–46.
de la Torre JA, Vilagut G, Ronaldson A, Serrano-Blanco A, Martín V, Peters M, et al. Prevalence and variability of current depressive disorder in 27 European countries: a population-based study. Lancet Public Health. 2021;6:e729–38.
Anacker C. Fresh approaches to antidepressant drug discovery. Expert Opin Drug Discov. 2014;9:407–21.
Millan MJ, Goodwin GM, Meyer-Lindenberg A, Ove ÖS. Learning from the past and looking to the future: emerging perspectives for improving the treatment of psychiatric disorders. Eur Neuropsychopharmacol. 2015;25:599–656.
Heninger GR, Delgado PL, Charney DS. The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry. 1996;29:2–11.
Charney DS. Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry. 1998;59(Suppl. 14):11–4.
Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin N Am. 1996;19:179–200.
Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64.
Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–42.
Fava M, Freeman MP, Flynn M, Judge H, Hoeppner BB, Cusin C, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020;25:1592–603.
Kavalali ET, Monteggia LM. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry. 2012;169:1150–6.
Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–49.
Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–57.
Russo SJ, Murrough JW, Han M-H, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–84.
Southwick SM, Charney DS. The science of resilience: implications for the prevention and treatment of depression. Science. 2012;338:79–82.
Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404.
Bagot RC, Cates HM, Purushothaman I, Lorsch ZS, Walker DM, Wang J, et al. Circuit-wide transcriptional profiling reveals brain region-specific gene networks regulating depression susceptibility. Neuron. 2016;90:969–83.
Han M-H, Nestler EJ. Neural substrates of depression and resilience. Neurotherapeutics. 2017;14:677–86.
Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, et al. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science. 2014;344:313–9.
Friedman AK, Juarez B, Ku SM, Zhang H, Calizo RC, Walsh JJ, et al. KCNQ channel openers reverse depressive symptoms via an active resilience mechanism. Nat Commun. 2016;7:11671.
Tan A, Costi S, Morris LS, Van Dam NT, Kautz M, Whitton AE, et al. Effects of the KCNQ channel opener ezogabine on functional connectivity of the ventral striatum and clinical symptoms in patients with major depressive disorder. Mol Psychiatry. 2020;25(6):1323–33.
Insel TR, Voon V, Nye JS, Brown VJ, Altevogt BM, Bullmore ET, et al. Innovative solutions to novel drug development in mental health. Neurosci Biobehav Rev. 2013;37:2438–44.
Golden SA, Covington HE, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–91.
Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–51.
Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–25.
Cao J-L, Covington HE, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, et al. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci. 2010;30:16453–8.
Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. 2016;321:138–62.
Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–9.
Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviours. Trends Neurosci. 2007;30:220–7.
Walsh JJ, Friedman AK, Sun H, Heller EA, Ku SM, Juarez B, et al. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci. 2014;17:27–9.
Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–6.
Xiong Q, Gao Z, Wang W, Li M. Activation of Kv7 (KCNQ) voltage-gated potassium channels by synthetic compounds. Trends Pharmacol Sci. 2008;29:99–107.
Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, et al. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–3.
Wulff H, Castle NA, Pardo LA. Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov. 2009;8:982–1001.
Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol. 2009;156:1185–95.
Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci. 2000;1:21–30.
Robbins J. KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacol Ther. 2001;90:1–19.
Gutman GA, Chandy KG, Adelman JP, Aiyar J, Bayliss DA, Clapham DE, et al. International Union of Pharmacology. XLI. Compendium of voltage-gated ion channels: potassium channels. Pharmacol Rev. 2003;55:583–6.
Greene DL, Hoshi N. Modulation of Kv7 channels and excitability in the brain. Cell Mol Life Sci. 2017;74:495–508.
MacKinnon R. Potassium channels. FEBS Lett. 2003;555:62–5.
Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev. 2008;88:1407–47.
Brown DA. M currents. In: Narahashi T, editor. Ion channels. Boston: Springer; 1988. p. 55–94.
Joshi S, Balan P, Gurney AM. Pulmonary vasoconstrictor action of KCNQ potassium channel blockers. Respir Res. 2006;7:31.
Zhang J, Shapiro MS. Activity-dependent transcriptional regulation of M-type (Kv7) K(+) channels by AKAP79/150-mediated NFAT actions. Neuron. 2012;76:1133–46.
Richards MC, Heron SE, Spendlove HE, Scheffer IE, Grinton B, Berkovic SF, et al. Novel mutations in the KCNQ2 gene link epilepsy to a dysfunction of the KCNQ2-calmodulin interaction. J Med Genet. 2004;41: e35.
Brodie MJ, Lerche H, Gil-Nagel A, Elger C, Hall S, Shin P, et al. Efficacy and safety of adjunctive ezogabine (retigabine) in refractory partial epilepsy. Neurology. 2010;75:1817–24.
French JA, Abou-Khalil BW, Leroy RF, Yacubian EMT, Shin P, Hall S, et al. Randomized, double-blind, placebo-controlled trial of ezogabine (retigabine) in partial epilepsy. Neurology. 2011;76:1555–63.
Porter RJ, Partiot A, Sachdeo R, Nohria V, Alves WM. Randomized, multicenter, dose-ranging trial of retigabine for partial-onset seizures. Neurology. 2007;68:1197–204.
Kovalchuk MO, Heuberger JAAC, Sleutjes BTHM, Ziagkos D, van den Berg LH, Ferguson TA, et al. Acute effects of riluzole and retigabine on axonal excitability in patients with amyotrophic lateral sclerosis: a randomized, double-blind, placebo-controlled, crossover trial. Clin Pharmacol Ther. 2018;104:1136–45.
Bialer M, Johannessen SI, Koepp MJ, Levy RH, Perucca E, Tomson T, et al. Progress report on new antiepileptic drugs: a summary of the Fourteenth Eilat conference on new antiepileptic drugs and devices (EILAT XIV). I. Drugs in preclinical and early clinical development. Epilepsia. 2018;59:1811–41.
Li L, Sun H, Ding J, Niu C, Su M, Zhang L, et al. Selective targeting of M-type potassium Kv 7.4 channels demonstrates their key role in the regulation of dopaminergic neuronal excitability and depression-like behaviour. Br J Pharmacol. 2017;174:4277–94.
Grupe M, Bentzen BH, Benned-Jensen T, Nielsen V, Frederiksen K, Jensen HS, et al. In vitro and in vivo characterization of Lu AA41178: a novel, brain penetrant, pan-selective Kv7 potassium channel opener with efficacy in preclinical models of epileptic seizures and psychiatric disorders. Eur J Pharmacol. 2020;887: 173440.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington: American Psychiatric Association; 2013.
Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43:76–87.
Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57:319–27.
Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, de Boer P, et al. Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry. 2013;73:639–45.
Uher R, Perlis RH, Henigsberg N, Zobel A, Rietschel M, Mors O, et al. Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychol Med. 2012;42:967–80.
Spijker J, Bijl RV, de Graaf R, Nolen WA. Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS). Acta Psychiatr Scand. 2001;103:122–30.
Pechtel P, Dutra SJ, Goetz EL, Pizzagalli DA. Blunted reward responsiveness in remitted depression. J Psychiatr Res. 2013;47:1864–9.
Bagot RC, Cates HM, Purushothaman I, Vialou V, Heller EA, Yieh L, et al. Ketamine and imipramine reverse transcriptional signatures of susceptibility and induce resilience-specific gene expression profiles. Biol Psychiatry. 2017;81:285–95.
Vialou V, Robison AJ, Laplant QC, Covington HE, Dietz DM, Ohnishi YN, et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–52.
Wilkinson MB, Xiao G, Kumar A, LaPlant Q, Renthal W, Sikder D, et al. Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. J Neurosci. 2009;29:7820–32.
Schmaal L, Pozzi E, Ho TC, van Velzen LS, Veer IM, Opel N, et al. ENIGMA MDD: seven years of global neuroimaging studies of major depression through worldwide data sharing. Transl Psychiatry. 2020;10:172.
Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, et al. Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG studies. Am J Psychiatry. 2018;175:1111–20.
Borsini A, Wallis ASJ, Zunszain P, Pariante CM, Kempton MJ. Characterizing anhedonia: a systematic review of neuroimaging across the subtypes of reward processing deficits in depression. Cogn Affect Behav Neurosci. 2020;20:816–41.
Morgan JK, Olino TM, McMakin DL, Ryan ND, Forbes EE. Neural response to reward as a predictor of increases in depressive symptoms in adolescence. Neurobiol Dis. 2013;52:66–74.
Pan PM, Sato JR, Salum GA, Rohde LA, Gadelha A, Zugman A, et al. Ventral striatum functional connectivity as a predictor of adolescent depressive disorder in a longitudinal community-based sample. Am J Psychiatry. 2017;174:1112–9.
Large CH, Sokal DM, Nehlig A, Gunthorpe MJ, Sankar R, Crean CS, et al. The spectrum of anticonvulsant efficacy of retigabine (ezogabine) in animal models: implications for clinical use. Epilepsia. 2012;53:425–36.
Costi S, Morris LS, Kirkwood K, Hoch M, Corniquel M, Vo-Le B, et al. Impact of the KCNQ2/3 channel opener ezogabine onreward circuit activity and clinical symptoms in depression: results from a randomized controlled trial. Am J Psychiatry. 2021;178(5):437–46.
Stoy M, Schlagenhauf F, Sterzer P, Bermpohl F, Hägele C, Suchotzki K, et al. Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. J Psychopharmacol. 2012;26:677–88.
Ossewaarde L, Verkes RJ, Hermans EJ, Kooijman SC, Urner M, Tendolkar I, et al. Two-week administration of the combined serotonin-noradrenaline reuptake inhibitor duloxetine augments functioning of mesolimbic incentive processing circuits. Biol Psychiatry. 2011;70:568–74.
Krystal AD, Pizzagalli DA, Smoski M, Mathew SJ, Nurnberger J, Lisanby SH, et al. A randomized proof-of-mechanism trial applying the “fast-fail” approach to evaluating κ-opioid antagonism as a treatment for anhedonia. Nat Med. 2020;26:760–8.
Premoli I, Beatch G, Rossini P, Abela E, Posadas K, Green L, et al. TMS-EEG and TMS-EMG to assess the pharmacodynamic profile of a novel potassium channel opener (XEN1101) on human cortical excitability. Brain Stimul. 2019;12:459.
Dencker D, Dias R, Pedersen ML, Husum H. Effect of the new antiepileptic drug retigabine in a rodent model of mania. Epilepsy Behav. 2008;12:49–53.
Sotty F, Damgaard T, Montezinho LP, Mørk A, Olsen CK, Bundgaard C, et al. Antipsychotic-like effect of retigabine [N-(2-amino-4-(fluorobenzylamino)-phenyl)carbamic acid ester], a KCNQ potassium channel opener, via modulation of mesolimbic dopaminergic neurotransmission. J Pharmacol Exp Ther. 2009;328:951–62.
Kim K-W, Kim K, Lee H, Suh B-C. Ethanol elevates excitability of superior cervical ganglion neurons by inhibiting Kv7 channels in a cell type-specific and PI(4,5)P2-dependent manner. Int J Mol Sci. 2019;20:4419.
McGuier NS, Griffin WC, Gass JT, Padula AE, Chesler EJ, Mulholland PJ. Kv7 channels in the nucleus accumbens are altered by chronic drinking and are targets for reducing alcohol consumption. Addict Biol. 2016;21:1097–112.
McGuier NS, Rinker JA, Cannady R, Fulmer DB, Jones SR, Hoffman M, et al. Identification and validation of midbrain Kcnq4 regulation of heavy alcohol consumption in rodents. Neuropharmacology. 2018;138:10–9.
Hansen HH, Andreasen JT, Weikop P, Mirza N, Scheel-Krüger J, Mikkelsen JD. The neuronal KCNQ channel opener retigabine inhibits locomotor activity and reduces forebrain excitatory responses to the psychostimulants cocaine, methylphenidate and phencyclidine. Eur J Pharmacol. 2007;570:77–88.
Parrilla-Carrero J, Buchta WC, Goswamee P, Culver O, McKendrick G, Harlan B, et al. Restoration of Kv7 channel-mediated inhibition reduces cued-reinstatement of cocaine seeking. J Neurosci. 2018;38:4212–29.
Arnsten AFT, Jin LE, Gamo NJ, Ramos B, Paspalas CD, Morozov YM, et al. Role of KCNQ potassium channels in stress-induced deficit of working memory. Neurobiol Stress. 2019;11: 100187.
Goldman AM, Glasscock E, Yoo J, Chen TT, Klassen TL, Noebels JL. Arrhythmia in heart and brain: KCNQ1 mutations link epilepsy and sudden unexplained death. Sci Transl Med. 2009;1:2ra6.
Bruce HA, Kochunov P, Paciga SA, Hyde CL, Chen X, Xie Z, et al. Potassium channel gene associations with joint processing speed and white matter impairments in schizophrenia. Genes Brain Behav. 2017;16:515–21.
FDA Approved Labeling Text. ezogabine package insert. 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022345s001lbl.pdf.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R61MH111932. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional funding for this article was provided by the Friedman Brain Institute and by the Ehrenkranz Laboratory for Human Resilience, both components of the Icahn School of Medicine at Mount Sinai and by the Gottesman Foundation. Additional research support was provided by the National Institute of Mental Health (R56MH115409, R01MH120637, Dr. Han).
Conflicts of Interest/Competing Interests
In the past 5 years, Dr. Murrough has provided consultation services and/or served on advisory boards for Allergan, Boehringer Ingelheim, Clexio Biosciences, FSV7, Global Medical Education (GME), Otsuka, and Sage Therapeutics. Dr. Murrough is named on a patent pending for neuropeptide Y as a treatment for mood and anxiety disorders and on a patent pending for the use of ezogabine and other KCNQ channel openers to treat depression and related conditions. The Icahn School of Medicine (employer of Dr. Murrough) is named on a patent and has entered into a licensing agreement and will receive payments related to the use of ketamine or esketamine for the treatment of depression. The Icahn School of Medicine is also named on a patent related to the use of ketamine for the treatment of post-traumatic stress disorder. Dr. Murrough is not named on these patents and will not receive any payments. Dr. Ming-Hu Han is named on a patent pending for the use of ezogabine (KCNQ channel opener) to treat depression and related conditions. Dr. Costi has provided consultation services for Guidepoint.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Authors’ Contributions
All authors devised the article. S.C. wrote the first draft of the article, M.H.H. drafted the figure, and all authors commented on drafts of the article.
Rights and permissions
About this article
Cite this article
Costi, S., Han, MH. & Murrough, J.W. The Potential of KCNQ Potassium Channel Openers as Novel Antidepressants. CNS Drugs 36, 207–216 (2022). https://doi.org/10.1007/s40263-021-00885-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-021-00885-y