Abstract
Background
Weight gain is a potentially concerning side effect of second-generation antipsychotics (SGAs). Metformin, a biguanide with antihyperglycemic effects, is used to manage weight gain in adults treated with SGAs.
Objective
The objective of this study was to perform the first systematic review and meta-analysis of randomized controlled trials (RCTs) assessing the effects of metformin on weight gain in children and adolescents treated with SGAs.
Methods
Based on a pre-registered protocol (PROSPERO–CRD42017074839), we searched the PubMed, EMBASE, PsychoINFO, BIOSIS, Science Direct, Cochrane Central, and ClinicalTrials.gov electronic databases through March 2018 (with no restrictions on language, date, or type of publication) for RCTs that assessed the effect of metformin or placebo on body weight in children or adolescents (< 18 years of age) treated with selected SGAs (risperidone, aripiprazole, olanzapine, and clozapine) for any psychiatric disorder. We also contacted relevant drug manufacturers for possible additional pertinent studies/data. A random effects model was used and the quality of the included RCTs was assessed using the Cochrane Risk of Bias tool.
Results
Five RCTs (205 participants in total) were included in the meta-analysis. We found a significant weight decrease in the metformin group compared with placebo after 4, 12, and 16 weeks of treatment {mean difference − 0.98 kg (95% confidence interval [CI] − 1.26, − 0.69); − 1.83 kg (95% CI − 2.47, − 1.18); and − 3.23 kg (95% CI − 5.59, − 0.86), respectively}. A weight decrease at weeks 2 and 8 did not reach statistical significance. The decrease in body mass index (BMI) paralleled that of weight, with a significant effect at weeks 4, 12, and 16. Overall, four studies were rated as unclear, and one study was rated as high, risk of bias.
Conclusion
Meta-analytical evidence shows that metformin might decrease weight in children/adolescents treated with SGAs but additional high-quality evidence is needed. Clinicians need to be aware that this use of metformin is currently off-label.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This is the first systematic review and meta-analysis exploring the efficacy of metformin to manage weight gain and body mass index (BMI) increase related to the administration of second-generation antipsychotics (SGAs) in the pediatric population. |
From 12 weeks of treatment, metformin was significantly more efficacious than placebo in reducing BMI in children and adolescents treated with SGAs. |
1 Introduction
In children and adolescents, second-generation antipsychotics (SGAs) are US FDA-approved for a number of indications in mental health conditions, including autism spectrum disorder, schizophrenia, and bipolar disorder, and are widely used off-label in Tourette’s syndrome and disruptive behavior associated with externalizing disorders [1,2,3,4]. Over the past decades, there has been a remarkable increase in the prescription of SGAs across several countries [5,6,7,8]. Indeed, SGAs are among the most commonly prescribed medications in children and adolescents in North America [4]. Unfortunately, these drugs are associated with important adverse effects, including weight gain and metabolic disturbances [9, 10]. In the Second-generation Antipsychotic Treatment Indications, Effectiveness and Tolerability in Youth (SATIETY), a large naturalistic cohort study conducted from 2001 to 2007, a weight gain of more than 7% was observed during the first 3 months for 85% of patients treated with olanzapine, 65% of patients treated with risperidone, and 60% of patients treated with aripiprazole [11]. These results have been confirmed by recent meta-analytic evidence [12].
Obesity in children and adolescents is associated with a dramatic increase in morbidity. Approximately 25% of youth with obesity present with metabolic syndrome and increased risk for atherosclerosis, heart diseases, and type 2 diabetes mellitus [13,14,15]. The risk of heart disease is tenfold higher in youth with obesity compared with healthy-weight peers [16]. Children and adolescents with obesity may also experience psychological distress related to being bullied, leading to poor self-image and possibly depression [17]; thus, careful screening and early interventions to prevent weight gain and metabolic syndrome are recommended in youth treated with SGAs [18]. Unfortunately, in clinical practice, careful metabolic parameter monitoring is often overlooked in youth treated with SGAs [19]. Non-pharmacological strategies for weight gain, such as educational or family-based interventions, are recommended but showed limited efficacy. Supporting evidence on their effects have been extrapolated from studies of children with obesity or type 2 diabetes mellitus [20]. In 1999, metformin was proposed as a promising pharmacological option to manage weight gain associated with the use of SGAs, although use for this purpose was, and remains, off-label [21]. Metformin is currently approved by both the FDA and the European Medicines Agency (EMA) for treating type 2 diabetes mellitus in children older than 10 years of age [22, 23]; however, in this specific indication, lifestyle interventions are more effective than metformin for preventing progress from pre-diabetes to diabetes, and inducing weight loss [24]. This drug is also effective in reducing the body mass index (BMI) of children with obesity [25]. Meta-analytic evidence shows that metformin combined with lifestyle interventions was efficacious (albeit with moderate effect size) and well tolerated in decreasing weight in children with obesity aged 10–16 years [26, 27]. Additionally, in adults, metformin has been found to be efficacious in improving both glycemic control and weight gain related to SGAs. It is important to note that this effect is reported in studies of relatively short duration, especially in patients who are already obese [28,29,30,31]. There is also preliminary but increasing evidence on the use of metformin in childhood to manage the metabolic disturbances induced by SGAs, including weigh gain [32, 33]. To our knowledge, no systematic review and meta-analysis has been conducted to estimate the efficacy of metformin in counteracting the effects of SGAs on weight in children and adolescents, and our study aimed to fill this gap. Given the exploratory nature of this meta-analysis, no a priory hypothesis was formulated.
2 Methods
2.1 Search Strategy
The protocol for the present systematic review/meta-analysis was registered on the international prospective register of systematic reviews (PROSPERO; https://www.crd.york.ac.uk/PROSPERO, protocol number CRD42017074839). This systematic review and meta-analysis was conducted and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations [34]. The PubMed (MEDLINE), EMBASE, PsychoINFO, BIOSIS, Science Direct, and Cochrane CENTRAL electronic databases were searched, with no restriction in terms of language, type of document, or date; the following search terms/syntax were used for Pubmed: metformin AND (antipsychotic* OR Risperidone OR Aripiprazole OR Olanzapine OR Clozapine) AND (child OR children OR adolesc* OR youth* OR pediatr* OR paediatr* OR early onset). In our search terms, we specified the risperidone, aripiprazole, olanzapine, and clozapine SGAs because these are the most widely used drugs of this class in the pediatric population and/or those most associated with metabolic side effects. The search terms/syntax were adapted accordingly for all the remaining databases. Reference lists of the retained articles and relevant review articles were hand-searched to retrieve any additional pertinent reports not detected via the electronic database search. Furthermore, we searched the ClinicalTrials.gov database to retrieve any pertinent studies not yet published as full-text articles at the time of our search. The last search was completed on 30 March 2018. Additionally, we contacted relevant drug manufacturers to inquire about any relevant published or unpublished studies not identified in our search.
2.2 Selection of Relevant Articles
Studies were included in our systematic review if they met the following criteria: (1) randomized controlled trials (RCTs), regardless of the level of blinding and follow-up time; (2) participants under the age of 18 years; (3) participants treated with any SGAs and randomized to metformin or placebo, whatever the type of psychiatric disorder for which they received the antipsychotic; and (4) weight and/or BMI values at baseline and study endpoint as study outcomes. All non-randomized studies were excluded, and no restrictions in terms of the ethnic origin of participants were applied.
2.3 Selection of Studies and Data Extraction
The eligibility process was conducted in two separate stages. First, two researchers (PE and RD) independently screened all non-duplicate references initially retrieved as potentially pertinent, and excluded those clearly not pertinent based on title or abstract. A final list was agreed on, with discrepancies resolved by consensus between the two authors. When consensus was not reached, a third senior researcher (SC) acted as arbitrator. Second, full-text versions of the articles passing stage 1 screening were downloaded and independently assessed for eligibility by the two researchers. Discrepancies were resolved by consensus between the two researchers and, if needed, the third senior researcher acted as arbitrator. When required, corresponding authors were contacted to clarify study eligibility.
2.4 Risk of Bias of Included Studies
Risk of bias for each study included in the meta-analysis was assessed using the Cochrane Risk of Bias Tool [35]. Risk of bias domains included selection bias (random sequence generation, allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other bias. As in the work by Cortese [36], the overall rating of risk of bias for each study was the lowest rating for any of the criteria (e.g. if any item was scored as high risk of bias, the entire study was scored as high risk of bias; if all items were scored as low risk, the entire study overall was rated as low risk).
2.5 Statistical Analyses
The mean difference (MD) for each study was first calculated as the mean pre- to post-treatment change in the intervention group minus the mean pre- to post-treatment change in the control group, divided by the pooled pre-test standard deviation with bias adjustment [37]. Analyses were conducted as per protocol data, with the exception of one study [38] that presented data for intention-to-treat analyses only. The MD for each trial was then combined using the inverse variance method. Given the inherent heterogeneity of studies, a random effects model was used. The I2 statistic (representing the percentage of variance due to between-study heterogeneity rather than sampling error [39]) was calculated to estimate between-trial MD heterogeneity. Analyses were performed using RevManager 5 (http://community.cochrane.org/help/tools-and-software/revman-5).
3 Results
3.1 Search Strategy
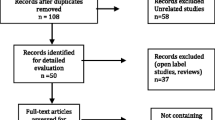
From an initial pool of 76 potentially relevant references, five studies were included in the meta-analysis [38, 40,41,42,43]. Figure 1 reports the PRISMA flowchart detailing the screening process, and Electronic Supplementary Table 1 reports the references discarded after reading the full text, with the specific reasons for exclusion.
3.2 Characteristics of Studies Included in the Meta-Analysis
Three studies were published as full-text reports in peer-reviewed journals [38, 40, 41] and two additional studies were found on ClinicalTrials.gov [42, 43] (Table 1). The duration of the studies varied from 16 to 26 weeks. For all studies, both weight and BMI values (at baseline and endpoint) were available. The age range of participants was between 11.25 and 14.2 years.
3.3 Results of the Meta-Analysis
3.3.1 Effect of Metformin on Weight
Meta-analysis results on weight gain are reported in Figs. 2, 3, and 4. The difference in weight change between metformin and placebo from baseline to weeks 2 and 8 did not reach statistical significance {MD − 0.29 (95% confidence interval [CI] − 1.00, 0.41); and − 1.54 (95% CI − 3.52, 0.45), respectively}. There was evidence of heterogeneity at week 2 [tau2 = 0.16, Chi2 = 2.67, degrees of freedom (df) = 1 (p = 0.10), I2 = 63%] and week 8 [tau2 = 1.28, Chi2 = 2.55, df = 1 (p = 0.11), I2 = 61%]. The difference between metformin and placebo in weight change from baseline to weeks 4, 12, and 16 was significant [− 0.98 (95% CI − 1.26, − 0.69); − 1.83 (95% CI − 2.47, − 1.18); and − 3.23 (95% CI − 5.59, − 0.86), respectively]. Heterogeneity values at weeks 4, 12, and 16 were tau2 = 0.01, Chi2 = 2.10, df = 2 (p = 0.35), I2 = 5%; tau2 = 0.00, Chi2 = 2.16, df = 3 (p = 0.54), I2 = 0%; and tau2 = 1.20, Chi2 = 1.41, df = 1 (p = 0.24), I2 = 29%, respectively.
3.3.2 Effect of Metformin on Body Mass Index
The results of the meta-analysis on BMI are reported in Figs. 5 and 6. Results paralleled those for weight, with no statistical significance at week 4 [− 0.29 (95% CI − 0.59, 0.01)], but significant values at weeks 12 and 16 [− 0.63 (95% CI − 0.86, − 0.40) and − 1.00 (95% CI − 1.54, − 0.46), respectively] between weight changes in the metformin and placebo groups. Heterogeneity values at weeks 4, 12, and 16 were tau2 = 0.04, Chi2 = 4.20, df = 1 (p = 0.04), I2 = 76%; tau2 = 0.00, Chi2 = 0.11, df = 2 (p = 0.95), I2 = 0%; and tau2 = 0.03, Chi2 = 1.16, df = 1 (p = 0.28), I2 = 14%, respectively.
3.4 Risk of Bias
As shown in Table 2, one study [43] was, overall, deemed as high risk of bias, whereas the others were rated as unclear risk of bias overall [37, 39,40,41].
3.5 Changes in Relation to the Original Protocol
Given the paucity of available data, we were unable to conduct the planned analysis on the effect of metformin on glucose and cholesterol parameters, or on the tolerability of metformin.
4 Discussion
To our knowledge, this is the first systematic review and meta-analysis exploring the efficacy of metformin in counteracting weight gain and BMI increase related to the administration of SGAs in the juvenile population. Our meta-analysis showed that from 12 weeks of treatment, metformin was significantly more efficacious than placebo in reducing BMI in children and adolescents treated with SGAs. Our results are in agreement with those results in adults where the efficacy of metformin in reducing weight gain was demonstrated by several meta-analyses [28, 44,45,46]. Although our meta-analysis could not provide additional information beyond week 16, preliminary evidence from an open-label study suggested that the effect was maintained after 16 weeks of treatment [47]. It is also fundamental to note that metformin is used off-label in this indication in psychiatry. In addition, a 10-year follow-up study of the Diabetes Prevention Program Research Group has shown that a lifestyle intervention program is more efficacious than metformin for weight loss [24]. Even if the efficacy of metformin in adult populations exposed to SGAs is more robust, it is recommended that it never be used before healthy lifestyle interventions [48, 49].
The exact mechanisms underlying the effects of metformin in reducing weight gain related to antipsychotic treatment remain to be elucidated. Metformin is a biguanide that has been found to inhibit hepatic glucose production, lower circulating free fatty acids, and, ultimately, reduce gluconeogenesis and intestinal absorption of glucose [50]. It also improves the uptake of glucose and its use by the muscle instead of adipocytes [51]. The main mechanism of weight loss may result from a reduction of insulin resistance and an increase in the sense of satiety [52]. Finally, one cannot exclude that gastrointestinal distress induced by metformin may also be one of the mechanisms leading to weight loss in patients. In the specific context of SGA use, the efficacy of metformin might be accounted for by these two mechanisms [53]. Of note, a recent study showed that only half of the weight gain observed in youth taking antipsychotics is fat [54]. Metformin attenuation of weight gain might represent some attenuation of fat increase and some attenuation of lean mass (muscle and/or water). Because these mechanisms are mostly speculative, further research is needed to gain insight into the precise mechanism of action of metformin in patients treated with SGAs.
Our results should be considered in light of the strengths and limitations of this study. Regarding the strengths, we performed a systematic search of several databases, without language restrictions, including ClinicalTrials.gov. We also contacted the studies’ authors and drug companies to gather additional unpublished data. However, a number of limitations should be taken into account. First, we could only retain a limited number of studies that explored the effects of metformin in the pediatric populations. However, there is no established minimum number of studies to be included in a meta-analysis [54], and, given the high clinical relevance of the topic, this first evidence synthesis in the field should hopefully encourage further methodologically sound investigations. Second, none of the included studies were, overall, rated as low risk of bias, with the majority (n = 4) of studies overall rated as unclear risk of bias, and one study rated as high risk of bias. However, it should be noted that we used stringent criteria to rate the risk of bias (a study had to present all items at low risk of bias in order to be rated, overall, as low risk of bias). Furthermore, many items were rated as unclear due to missing information in the publication, which calls for better and more complete reporting in the field. Third, due to the paucity of data, we did not perform subgroup analysis based on the type of SGA. For similar reasons, we were unable to perform subgroup analysis by preventative or curative use of metformin (whatever the specific mental health conditions for which SGAs were prescribed). However, unlike studies in adults, all SGAs have been associated with significant metabolic alterations in children and adolescents [55]. Furthermore, we were not able to meta-analyze the outcome on the tolerability of metformin; however, evidence from non-randomized studies is available to inform prescribers on this relevant issue. For instance, in a long-term follow-up study of 6.5 years on 700 pediatric patients treated with metformin, 40% presented with mild gastrointestinal symptoms (such as abdominal pain, nausea, metallic taste, bloating, and diarrhea), 20% presented with anemia, and 5.7% showed elevated liver transaminases [56]. Clinicians must be aware of the high rate of gastrointestinal distress, which can be a major issue in treatment adherence. Long-term use of metformin was also associated with a decreased in vitamin B12 [32]. The overall adverse event rate was estimated at 5.6% participant-years of exposure [56]. In adults, a study on 47,597 patients during a follow-up period of 7.2 ± 3.2 years showed that the long-term use of metformin was associated with a significant decrease in colorectal cancer occurrence [57]. These results were replicated in another cohort [58]. More generally, in a cohort including 82,720 adult metformin users, long-term adherence to metformin was associated with decreased risks of all-cause mortality after a 2.4-year follow-up period [59]. However, caution is required in the pediatric context, especially in relation to the use of metformin for weight gain prevention related to SGA treatment, considering the possible risks of metformin and the fact that the non-pharmacological alternatives have been found to be effective. However, in situations where non-pharmacological approaches are not feasible or are unsuccessful, metformin may be considered as a possible intervention, supported by preliminary evidence, as shown in this meta-analysis. Of note, no serious adverse events were reported in any of the studies included in our meta-analysis.
5 Conclusions
Our study provided meta-analytic evidence that metformin may be beneficial, in the short term, to counteract weight gain in children and adolescents treated with SGAs. However, our findings should be replicated in larger trials before being considered for supporting clinical recommendations, and should be considered with caution due to the low number of studies (with a low number of participants) included, the overall heterogeneity between studies, and the off-label use of metformin [43, 60,61,62,63,64,65]. If replicated in further large, high-quality trials, our findings suggest that since it took approximately 4 weeks for significant effects to be observed, early administration of metformin, soon after the patient is started on an SGA, would be warranted, reflecting the currently available guidelines for adults [66]. It is important to bear in mind that the American Psychiatric Association recommends that antipsychotic medications should not be routinely prescribed as a first-line intervention for children and adolescents, which is actually the best way to avoid metabolic side effects [67]. Multimodal treatment strategies encompassing metformin and non-pharmacological strategies (such as diet and educational or family-based interventions) are likely to represent the most efficacious intervention to counteract weight gain associated with SGAs in youth, and should be a research priority in this field.
References
Kurlan R. Clinical practice. Tourette’s syndrome. N Engl J Med. 2010;363:2332–8.
McCracken JT, McGough J, Shah B, Cronin P, Hong D, Aman MG, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347:314–21.
Birnbaum ML, Saito E, Gerhard T, Winterstein A, Olfson M, Kane JM, et al. Pharmacoepidemiology of antipsychotic use in youth with ADHD: trends and clinical implications. Curr Psychiatry Rep. 2013;15:382.
Olfson M, Blanco C, Liu S-M, Wang S, Correll CU. National trends in the office-based treatment of children, adolescents, and adults with antipsychotics. Arch Gen Psychiatry. 2012;69:1247–56.
Prah P, Petersen I, Nazareth I, Walters K, Osborn D. National changes in oral antipsychotic treatment for people with schizophrenia in primary care between 1998 and 2007 in the United Kingdom. Pharmacoepidemiol Drug Saf. 2012;21:161–9.
Bernardo M, Coma A, Ibáñez C, Zara C, Bari JM, Serrano-Blanco A. Antipsychotic polypharmacy in a regional health service: a population-based study. BMC Psychiatry. 2012;12:42.
Jaracz J, Tetera-Rudnicka E, Kujath D, Raczyńska A, Stoszek S, Czernaś W, et al. The prevalence of antipsychotic polypharmacy in schizophrenic patients discharged from psychiatric units in Poland. Pharmacol Rep. 2014;66:613–7.
Verdoux H, Pambrun E, Tournier M, Bezin J, Pariente A. Antipsychotic long-acting injections: a community-based study from 2007 to 2014 of prescribing trends and characteristics associated with initiation. Schizophr Res. 2016;178:58–63.
Bak M, Fransen A, Janssen J, van Os J, Drukker M. Almost all antipsychotics result in weight gain: a meta-analysis. PLoS ONE. 2014;9:e94112.
Burghardt KJ, Seyoum B, Mallisho A, Burghardt PR, Kowluru RA, Yi Z. Atypical antipsychotics, insulin resistance and weight; a meta-analysis of healthy volunteer studies. Prog Neuropsychopharmacol Biol Psychiatry. 2018;83:55–63.
Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302:1765–73.
Almandil NB, Liu Y, Murray ML, Besag FMC, Aitchison KJ, Wong ICK. Weight gain and other metabolic adverse effects associated with atypical antipsychotic treatment of children and adolescents: a systematic review and meta-analysis. Paediatr Drugs. 2013;15:139–50.
Tirosh A, Shai I, Afek A, Dubnov-Raz G, Ayalon N, Gordon B, et al. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med. 2011;364:1315–25.
Sabin MA, Ford AL, Holly JMP, Hunt LP, Crowne EC, Shield JPH. Characterisation of morbidity in a UK, hospital based, obesity clinic. Arch Dis Child. 2006;91:126–30.
Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365:1876–85.
Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the Princeton Lipid Research Clinics Follow-up Study. Pediatrics. 2007;120:340–5.
US Preventive Services Task Force, Grossman DC, Bibbins-Domingo K, Curry SJ, Barry MJ, Davidson KW, et al. Screening for Obesity in Children and Adolescents: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317:2417–26
Curtis J, Newall HD, Samaras K. The heart of the matter: cardiometabolic care in youth with psychosis. Early Interv Psychiatry. 2012;6:347–53.
Nolt VD, Kibler AV, Wilkening GL, Fabian TJ. Second-Generation antipsychotic utilization and metabolic parameter monitoring in an inpatient pediatric population: a retrospective analysis. Pediatr Drugs. 2017;19:139–46.
O’Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:2427–44.
Baptista T. Body weight gain induced by antipsychotic drugs: mechanisms and management. Acta Psychiatr Scand. 1999;100:3–16.
TODAY Study Group, Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366:2247–56
European Medicines Agency. -Human medicines—EMEA-002249-PIP01-17. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/pips/EMEA-002249-PIP01-17/pip_001818.jsp&mid=WC0b01ac058001d129. Accessed 29 July 2018.
Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–86.
Baur LA, Hazelton B, Shrewsbury VA. Assessment and management of obesity in childhood and adolescence. Nat Rev Gastroenterol Hepatol. 2011;8:635–45.
McDonagh MS, Selph S, Ozpinar A, Foley C. Systematic review of the benefits and risks of metformin in treating obesity in children aged 18 years and younger. JAMA Pediatr. 2014;168:178–84.
Mead E, Atkinson G, Richter B, Metzendorf M-I, Baur L, Finer N, et al. Drug interventions for the treatment of obesity in children and adolescents. Cochrane Database Syst Rev. 2016;(11):CD012436.
de Silva VA, Suraweera C, Ratnatunga SS, Dayabandara M, Wanniarachchi N, Hanwella R. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. 2016;16:341.
Wu R-R, Zhang F-Y, Gao K-M, Ou J-J, Shao P, Jin H, et al. Metformin treatment of antipsychotic-induced dyslipidemia: an analysis of two randomized, placebo-controlled trials. Mol Psychiatry. 2016;21:1537–44.
Taylor J, Stubbs B, Hewitt C, Ajjan RA, Alderson SL, Gilbody S, et al. The Effectiveness of Pharmacological and Non-Pharmacological Interventions for Improving Glycaemic Control in Adults with Severe Mental Illness: a Systematic Review and Meta-Analysis. PLoS One. 2017;12:e0168549.
Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medications used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. 2010;35:1520–30.
Andrade C. Metformin as a possible intervention for cardiometabolic risks in pediatric subjects exposed to antipsychotic drugs. J Clin Psychiatry. 2016;77:1362–4.
Walkup JT, Cottingham E. Antipsychotic-Induced Weight Gain and Metformin. J Am Acad Child Adolesc Psychiatry. 2017;56:808–10.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Cochrane Handbook for Systematic Reviews of Interventions. http://handbook-5-1.cochrane.org/. Accessed 18 May 2018.
Cortese S. Meta-analyses in child and adolescent psychiatry: getting closer to clinical practice. J Am Acad Child Adolesc Psychiatry. 2018;57:229–30.
Sonuga-Barke EJS, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, et al. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry. 2013;170:275–89.
Anagnostou E, Aman MG, Handen BL, Sanders KB, Shui A, Hollway JA, et al. Metformin for treatment of overweight induced by atypical antipsychotic medication in young people with autism spectrum disorder: a randomized clinical trial. JAMA Psychiatry. 2016;73:928–37.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Klein DJ, Cottingham EM, Sorter M, Barton BA, Morrison JA. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry. 2006;163:2072–9.
Arman S, Sadramely MR, Nadi M, Koleini N. A randomized, double-blind, placebo-controlled trial of metformin treatment for weight gain associated with initiation of risperidone in children and adolescents. Saudi Med J. 2008;29:1130–4.
Strategies to reduce antipsychotic-associated weight gain in youth (PREVENT). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT00617240. Accessed 7 May 2018.
Reducing weight gain and improving metabolic function in children being treated with antipsychotics (IMPACT). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT00806234. Accessed 7 May 2018.
Siskind DJ, Leung J, Russell AW, Wysoczanski D, Kisely S. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11:e0156208.
Zheng W, Li X-B, Tang Y-L, Xiang Y-Q, Wang C-Y, de Leon J. Metformin for weight gain and metabolic abnormalities associated with antipsychotic treatment: meta-analysis of randomized placebo-controlled trials. J Clin Psychopharmacol. 2015;35:499–509.
Zheng W, Zhang Q-E, Cai D-B, Yang X-H, Ungvari GS, Ng CH, et al. Combination of metformin and lifestyle intervention for antipsychotic-related weight gain: a meta-analysis of randomized controlled trials. Pharmacopsychiatry. Epub 27 Feb 2018. https://doi.org/10.1055/s-0044-101466.
Handen BL, Anagnostou E, Aman MG, Sanders KB, Chan J, Hollway JA, et al. A randomized, placebo-controlled trial of metformin for the treatment of overweight induced by antipsychotic medication in young people with autism spectrum disorder: open-label extension. J Am Acad Child Adolesc Psychiatry. 2017;56(849–856):e6.
Hasnain M, Vieweg WVR, Fredrickson SK. Metformin for atypical antipsychotic-induced weight gain and glucose metabolism dysregulation: review of the literature and clinical suggestions. CNS Drugs. 2010;24:193–206.
Hasnain M, Fredrickson SK, Vieweg WVR. Metformin for obesity and glucose dysregulation in patients with schizophrenia receiving antipsychotic drugs. J Psychopharmacol. 2011;25:715–21.
Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–9.
Minamii T, Nogami M, Ogawa W. Mechanisms of metformin action: in and out of the gut. J Diabetes Investig. 2018;9(4):701–3.
Lee A, Morley JE. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes Res. 1998;6:47–53.
Dayabandara M, Hanwella R, Ratnatunga S, Seneviratne S, Suraweera C, de Silva VA. Antipsychotic-associated weight gain: management strategies and impact on treatment adherence. Neuropsychiatr Dis Treat. 2017;13:2231–41.
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Chichester: Wiley; 2009 [cited 5 Sep 2016]. https://doi.org/10.1002/9780470743386.
Solmi M, Murru A, Pacchiarotti I, Undurraga J, Veronese N, Fornaro M, et al. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther Clin Risk Manag. 2017;13:757–77.
TODAY Study Group. Safety and tolerability of the treatment of youth-onset type 2 diabetes: the TODAY experience. Diabetes Care. 2013;36:1765–71.
Chang Y-T, Tsai H-L, Kung Y-T, Yeh Y-S, Huang C-W, Ma C-J, et al. Dose-Dependent relationship between metformin and colorectal cancer occurrence among patients with type 2 diabetes: a nationwide cohort study. Transl Oncol. 2018;11:535–41.
Bradley MC, Ferrara A, Achacoso N, Ehrlich SF, Quesenberry CP, Habel LA. A cohort study of metformin and colorectal cancer risk among patients with diabetes mellitus. Cancer Epidemiol Biomark Prev. 2018;27:525–30.
Simard P, Presse N, Roy L, Dorais M, White-Guay B, Räkel A, et al. Association between metformin adherence and all-cause mortality among new users of metformin: a nested case-control study. Ann Pharmacother. 2018;52:305–13.
Morrison JA, Cottingham EM, Barton BA. Metformin for weight loss in pediatric patients taking psychotropic drugs. Am J Psychiatry. 2002;159:655–7.
Shin L, Bregman H, Breeze JL, Noyes N, Frazier JA. Metformin for weight control in pediatric patients on atypical antipsychotic medication. J Child Adolesc Psychopharmacol. 2009;19:275–9.
Wink LK, Adams R, Pedapati EV, Dominick KC, Fox E, Buck C, et al. Brief report: metformin for antipsychotic-induced weight gain in youth with autism spectrum disorder. J Autism Dev Disord. 2017;47:2290–4.
Metformin for weight control in adolescents taking atypical antipsychotics. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT00845936. Accessed 30 July 2018.
Metformin for Overweight & OBese ChILdren and Adolescents With BDS Treated With SGAs (MOBILITY). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02515773. Accessed 30 July 2018.
Improving metabolic parameters of antipsychotic child treatment with ziprasidone, aripiprazole, and clozapine (ZAC). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT00617058. Accessed 30 July 2018.
Hendrick V, Dasher R, Gitlin M, Parsi M. Minimizing weight gain for patients taking antipsychotic medications: the potential role for early use of metformin. Ann Clin Psychiatry. 2017;29:120–4.
American Psychiatric Association. Choosing Wisely. https://www.psychiatry.org/psychiatrists/practice/quality-improvement/choosing-wisely. Accessed 29 July 2018.
Acknowledgements
The authors would like to thank the following authors who kindly provided additional unpublished information/data: E. Anagnostou and colleagues, Department of Pediatrics, University of Toronto, Toronto, ON, Canada; C.U. Correll and colleagues, Hofstra Northwell School of Medicine, New York, NY, USA; S. Arman and colleagues, Department of Psychiatry, Isfahan University of Medical Sciences, Iran; M.A. Riddle and colleagues, Division of Child and Adolescent Psychiatry, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, MD, USA; L. Sikich and colleagues, Division of Child and Adolescent Psychiatry, School of Medicine, University of Maryland, Baltimore, MD, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this manuscript.
Conflicts of interest
Pierre Ellul and Richard Delorme declare no conflicts of interest. Samuele Cortese has received fees from the Association for Child and Mental Health (ACAMH; a non-profit organization) and Healthcare Convention for educational activity on attention-deficit hyperactivity disorder.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ellul, P., Delorme, R. & Cortese, S. Metformin for Weight Gain Associated with Second-Generation Antipsychotics in Children and Adolescents: A Systematic Review and Meta-Analysis. CNS Drugs 32, 1103–1112 (2018). https://doi.org/10.1007/s40263-018-0571-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-018-0571-z