Abstract
Background
Poor adherence to oral antipsychotics is common in patients with schizophrenia; nonetheless, there has been no systematic review or meta-analysis on medication adherence measured by electronic adherence monitoring (EAM), considered by many as the ‘gold standard’ assessment.
Methods
We systematically searched MEDLINE and Embase to identify studies investigating adherence to oral antipsychotics using EAM in patients with schizophrenia spectrum disorder. There were no exclusion criteria. We looked at the methodology in each study and defined which type of adherence was used in the study. Data on medication adherence, definition of satisfactory adherence (i.e., the threshold set in terms of the percentage of times medication was taken as prescribed), and factors associated with adherence were extracted for the included studies. Further, data on the rates of medication adherence were quantitatively synthesized.
Results
A total of 19 studies involving 2184 patients were included. EAM-measured medication adherence was classified into three outcome types: taking adherence, regimen adherence, and timing adherence. The meta-analysis yielded oral antipsychotic adherence rates (defined as a continuous variable) of 71.1% for taking adherence [from seven studies, n = 256, 95% confidence interval (CI) 58.0–84.1], 70.0% for regimen adherence (from five studies, n = 174, 95% CI = 63.6–76.4), and 64.9% for timing adherence (from four studies, n = 212, 95% CI 53.2–76.6), respectively. The proportions of patients with oral antipsychotic adherence, when defined as a dichotomous variable, ranged from 50 to 78.3% for the 70% threshold for satisfactory adherence, 29.8–75.7% for the 75% threshold, and 47.8–75.7% for the 80% threshold. Factors associated with poor medication adherence were greater symptom severity, more frequent dosing regimen, poorer insight, and more negative drug attitude.
Conclusions
Oral antipsychotic adherence rates in schizophrenia, defined as a continuous variable and measured by EAM, were in the range of 70%, lower than the 80% threshold used widely to define satisfactory adherence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This is the first systematic review and meta-analysis of medication adherence, as measured by electronic adherence monitoring (EAM) (considered the gold standard for assessment of adherence) in schizophrenia. |
EAM-measured medication adherence was classified into taking adherence, regimen adherence, and timing adherence: the meta-analysis yielded oral antipsychotic adherence rates (defined as a continuous variable) of 71.1%, 70.0%, and 64.9%, respectively. |
Thresholds of 70%, 75%, and 80% were used across studies to define satisfactory medication adherence: the proportions of patients with oral antipsychotic adherence (defined as a dichotomous variable) ranged from 50% to 78.3%, 29.8% to 75.7%, and 47.8% to 75.7%, respectively. |
Oral antipsychotic adherence rates in schizophrenia, defined as a continuous variable and measured by EAM, were in the range of 70%, lower than the 80% threshold used widely to define satisfactory adherence. |
1 Introduction
Treatment with antipsychotic medications is necessary in both the acute and maintenance phases of schizophrenia to improve symptoms and prevent relapse [1,2,3]. However, in schizophrenia, poor adherence to oral antipsychotics (e.g., defined as taking medications as prescribed 75% of the time or less) is common, with reported rates in the range of 50% [4, 5], and it is associated with an increased risk of relapse, re-hospitalization, and suicide [6]. Factors related to poor medication adherence include lack of insight into illness, substance abuse, negative attitude to medication, cognitive impairment, and poor therapeutic alliance [7,8,9].
Medication adherence is evaluated by various measures such as self-report, patient rating scales (e.g., Drug Attitude Inventory (DAI) [10], Brief Adherence Rating Scale (BARS) [11]), clinician/caregiver rating scales (e.g., Brief Evaluation of Medication Influences and Beliefs (BEMIB) [12]), pill count, blood drug concentrations, and electronic monitoring. While patients’ or clinicians’ ratings are the most commonly used, these often overestimate medication adherence [13]. Notably, an expert consensus guideline identified electronic monitoring, pill count, and plasma drug levels as reliable assessments [14]. Pill counts can be complicated in tracking dispensed medication, while therapeutic drug monitoring (TDM) struggles with precise thresholds for individual antipsychotics and inter- as well as intra-individual variability [15]. Among the different options for monitoring medication adherence, electronic adherence monitoring (EAM) such as the Medication Event Monitoring System (MEMS®) is considered the gold standard [16]. This consists of a medication bottle cap with a microprocessor that records the occurrence and time of each bottle opening.
There have been a number of investigations evaluating medication adherence in schizophrenia using EAM [17,18,19]. However, there are substantial differences between studies in how medication adherence is calculated, in addition to how satisfactory medication adherence is defined. Moreover, to our knowledge there has been no meta-analysis to summarize EAM-measured adherence to oral antipsychotics in schizophrenia. In light of this, we conducted a systematic review and meta-analysis to (1) summarize the methodology of EAM studies, (2) identify factors related to EAM-measured medication adherence, and (3) estimate overall EAM-measured adherence to oral antipsychotics in schizophrenia.
2 Methods
2.1 Literature Search and Study Selection
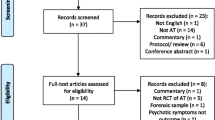
Two authors (H.Y. and S.K.) independently conducted a systematic literature search in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [20]. MEDLINE (1946–present) and Embase (1947–present) were searched using the following keywords (schizophreni* OR schizoaffective OR psychosis OR antipsychotic* OR neuroleptic*) AND ((electronic* AND monitor*) OR MEMS OR medication event monitoring system), with a limitation of English language (first search: August 5, 2018; last search: March 20, 2019). Studies that met the following eligibility criteria were selected: (1) investigating adherence to oral antipsychotics; (2) using EAM; and (3) including patients with schizophrenia spectrum disorders (i.e., schizophrenia, schizoaffective disorder, schizophreniform disorder, and delusional disorder). There were no exclusion criteria. Study selection was performed as follows: first, records were screened according to title and abstract, at which point the full text of articles was assessed for eligibility. Any disagreements in study selection were resolved by discussion with the senior corresponding author (H.T.).
2.2 Data Extraction
Two authors (H.Y. and S.K.) independently extracted the methodology from selected studies, including the definition of satisfactory medication adherence as well as the following clinical outcome data: (1) oral antipsychotic adherence rates when defined as a continuous variable; (2) adherence rates as per dichotomous variable definition; and (3) factors associated with medication adherence/non-adherence. We looked at the methodology in each study and defined which type of adherence was used in the study. Any disagreements in data extraction were resolved by discussion with the senior corresponding author (H.T.). It should be noted that for a randomized controlled trial (RCT) comparing medication adherence between an intervention group (i.e., using EAM with an alarm function) and a treatment-as-usual (TAU) group (i.e., using EAM without the alarm function) [21], we only included the TAU group, given the possibility that the alarm function inflated medication adherence.
2.3 Data Analysis
We performed a meta-analysis of rates of adherence to oral antipsychotics as a continuous variable from studies if the data were provided. Pooled estimates of mean rates of EAM-measured adherence to oral antipsychotics with two-sided 95% confidence intervals (CIs) were calculated using a random-effects model [22] by Comprehensive Meta-Analysis Version 2.0. The random-effects model was chosen because underlying true effects were assumed to vary between studies. Study heterogeneity was quantified using the I2 statistic, with an I2 value ≥ 50% indicating significant heterogeneity. Funnel plots were visually inspected to assess the likelihood of overt publication bias [23].
3 Results
3.1 Characteristics of Included Studies
A total of 19 studies, involving 2184 patients and detailed in 26 articles [11, 13, 17,18,19, 21, 24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] met eligibility criteria and were included in the systematic review (Fig. 1). Tables 1 and 2 summarize study characteristics and information on antipsychotics. The studies were published between 2004 and 2018, with study duration ranging from 1.5 to 12 months. Two types of EAM were used in the studies; one was a bottle cap type such as MEMS® (N = 16) [11, 13, 17,18,19, 24, 25, 27,28,29,30,31,32, 34,35,36,37,38,39,40,41,42,43] and the other was a pill container type such as DoPill® with or without an alarm function (N = 2 [26, 33] or N = 1 [21], respectively). The former embeds a microprocessor that records occurrence and time of each bottle opening in the bottle cap. The latter is an electronic dispenser with 28 compartments that can contain multiple pills and often has sensors that record the time of opening, but also visual and sound alarms that alert the user when it is time to take medications. Only two studies [24, 43] were conducted in a blind fashion, where medication adherence was measured without disclosure to participants. Most participants were diagnosed with schizophrenia or schizoaffective disorder, and only one study [41] recruited patients with first-episode schizophrenia. Second-generation antipsychotics (SGAs) were administered more frequently than first-generation antipsychotics (FGAs) in all studies; antipsychotic details were not reported in five studies [13, 33, 35, 36, 43], and daily dose was reported in only one study [42]. Patients receiving clozapine and long-acting injectable antipsychotics (LAIs) were excluded in two [24, 32] and seven studies [17, 21, 24, 25, 29, 30, 32, 36, 43], respectively, while information regarding dosing regimen was provided in eight studies [13, 19, 24, 27,28,29, 32, 37,38,39].
3.2 Medication Adherence
Electronic adherence monitoring-measured medication adherence can be classified in three dimensions: taking adherence, regimen adherence, and timing adherence [44]. Collectively, they represent three levels of adherence. The most liberal, taking adherence, was defined as the number of bottle cap openings divided by the number of prescribed doses during the monitoring period. At the next level, regimen adherence was defined as the percentage of days that the correct number of doses was taken, a more stringent definition. Timing adherence, the most stringent definition, was defined as the percentage of doses taken within an assigned time window. The majority of included studies did not clarify whether adherence data represented the primary or secondary outcome.
Table 3 addresses the methods of evaluating medication adherence. There were two strategies; the first assesses medication adherence as a continuous variable (N = 14) [11, 13, 21, 24,25,26,27,28,29, 31, 32, 34,35,36,37,38,39,40]. Figure 2 summarizes the results of the meta-analysis for oral antipsychotic adherence rates when defined as a continuous variable. The combined rates of taking adherence, regimen adherence, and timing adherence were 71.1% (N = 7 [11, 26, 32, 34, 36, 37, 39], n = 256, 95% CI 58.0–84.1, I2 = 94%), 70.0% (N = 5 [13, 24, 34, 36, 37], n = 174, 95% CI 63.6–76.4, I2 = 59%), and 64.9% (N = 4 [25, 27, 34, 37], n = 212, 95% CI 53.2–76.6, I2 = 95%), respectively. We performed a sensitivity analysis excluding one study [26] using the container type of EAM; the combined rate for taking adherence was 71.8% (N = 6 [11, 32, 34, 36, 37, 39], n = 230, 95% CI 57.2–86.3). Also, we performed a sensitivity analysis excluding one study [32] with an outlying result; the combined rate for taking adherence was 77.0% (N = 6 [11, 26, 34, 36, 37, 39], n = 207, 95% CI 67.3–86.8). All funnel plots were symmetrical, indicating no overt publication bias with regard to the outcomes. The second strategy is to treat medication adherence as a dichotomous variable and calculate proportion of patients with satisfactory medication adherence (N = 13) [11,11,,13, 17,18,19, 24,25,26,27, 29,30,31, 33,34,35,36, 39, 42, 43], defined using an arbitrary threshold. For example, it has been suggested by an expert consensus guideline that satisfactory adherence is defined as a patient taking ≥ 80% of prescribed medications [14]. Amongst the 13 studies addressing this issue, we identified three thresholds of dichotomous medication adherence, above which medication adherence had been defined as satisfactory: 70%, 75%, and 80% were used in five [11, 13, 26, 31, 33, 39], four [18, 19, 36, 43], and six studies [17, 24, 25, 27, 29, 30, 39, 42], respectively. The proportions of patients with satisfactory oral antipsychotic adherence, defined as a dichotomous variable, ranged from 50 to 78.3% for the 70% threshold, 29.8–75.7% for the 75% threshold, and 47.8–75.7% for the 80% threshold of satisfactory adherence.
Forest plots of the rates of adherence to antipsychotics. A Taking adherencea. B Regimen adherenceb. C Timing adherencec. CI confidence interval. aNumber of bottle cap openings divided by the prescribed number of doses. bPercentage of days with the appropriate number of doses taken. cPercentage of doses taken within assigned intervals
Medication adherence measures other than EAM included pill count (N = 9) [18, 21, 24, 25, 29, 34, 35, 41, 42], TDM (N = 5) [30, 34, 35, 41, 42], and patient- or clinician-rating scales [e.g., BARS, BEMIB, DAI, Visual Analogue Scale (VAS)] (N = 11) [11, 13, 17, 18, 24,25,26, 29, 31, 34, 36, 41, 42]. Five studies examined the relationship between medication adherence measures: pill count, BARS, and self-report were associated with EAM in three studies [24, 30, 42], one study [11], and one study [34], respectively.
In terms of factors related to poor medication adherence, greater symptom severity, more frequent dosing regimen, poorer insight, and negative attitude to medication were identified in six [11, 19, 24, 25, 40, 41], three [24, 32, 37], two [18, 19], and two [17, 25] studies, respectively.
4 Discussion
To our knowledge, this is the first systematic review and meta-analysis of medication adherence as measured by EAM (considered the gold standard for assessment of adherence) in schizophrenia. The main findings are (1) rates of EAM-measured adherence to oral antipsychotics, when defined as a continuous variable, approximated 70%; (2) thresholds of 70%, 75%, and 80% were used across studies to define satisfactory medication adherence; and (3) factors associated with poor medication adherence included greater symptom severity, more frequent dosing regimen, poor insight, and negative attitude toward medication.
The current meta-analysis revealed that the rates of EAM-measured adherence to oral antipsychotics in schizophrenia, defined as a continuous variable, were in the range of 70%. More specifically, the combined rates were 71.1%, 70.0%, and 64.9% for taking adherence, regimen adherence, and timing adherence, respectively, which notably diminishes as the stringency of definition increases. For comparison purposes, a meta-analysis evaluating EAM-measured medication adherence in chronic cardiovascular diseases reported ranges of 80.1–93.1% for taking adherence, 65.4–84.9% for regimen adherence, and 57.1–76.3% for timing adherence [44]. Further to this point, an observational study examining EAM-measured medication adherence in depressive disorders reported a mean of 68.5% for taking adherence [45]. It remains, however, that there are few studies measuring medication adherence with EAM in psychiatric disorders other than schizophrenia. Summarizing, medication adherence in schizophrenia may align with other psychiatric disorders such as depression, but together may be poorer than has been established in medical illnesses such as chronic cardiovascular disease. We would add that the paucity of EAM studies across psychiatry prevents clear conclusions regarding medication adherence in schizophrenia versus other diagnoses.
Notably, oral antipsychotic adherence rates in schizophrenia, defined as a continuous variable and measured by EAM, were in the range of 70%, lower than the 80% threshold recommended by expert consensus and used widely to define satisfactory adherence. Therefore, the results of the current meta-analysis indicate wide-spread non-adherence to oral antipsychotics even using the most liberal classification of adherence, taking adherence. Further, adherence in the global population of antipsychotic-taking patients may be even lower, as the current meta-analysis would have excluded a considerable proportion of patients already identified as non-adherent and receiving LAIs. In addition, EAM may actually be associated with increased medication adherence as participants may be aware adherence is being monitored, as was the case in 17 of the 19 studies included in our meta-analysis. However, one of the remaining two studies [24, 43] where EAM was not disclosed reported the mean rate of medication adherence as 66.1%, which calls into question this hypothesis [24]. This said, it would be valuable to use EAM in clinical practice if it is proven that patient awareness of monitoring improves adherence. It is also possible that the antipsychotic type may influence results; of note, SGAs were used more than FGAs in all included studies. Some studies reported that patients receiving SGAs demonstrated greater medication adherence and/or a more positive attitude toward medications than those receiving FGAs [46,47,48], while other studies did not [38, 49]. Because of inconclusive evidence, the relationship between the type of antipsychotics and medication adherence remains controversial.
The studies included in this systematic review used one of three cut-off points, 70%, 75%, and 80%, above which medication adherence was defined as satisfactory. The threshold of 70% was derived from research demonstrating that adherence to antipsychotics below 70% is associated with a greater risk of hospitalization in schizophrenia [50]. The threshold of 80% is recommended by an expert consensus survey on medication adherence in schizophrenia [14], and was used most frequently in the included studies. Given that both continuous and dichotomous medication adherence data provide useful information, we suggest that both be reported. In doing so, we can explore how adherence rates, when defined as a continuous variable, correspond to figures where adherence is defined as a dichotomous variable, and vice versa.
The current systematic review identified that poor oral antipsychotic adherence, as measured by EAM in schizophrenia, was associated with poorer insight into illness, greater illness severity, and greater complexity of dosing regimen, which aligns with the previous literature, which includes various adherence measures. A systematic review found that the main risk factors for medication non-adherence in schizophrenia were younger age, substance abuse, poor insight, cognitive impairments, low level of education, minority ethnicity, poor therapeutic alliance, experience of barriers to care, high intensity of delusional symptoms and suspiciousness, and low socioeconomic status [5]. In addition to these factors, an expert consensus survey on medication adherence in schizophrenia listed treatment-associated factors such as fear of side effect, lack of efficacy, and complexity of treatment regimen [14]. Certainly, simplifying dosing regimens may enhance medication adherence; for example, a meta-analysis of RCTs revealed that a once-daily dosing regimen was associated with better medication adherence than twice-daily dosing in chronic cardiovascular disease [51]. Similarly, another meta-analysis indicated a consistent trend of better medication adherence with less frequent dosing regimens in chronic psychiatric disorders [52]. Indeed, at least some studies included in the current systematic review showed that dosing frequency was a better predictor of medication adherence than antipsychotic type [24, 32, 37]. To our knowledge, though, no RCTs have been conducted to examine if simplifying dosing regimens improves medication adherence in psychiatric disorders.
It should be noted that EAM is not without its own limitations; for example, because confirmation that the bottle was opened does not guarantee that the medication was taken, EAM may overestimate medication adherence. On the other hand, in the case where a patient moves medications from EAM to a pill case, EAM can also underestimate the medication adherence. Nonetheless, EAM provides important and useful information that can shed light on the type of gaps that may be occurring [16]. In addition, a systematic review reported that self-report, self-rating/clinician rating, and pill count overestimated medication adherence by 17%, 6%, and 8%, respectively, compared to EAM [53]. As noted in Sect. 1, plasma concentrations of antipsychotics also offer only indirect information regarding actual adherence, and are subject to marked intra-, as well as inter-individual, variability [29]. Accordingly, at present, EAM remains the gold standard in measuring medication adherence. This may, of course, change as the field advances; for example, novel methods, such as the Proteus Digital Health®, that can capture medication adherence more directly are now accessible as options. This particular system consists of ingestible sensors and a wearable sensor patch, which can provide confirmation that the medication was ingested [54, 55]. As of yet, though, there are not enough data available to closely evaluate this system [56]. In addition, systems of this sort call into play ethical issues related to autonomy, confidentiality, and privacy that are yet to be resolved [57, 58].
The present findings need to be interpreted in the context of several limitations. First, only studies published in English were included in the current systematic review, so there may be additional studies relevant to the topic. Second, the time course of medication adherence is another important topic, and is not addressed in this study. Third, a small number of studies were included in the meta-analysis to estimate overall EAM-measured adherence to antipsychotics. Moreover, significant study heterogeneity was identified for all three outcomes. Fourth, results of the current meta-analysis may not be representative of the global population; for example, a considerable proportion of patients already identified as non-adherent and receiving LAIs were excluded. Fifth, the findings may not be applied to specific patient populations such as first-episode and treatment-resistant schizophrenia; to this point, in the present investigation, only one study [41] and none exclusively included these specific populations, respectively. In line with this, the findings cannot expand to clozapine as no studies investigating adherence to clozapine with EAM were identified in the literature search. While clozapine is associated with a higher rate of treatment continuation than other antipsychotics [59], it is surprising that only one study addressed clozapine adherence, using medication possession ratio (MPR) and reporting a range of 66–75% [60]. Similarly, there have been no studies focusing on adherence to concomitant oral antipsychotics separately in patients receiving LAIs with EAM, whereas there have been a few investigating adherence to oral antipsychotics and LAIs together [18, 28].
5 Conclusions
Oral antipsychotic adherence rates in schizophrenia, when defined as a continuous variable and measured by EAM, were in the range of 70%, lower than the 80% threshold used widely to define satisfactory adherence. There remain important questions that require further investigation, e.g., what constitutes a threshold that impacts response and negative outcomes (e.g., relapse, hospitalization, and suicide), what is the impact of patterns of nonadherence, and what are the possible differences in nonadherence as a function of antipsychotic type. EAM continues to provide benefits in addressing such questions.
References
Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–51. https://doi.org/10.1016/s0140-6736(19)31135-3.
Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–62. https://doi.org/10.1016/s0140-6736(13)60733-3.
Takeuchi H, Kantor N, Sanches M, Fervaha G, Agid O, Remington G. One-year symptom trajectories in patients with stable schizophrenia maintained on antipsychotics versus placebo: meta-analysis. Br J Psychiatry. 2017;211(3):137–43. https://doi.org/10.1192/bjp.bp.116.186007.
Lacro JP, Dunn LB, Dolder CR, Leckband SG, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):892–909.
Garcia S, Martinez-Cengotitabengoa M, Lopez-Zurbano S, Zorrilla I, Lopez P, Vieta E, et al. Adherence to antipsychotic medication in bipolar disorder and schizophrenic patients: a systematic review. J Clin Psychopharmacol. 2016;36(4):355–71. https://doi.org/10.1097/jcp.0000000000000523.
Higashi K, Medic G, Littlewood KJ, Diez T, Granstrom O, De Hert M. Medication adherence in schizophrenia: factors influencing adherence and consequences of nonadherence, a systematic literature review. Ther Adv Psychopharmacol. 2013;3(4):200–18. https://doi.org/10.1177/2045125312474019.
Leclerc E, Noto C, Bressan RA, Brietzke E. Determinants of adherence to treatment in first-episode psychosis: a comprehensive review. Braz J Psychiatry. 2015;37(2):168–76. https://doi.org/10.1590/1516-4446-2014-1539.
Velligan DI, Sajatovic M, Hatch A, Kramata P, Docherty JP. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449–68. https://doi.org/10.2147/ppa.s124658.
Wade M, Tai S, Awenat Y, Haddock G. A systematic review of service-user reasons for adherence and nonadherence to neuroleptic medication in psychosis. Clin Psychol Rev. 2017;51:75–95. https://doi.org/10.1016/j.cpr.2016.10.009.
Hogan TP, Awad AG, Eastwood R. A self-report scale predictive of drug compliance in schizophrenics: reliability and discriminative validity. Psychol Med. 1983;13(1):177–83. https://doi.org/10.1017/s0033291700050182.
Byerly MJ, Nakonezny PA, Rush AJ. The Brief Adherence Rating Scale (BARS) validated against electronic monitoring in assessing the antipsychotic medication adherence of outpatients with schizophrenia and schizoaffective disorder. Schizophr Res. 2008;100(1–3):60–9. https://doi.org/10.1016/j.schres.2007.12.470.
Dolder CR, Lacro JP, Warren KA, Golshan S, Perkins DO, Jeste DV. Brief evaluation of medication influences and beliefs: development and testing of a brief scale for medication adherence. J Clin Psychopharmacol. 2004;24(4):404–9. https://doi.org/10.1097/01.jcp.0000130554.63254.3a.
Byerly M, Fisher R, Whatley K, Holland R, Varghese F, Carmody T, et al. A comparison of electronic monitoring vs. clinician rating of antipsychotic adherence in outpatients with schizophrenia. Psychiatry Res. 2005;133(2–3):129–33. https://doi.org/10.1016/j.psychres.2004.11.002.
Velligan DI, Weiden PJ, Sajatovic M, Scott J, Carpenter D, Ross R, et al. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(Suppl 4):1–46 (quiz 7–8).
Velligan DI, Lam YW, Glahn DC, Barrett JA, Maples NJ, Ereshefsky L, et al. Defining and assessing adherence to oral antipsychotics: a review of the literature. Schizophr Bull. 2006;32(4):724–42. https://doi.org/10.1093/schbul/sbj075.
Vrijens B, Urquhart J. Methods for measuring, enhancing, and accounting for medication adherence in clinical trials. Clin Pharmacol Ther. 2014;95(6):617–26. https://doi.org/10.1038/clpt.2014.59.
Brain C, Allerby K, Sameby B, Quinlan P, Joas E, Karilampi U, et al. Drug attitude and other predictors of medication adherence in schizophrenia: 12 months of electronic monitoring (MEMS((R))) in the Swedish COAST-study. Eur Neuropsychopharmacol. 2013;23(12):1754–62. https://doi.org/10.1016/j.euroneuro.2013.09.001.
Gutierrez-Casares JR, Canas F, Rodriguez-Morales A, Hidalgo-Borrajo R, Alonso-Escolano D. Adherence to treatment and therapeutic strategies in schizophrenic patients: the ADHERE study. CNS Spectr. 2010;15(5):327–37. https://doi.org/10.1017/s1092852900027553.
Acosta FJ, Bosch E, Sarmiento G, Juanes N, Caballero-Hidalgo A, Mayans T. Evaluation of noncompliance in schizophrenia patients using electronic monitoring (MEMS) and its relationship to sociodemographic, clinical and psychopathological variables. Schizophr Res. 2009;107(2–3):213–7. https://doi.org/10.1016/j.schres.2008.09.007.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. https://doi.org/10.1136/bmj.b2535.
Velligan D, Mintz J, Maples N, Xueying L, Gajewski S, Carr H, et al. A randomized trial comparing in person and electronic interventions for improving adherence to oral medications in schizophrenia. Schizophr Bull. 2013;39(5):999–1007. https://doi.org/10.1093/schbul/sbs116.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Remington G, Kwon J, Collins A, Laporte D, Mann S, Christensen B. The use of electronic monitoring (MEMS) to evaluate antipsychotic compliance in outpatients with schizophrenia. Schizophr Res. 2007;90(1–3):229–37. https://doi.org/10.1016/j.schres.2006.11.015.
Yang J, Ko YH, Paik JW, Lee MS, Han C, Joe SH, et al. Symptom severity and attitudes toward medication: impacts on adherence in outpatients with schizophrenia. Schizophr Res. 2012;134(2–3):226–31. https://doi.org/10.1016/j.schres.2011.11.008.
Stip E, Vincent PD, Sablier J, Guevremont C, Zhornitsky S, Tranulis C. A randomized controlled trial with a Canadian electronic pill dispenser used to measure and improve medication adherence in patients with schizophrenia. Front Pharmacol. 2013;4:100. https://doi.org/10.3389/fphar.2013.00100.
Acosta FJ, Ramallo-Farina Y, Bosch E, Mayans T, Rodriguez CJ, Caravaca A. Antipsychotic treatment dosing profile in patients with schizophrenia evaluated with electronic monitoring (MEMS(R)). Schizophr Res. 2013;146(1–3):196–200. https://doi.org/10.1016/j.schres.2013.02.016.
Acosta FJ, Ramallo-Farina Y, Siris SG. Should full adherence be a necessary goal in schizophrenia? Full versus non-full adherence to antipsychotic treatment. Compr Psychiatry. 2014;55(1):33–9. https://doi.org/10.1016/j.comppsych.2013.09.005.
Brain C, Sameby B, Allerby K, Quinlan P, Joas E, Lindstrom E, et al. Stigma, discrimination and medication adherence in schizophrenia: results from the Swedish COAST study. Psychiatry Res. 2014;220(3):811–7. https://doi.org/10.1016/j.psychres.2014.10.016.
Brain C, Sameby B, Allerby K, Lindstrom E, Eberhard J, Burns T, et al. Twelve months of electronic monitoring (MEMS(R)) in the Swedish COAST-study: a comparison of methods for the measurement of adherence in schizophrenia. Eur Neuropsychopharmacol. 2014;24(2):215–22. https://doi.org/10.1016/j.euroneuro.2013.11.013.
Byerly MJ, Thompson A, Carmody T, Bugno R, Erwin T, Kashner M, et al. Validity of electronically monitored medication adherence and conventional adherence measures in schizophrenia. Psychiatr Serv. 2007;58(6):844–7. https://doi.org/10.1176/ps.2007.58.6.844.
Diaz E, Neuse E, Sullivan MC, Pearsall HR, Woods SW. Adherence to conventional and atypical antipsychotics after hospital discharge. J Clin Psychiatry. 2004;65(3):354–60. https://doi.org/10.4088/jcp.v65n0311.
Guevremont C, Sablier J, Lum M, Vincent P, Tranulis C, Marcotte FN, et al. Dopill, an electronic pill dispenser, helping evaluate and control medication adherence in patients with schizophrenia. Schizophr Res. 2010;117(2):278. https://doi.org/10.1016/j.schres.2010.02.450.
Kozuki Y, Poupore E, Schepp K. Visual feedback therapy to enhance medication adherence in psychosis. Arch Psychiatr Nurs. 2005;19(2):70–80. https://doi.org/10.1016/j.apnu.2005.02.008.
Kozuki Y, Schepp KG. Visual-feedback therapy for antipsychotic medication adherence. Int Clin Psychopharmacol. 2006;21(1):57–61. https://doi.org/10.1097/01.yic.0000177016.59484.ce.
Lee H, Kane I, Sereika SM, Cho RY, Jolley CJ. Medication-taking behaviours in young adults with schizophrenia: a pilot study. J Psychiatr Ment Health Nurs. 2011;18(5):418–24. https://doi.org/10.1111/j.1365-2850.2011.01689.x.
Misdrahi D, Tessier A, Husky M, Lange AC, Vrijens B, Llorca PM, et al. Evaluation of adherence patterns in schizophrenia using electronic monitoring (MEMS(R)): a six-month post-discharge prospective study. Schizophr Res. 2018;193:114–8. https://doi.org/10.1016/j.schres.2017.06.026.
Nakonezny PA, Byerly MJ. Electronically monitored adherence in outpatients with schizophrenia or schizoaffective disorder: a comparison of first- vs. second-generation antipsychotics. Schizophr Res. 2006;82(1):107–14. https://doi.org/10.1016/j.schres.2005.10.015.
Nakonezny PA, Byerly MJ, Pradhan A. The effect of providing patient-specific electronically monitored antipsychotic medication adherence results on the treatment planning of prescribers of outpatients with schizophrenia. Psychiatry Res. 2013;208(1):9–14. https://doi.org/10.1016/j.psychres.2013.02.015.
Nakonezny PA, Byerly MJ, Rush AJ. Electronic monitoring of antipsychotic medication adherence in outpatients with schizophrenia or schizoaffective disorder: an empirical evaluation of its reliability and predictive validity. Psychiatry Res. 2008;157(1–3):259–63. https://doi.org/10.1016/j.psychres.2007.05.001.
Subotnik KL, Ventura J, Gretchen-Doorly D, Hellemann GS, Agee ER, Casaus LR, et al. The impact of second-generation antipsychotic adherence on positive and negative symptoms in recent-onset schizophrenia. Schizophr Res. 2014;159(1):95–100. https://doi.org/10.1016/j.schres.2014.07.008.
Velligan DI, Wang M, Diamond P, Glahn DC, Castillo D, Bendle S, et al. Relationships among subjective and objective measures of adherence to oral antipsychotic medications. Psychiatr Serv. 2007;58(9):1187–92. https://doi.org/10.1176/ps.2007.58.9.1187.
Zhao Y, Moriwaki M, Kinoshita Y, Kawashima K, Ohga H, Tabuse H, et al. Maintaining favourable adherence by consistent self-administration of medication—Medication Event Monitoring System (MEMS) trial to evaluate the compliance of patients with schizophrenia in Japan. Schizophr Res. 2010;117(2):269. https://doi.org/10.1016/j.schres.2010.02.429.
Coleman CI, Roberts MS, Sobieraj DM, Lee S, Alam T, Kaur R. Effect of dosing frequency on chronic cardiovascular disease medication adherence. Curr Med Res Opin. 2012;28(5):669–80. https://doi.org/10.1185/03007995.2012.677419.
Lee MS, Lee HY, Kang SG, Yang J, Ahn H, Rhee M, et al. Variables influencing antidepressant medication adherence for treating outpatients with depressive disorders. J Affect Disord. 2010;123(1–3):216–21. https://doi.org/10.1016/j.jad.2009.10.002.
Dolder CR, Lacro JP, Dunn LB, Jeste DV. Antipsychotic medication adherence: is there a difference between typical and atypical agents? Am J Psychiatry. 2002;159(1):103–8. https://doi.org/10.1176/appi.ajp.159.1.103.
Karthik MS, Warikoo N, Chakrabarti S, Grover S, Kulhara P. Attitudes towards antipsychotics among patients with schizophrenia on first- or second-generation medications. Indian J Psychol Med. 2014;36(3):288–93. https://doi.org/10.4103/0253-7176.135382.
Warikoo N, Chakrabarti S, Grover S. Adherence and continuation of treatment with first- and second-generation antipsychotics in schizophrenia. Indian J Psychol Med. 2014;36(1):33–9. https://doi.org/10.4103/0253-7176.127244.
Sendt KV, Tracy DK, Bhattacharyya S. A systematic review of factors influencing adherence to antipsychotic medication in schizophrenia-spectrum disorders. Psychiatry Res. 2015;225(1–2):14–30. https://doi.org/10.1016/j.psychres.2014.11.002.
Weiden PJ, Kozma C, Grogg A, Locklear J. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886–91. https://doi.org/10.1176/appi.ps.55.8.886.
Weeda ER, Coleman CI, McHorney CA, Crivera C, Schein JR, Sobieraj DM. Impact of once- or twice-daily dosing frequency on adherence to chronic cardiovascular disease medications: a meta-regression analysis. Int J Cardiol. 2016;216:104–9. https://doi.org/10.1016/j.ijcard.2016.04.082.
Medic G, Higashi K, Littlewood KJ, Diez T, Granstrom O, Kahn RS. Dosing frequency and adherence in chronic psychiatric disease: systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2013;9:119–31. https://doi.org/10.2147/ndt.s39303.
El Alili M, Vrijens B, Demonceau J, Evers SM, Hiligsmann M. A scoping review of studies comparing the Medication Event Monitoring System (MEMS) with alternative methods for measuring medication adherence. Br J Clin Pharmacol. 2016;82(1):268–79. https://doi.org/10.1111/bcp.12942.
Belknap R, Weis S, Brookens A, Au-Yeung KY, Moon G, DiCarlo L, et al. Feasibility of an ingestible sensor-based system for monitoring adherence to tuberculosis therapy. PLoS One. 2013;8(1):e53373. https://doi.org/10.1371/journal.pone.0053373.
DiCarlo LA, Weinstein RL, Morimoto CB, Savage GM, Moon GL, Au-Yeung K, et al. Patient-centered home care using digital medicine and telemetric data for hypertension: feasibility and acceptability of objective ambulatory assessment. J Clin Hypertens (Greenwich). 2016;18(9):901–6. https://doi.org/10.1111/jch.12787.
Burnier M. Is there a threshold for medication adherence? Lessons learnt from electronic monitoring of drug adherence. Front Pharmacol. 2018;9:1540. https://doi.org/10.3389/fphar.2018.01540.
Klugman CM, Dunn LB, Schwartz J, Cohen IG. The ethics of smart pills and self-acting devices: autonomy, truth-telling, and trust at the dawn of digital medicine. Am J Bioethics. 2018;18(9):38–47. https://doi.org/10.1080/15265161.2018.1498933.
Campbell JI, Eyal N, Musiimenta A, Haberer JE. Ethical questions in medical electronic adherence monitoring. J Gen Intern Med. 2016;31(3):338–42. https://doi.org/10.1007/s11606-015-3502-4.
Chan HY, Pan YJ, Chen JJ, Chen CH. Time to discontinuation of second-generation antipsychotics versus haloperidol and sulpiride in people with schizophrenia: a naturalistic, comparative study. J Clin Psychopharmacol. 2017;37(1):13–20. https://doi.org/10.1097/jcp.0000000000000623.
Patel NC, Crismon ML, Miller AL, Johnsrud MT. Drug adherence: effects of decreased visit frequency on adherence to clozapine therapy. Pharmacotherapy. 2005;25(9):1242–7. https://doi.org/10.1592/phco.2005.25.9.1242.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of interest
Dr. Yaegashi has received speaker’s fees from Eisai, Meiji Seika Pharma, Janssen, Otsuka, Pfizer, and Sumitomo Dainippon Pharma. Dr. Kirino has received speaker’s fees from Novartis Pharma. Dr. Remington has received research support from the Canadian Institutes of Health Research (CIHR), HLS Therapeutics, Novartis Canada, and Research Hospital Fund–Canada Foundation for Innovation (RHF-CFI); conference support from Neurocrine Biosciences for data presentation; and advisory board support from HLS Therapeutics. Dr. Misawa has received speaker’s fees from Eli Lilly, Janssen, Novartis Pharma, Otsuka, Pfizer, and Sumitomo Dainippon Pharma. Dr. Takeuchi has received fellowship grants from Astellas Foundation for Research on Metabolic Disorders, the Canadian Institutes of Health Research (CIHR), Centre for Addiction and Mental Health (CAMH) Foundation, and the Japanese Society of Clinical Neuropsychopharmacology; speaker’s fees from Meiji Seika Pharma, Mochida, Otsuka, Sumitomo Dainippon Pharma, and Yoshitomiyakuhin; and manuscript fees from Sumitomo Dainippon Pharma.
Rights and permissions
About this article
Cite this article
Yaegashi, H., Kirino, S., Remington, G. et al. Adherence to Oral Antipsychotics Measured by Electronic Adherence Monitoring in Schizophrenia: A Systematic Review and Meta-analysis. CNS Drugs 34, 579–598 (2020). https://doi.org/10.1007/s40263-020-00713-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-020-00713-9