Abstract
Background
Intranasal and buccal midazolam have recently emerged as possible alternatives to intravenous or rectal diazepam or intravenous lorazepam in the treatment of early status epilepticus (SE). However, to date no randomized controlled trial (RCT) has directly compared intranasal midazolam with buccal midazolam.
Objective
The aim of this study was to indirectly compare intranasal midazolam with buccal midazolam in the treatment of early SE using common reference-based indirect comparison meta-analyses.
Methods
RCTs comparing intranasal or buccal midazolam versus either intravenous or rectal diazepam for early SE were systematically searched. Random-effects Mantel–Haenszel meta-analyses were performed to obtain odds ratios (ORs) for the efficacy and safety of intranasal or buccal midazolam versus either intravenous or rectal diazepam. Adjusted indirect comparisons were then made between intranasal and buccal midazolam using the obtained results.
Results
Fifteen studies, with a total of 1662 seizures in 1331 patients (some studies included patients with more than one episode of SE) were included; 1303 patients were younger than 16 years. Indirect comparisons showed no difference between intranasal and buccal midazolam for seizure cessation (OR 0.98, 95 % CI 0.32–3.01, comparator: intravenous diazepam; OR 0.87, 95 % CI 0.46–1.64, comparator: rectal diazepam). For serious adverse effects, we found a large width and asymmetrical distribution of confidence intervals around the obtained OR of 2.81 (95 % CI 0.39–20.12; comparator: rectal diazepam). No data were available for OR using intravenous diazepam as the comparator.
Conclusions
Indirect comparisons suggest that intranasal and buccal midazolam share similar efficacy in the treatment of early SE in children. Intranasal midazolam should be used with caution and under clinical monitoring of vital functions. RCTs directly comparing intranasal midazolam with buccal midazolam are required to confirm these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
No randomized controlled trial (RCT) has directly compared intranasal midazolam with buccal midazolam, therefore we indirectly compared these two formulations for early status epilepticus (SE) using indirect comparison meta-analysis. |
Intranasal and buccal midazolam have similar efficacy in the treatment of early SE in children; however, intranasal midazolam should be used with caution and under clinical monitoring of vital functions. |
RCTs directly comparing intranasal midazolam with buccal midazolam are required to confirm these findings. |
1 Introduction
Status epilepticus (SE) represents a medical and neurological emergency associated with high morbidity and mortality in adults [1, 2] and children [3, 4], requiring prompt recognition and treatment with antiepileptic drugs (AEDs) to prevent death or irreversible brain damage. Early treatment is a relevant prognostic factor, associated with lower morbidity and mortality, fewer drugs required in hospital and shorter overall seizure duration [5, 6].
Several AEDs are available as alternative and competing interventions for the treatment of early SE, which can be defined pragmatically as seizure activity lasting longer than 5 min [7]. Diazepam, lorazepam and midazolam are commonly used as acute rescue treatment in patients with early (stage I) SE. Diazepam can be administered either intravenously or rectally, whereas lorazepam is administered intravenously (although preclinical data on its bioavailability and pharmacokinetics after intramuscular and intranasal administration are available [8]). Both these routes of administration may result in a treatment delay as rectal administration requires removal of clothes and adequate positioning of the patient and intravenous administration requires an intravenous access, which may prove difficult in the prehospital setting as it requires some expertise. Furthermore, rectal administration can be socially unacceptable. Conversely, midazolam can be administered by both intravenous and non-intravenous routes (intramuscular, buccal, and intranasal) due to its water solubility.
A recent review of the literature has shown that non-intravenous midazolam is as effective and safe as intravenous or rectal diazepam in terminating early SE in children, and probably also in adults, and that it can be administered more rapidly than intravenous or rectal diazepam [9]. Most information in the literature regarding the role of midazolam in the treatment of early SE derives from clinical trials comparing this AED with intravenous or rectal diazepam [9, 10]. These studies therefore provide only a partial fragment of the whole picture. Knowing the efficacy and safety of midazolam compared with intravenous or rectal diazepam is useful clinical information; however, it would be ideal to know how all the different options rank against each other and how vital these differences are in effect size between all the available drugs [11, 12].
Although intranasal and buccal midazolam have recently emerged as possible alternatives to intravenous or rectal diazepam or to intravenous lorazepam in the treatment of early SE, to date no randomized controlled trial (RCT) has directly compared intranasal midazolam with buccal midazolam in this condition. Hence, from the available literature, no information has been obtained on the efficacy and safety of these two drugs derived from comparative trials. Until further data from direct head-to-head clinical trials comparing intranasal midazolam with buccal midazolam are available, other methods might be used to make comparisons between these AEDs in the treatment of early SE.
Conventional meta-analyses of RCTs focus on direct, pair-wise comparisons between two treatments (e.g. treatment A vs. treatment B). Unfortunately, direct head-to-head comparisons are not available for all treatments of interest in early SE. In such cases, definite data on treatment effect cannot be estimated. Despite this situation, it is possible to estimate the indirect effect of treatment A versus treatment B using evidence from trials comparing treatment A with treatment C, and trials comparing treatment B with treatment C [13]. The key assumption for this indirect comparison based on a common comparator (treatment C) is that of exchangeability of the treatment effect across all included trials [14].
Indirect comparisons based on a common comparator (also known as ‘adjusted indirect comparison’ [15] or ‘common reference-based indirect comparison’ [14]) represent a useful tool to provide information on relative efficacy of competing interventions where data from direct comparisons are not available [15], and their use has been recommended in case of lack of direct evidence [15].
We therefore decided to undertake a systematic review with meta-analysis of intranasal midazolam compared with buccal midazolam in the treatment of early SE in patients of any age, indirectly estimating their efficacy and safety through indirect comparison meta-analyses using intravenous and rectal diazepam as the common comparator.
2 Methods
This review was guided by a written prespecified protocol describing research questions, review methods, and a plan for data extraction and synthesis. The protocol is available online at: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015019540.
2.1 Criteria for Considering Studies for this Review
RCTs comparing intranasal or buccal midazolam against intravenous or rectal diazepam in the treatment of early SE were included in the meta-analysis. We included all RCTs, blinded or not blinded, and excluded uncontrolled and nonrandomized trials. Patients from any age group who presented to a hospital or emergency medical department, and who were diagnosed with early SE, were included. Early SE was defined as either seizures lasting >5 min [7] or as seizures at arrival to the Emergency Department or at the arrival of paramedics (for studies conducted in prehospital settings).
We considered all trials in which intranasal or buccal midazolam were compared with either intravenous or rectal diazepam, and which have been included in a previously published systematic review [9]. Trials were not excluded on the basis of dose, duration of treatment, or length of follow-up.
2.2 Search Methods
A comprehensive review of the literature of computerized databases, as well as searches to find unpublished trials, were performed to minimize publication bias. The following electronic databases and data sources were searched:
-
1.
MEDLINE (January 1966–21 July 2015), accessed through PubMed
-
2.
Cochrane Central Register of Controlled Trials (CENTRAL) [Issue 12, The Cochrane Library, December 2014], accessed 21 July 2015
-
3.
ClinicalTrials.gov (https://clinicaltrials.gov/), accessed 21 July 2015
-
4.
EMBASE, accessed 21 July 2015
-
5.
LILACS (http://lilacs.bvsalud.org/en/), accessed 21 July 2015
-
6.
Hand searching of the references quoted in the identified trials and in reviews
-
7.
Conference proceedings of the London-Innsbruck Colloquia (2007–2015) published in Epilepsia (the official journal of the International League Against Epilepsy [ILAE]) and Epilepsy and Behavior
-
8.
Contact with pharmaceutical companies (Viropharma, Upsher-Smith and Accord Healthcare) to identify unpublished trials or data missing from articles (April 2015)
-
9.
Contact with authors and known experts to identify any additional data.
The search strategies adopted for all databases mentioned above are reported in the electronic supplementary material, part 1. All resulting titles and abstracts were evaluated, and any relevant article was considered. There were no language restrictions.
2.3 Study Selection
Retrieved articles were independently assessed for inclusion by two review authors; any disagreement was resolved through discussion.
2.4 Methodological Quality Assessment
Trials were scrutinized, and the methodological quality of all included studies was evaluated. The randomized trials were judged on the reported method of allocation concealment and on the risk of bias as outlined in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) [16]. We also evaluated whether authors disclosed their conflicts of interest and whether pharmaceutical companies sponsored the studies.
2.5 Data Extraction
The following trial data were extracted: main study author and age of publication; country; definition of SE applied in the study; type of participants (children, adults, or both); total number, age, and sex of participants for each treatment group; seizure type; intervention details (dose, route of administration); definition of successful treatment adopted in each trial; proportion of seizures controlled after drug administration in each treatment group; and proportion of serious adverse effects (respiratory depression and/or hypotension) in each group.
2.6 Types of Outcome Measures
We chose dichotomous primary outcomes in order to have hard outcome measures of both treatment efficacy and safety. Odds ratios (ORs) for binary outcomes were chosen because they are associated with less heterogeneity in meta-analysis than risk differences or relative risks [17]. The following outcomes (reported in studies meeting the inclusion criteria) relevant to the efficacy and safety of the intervention drug (intranasal or buccal midazolam vs. intravenous or rectal diazepam) were collected.
2.6.1 Efficacy
Efficacy was assessed as the number of patients with clinical seizure cessation within 15 min after the start of drug administration.
2.6.2 Tolerability and Safety
Tolerability and safety were assessed as the number of patients experiencing serious adverse effects (respiratory depression and/or hypotension).
2.7 Statistical Analysis
For each outcome, an intention-to-treat primary analysis was made to include all patients in the treatment group to which they were allocated, irrespective of the treatment they actually received.
Analyses were conducted using RevMan 5 (conventional meta-analysis for each AED), Excel and R 2.15.1 (common reference-based indirect comparison meta-analysis).
2.8 Conventional Meta-Analysis Per Antiepileptic Drug (AED)
Conventional meta-analyses of comparisons between intranasal or buccal midazolam and intravenous or rectal diazepam were carried out. Random effects, inverse variance, and weighted meta-analysis were used to pool the results from individual trials for each AED (intranasal and buccal midazolam, both were compared against either intravenous or rectal diazepam) [18].
Each outcome was analyzed by calculating ORs with 95 % confidence intervals (CIs). For each outcome, a weighted treatment effect across trials was calculated. The Mantel–Haenszel method was used to estimate the OR statistic and to combine ORs [19].
2.8.1 Random Effects Model
Pair-wise meta-analyses were performed by synthesizing studies that compare the same interventions using a random effects model [18] to incorporate the assumption that the different studies are estimating different, yet related, treatment effects [16]. The random effects model was used for the quantitative pooling [18] as adjusted indirect comparison using the fixed-effect model tended to underestimate standard errors of pooled estimates [14, 20].
2.8.2 Assessment of Heterogeneity
Visual inspection of the forest plots was used to investigate the possibility of statistical heterogeneity. Homogeneity among trial results was evaluated using a standard Chi squared test, and the hypothesis of homogeneity was rejected if the p value was less than 0.10.
Assessment of statistical heterogeneity was supplemented using the I-squared (I 2) statistic, which provides an estimate of the percentage of variability due to heterogeneity rather than a sampling error [21]. The interpretation of I 2 for heterogeneity was performed according to Higgins and Green [16].
Possible sources of heterogeneity were assessed and discussed narratively.
2.8.3 Suitability of Indirect Comparisons
The suitability of indirect comparisons was investigated, considering whether studies were suitably similar by adopting the framework for assessing exchangeability assumption proposed by the Indirect Comparisons Working Group to the Pharmaceutical Benefits Advisory Committee [14].
2.9 Common Reference-Based Indirect Comparisons by Combining Meta-Analyses of AEDs
2.9.1 Comparison Method
We conducted a common reference-based indirect comparison meta-analysis to synthesize information from trials addressing the same question but involving different interventions. For a given comparison, e.g. A versus B, direct evidence is provided by studies that compare these two treatments head-to-head. In other terms, for the direct comparisons, comparison of the result of group A with the result of group B within an RCT gives an estimate of the efficacy of intervention A versus B. However, indirect evidence is provided when studies that compare A versus C and B versus C are analyzed jointly.
As none of the included trials directly compared intranasal midazolam with buccal midazolam, an adjusted method of indirect comparison between intranasal midazolam and buccal midazolam was performed using the results of the following four meta-analyses:
-
1.
Intranasal midazolam versus intravenous diazepam.
-
2.
Intranasal midazolam versus rectal diazepam.
-
3.
Buccal midazolam versus intravenous diazepam.
-
4.
Buccal midazolam versus rectal diazepam.
2.9.2 Statistical Analysis
We carried out common reference-based indirect comparisons using the method suggested by Bucher et al. [22] and adopted in previous reviews on AEDs [12, 23, 24]; the indirect comparison of intranasal and buccal midazolam was adjusted by the results of their direct comparisons with either intravenous or rectal diazepam (common intervention).
Using this adjusted method, it was possible to overcome the potential problem of different prognostic characteristics between study participants across different trials; it is valid if the relative efficacy of interventions is consistent among studies. In order for this indirect comparison to be valid, the overall characteristics of the trials included in the meta-analyses should not differ systematically [15].
The comparison between each AED and other AEDs was performed using the ORs derived from the conventional meta-analyses described above.
Comparison of each binary outcome measure was performed using the log of OR and its variance derived from the conventional meta-analyses [22]. Since the logs of the OR of each meta-analysis are asymptotically normally distributed and statistically independent, the estimate of the treatment effect (i.e. intranasal midazolam vs. buccal midazolam) was calculated by the difference (diff) between the logs of the two ORs:
The 95 % CI of this estimated effect was derived from the standard error of the difference:
where SE (diff) = (variance (ln ORIN MDZ) + variance (ln ORB MDZ))1/2. Back transformation was then performed to give the OR and its 95 % CIs for the indirect comparisons.
By convention, ORs >1 indicate that the outcome is more likely to occur in the intranasal midazolam group than in the group receiving either intravenous or rectal diazepam. The same was applied for buccal midazolam. For the indirect comparisons, an OR >1 indicates that the outcome is more likely after intranasal midazolam than after rectal midazolam administration. A p value of 0.05 was considered to be statistically significant. No heterogeneity was assessed for indirect comparisons.
3 Results
The search strategy described above yielded 913 results (255 MEDLINE, 50 CENTRAL, 45 ClinicalTrials.gov, 558 EMBASE, 5 LILACS, and 0 conference proceedings of the London-Innsbruck Colloquia). The pharmaceutical companies Viropharma and Accord Healthcare were contacted (April 2015) but no additional unpublished trials were found.
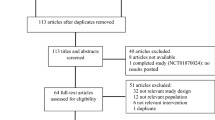
After excluding duplicate studies (60) and reading the abstract, 29 studies were provisionally selected according to the inclusion and exclusion criteria provided above. After having read the full text, we further excluded 14 studies (see electronic supplementary material, part 2, for details on excluded studies and reasons for exclusion). Hence, 15 studies (Fig. 1), with a total of 1662 seizures in 1331 patients (some studies included patients with more than one episode of SE) were included [25–39] (Tables 1, 2). Most patients who were included (1303) were aged less than 16 years. One study [35] was entirely conducted in adults, whereas one study [25] was conducted in both adults and children.
Study flow process (based on the example in Moher et al. [50])
3.1 Risk of Bias in the Included Studies
Details on the risk of bias for each included study are provided in Table 3. All studies were described as an RCT. Two studies used a systematic, nonrandom approach (sequence generation was obtained by a rule based on the day of admission) [27, 29]. Three RCTs used block randomization [32, 36, 37], whereas two used a random number table or generated the sequence of randomization by shuffling envelopes [25, 31]. In the remaining RCTs, the method used to generate random sequence was not reported.
Allocation concealment was adequate in five studies [26, 28, 32, 36, 39] and probably adequate in two studies not reporting whether an opaque envelope with the name of the drug was used [25, 31]. One study specified that allocation was not concealed [30], and the remaining studies did not provide enough data to evaluate the quality of allocation concealment.
Four RCTs were reported to be not blinded [34, 36–38], one study was defined as single-masked [31], and in other RCTs, blinding was not explicitly specified. However, it is possible that the ‘hard’ outcomes chosen in all studies were not influenced by lack of blinding [9, 40–42]. As a consequence, all studies have a low risk of performance and detection bias.
Seven RCTs explicitly detailed the authors’ conflicts of interest, and none were funded or sponsored by pharmaceutical companies [26, 30, 32, 35, 36, 38, 39]; the remaining studies did not report conflicts of interest.
3.2 Conventional Meta-Analysis Per AED
3.2.1 Intranasal Midazolam versus Either Intravenous or Rectal Diazepam
3.2.1.1 Intranasal Midazolam versus Intravenous Diazepam: Clinical Seizure Cessation After Drug Administration
Four studies with 232 seizures were included (Fig. 2a). No significant statistical heterogeneity among trials was detected, and there was no statistically significant difference in clinical seizure cessation after drug administration between the intranasal midazolam and intravenous diazepam groups (OR 0.92, 95 % CI 0.34–2.50).
Conventional meta-analyses of studies comparing intranasal midazolam versus either intravenous or rectal diazepam. a Intranasal midazolam versus intravenous diazepam: clinical seizure cessation after drug administration; b intranasal midazolam versus intravenous diazepam: serious adverse effects; c intranasal midazolam versus rectal diazepam: clinical seizure cessation after drug administration; d intranasal midazolam versus rectal diazepam: serious adverse effects. CI confidence intervals, df degrees of freedom, DZP diazepam, IN intranasal, IV intravenous, MDZ midazolam, M–H Mantel–Haenszel meta-analysis, R rectal
3.2.1.2 Intranasal Midazolam versus Intravenous Diazepam: Serious Adverse Effects
Four studies with 232 seizures (Fig. 2b) were included. Statistical heterogeneity among trials was not evaluable, and there was no statistically significant difference in serious adverse effects between the intranasal midazolam and intravenous diazepam groups (OR 0.27, 95 % CI 0.01–7.02).
3.2.1.3 Intranasal Midazolam versus Rectal Diazepam: Clinical Seizure Cessation After Drug Administration
Four studies with 449 seizures were included (Fig. 2c). Compared with rectal diazepam, intranasal midazolam had no statistically significant difference in seizure cessation after drug administration (OR 1.71, 95 % CI 0.65–4.52). Significant statistical heterogeneity among trials (p = 0.005; I 2 = 62 %) was observed. After repeating pooled analysis by excluding the study conducted in adults by de Haan et al. [35], significant statistical heterogeneity was no longer detected (p = 0.26; I 2 = 26 %).
3.2.1.4 Intranasal Midazolam versus Rectal Diazepam: Serious Adverse Effects
Four studies with 449 seizures were included (Fig. 2d). No significant statistical heterogeneity among trials was detected, and there was no statistically significant difference in serious adverse effects between intranasal midazolam and rectal diazepam (OR 1.49, 95 % CI 0.16–13.68).
3.2.2 Buccal Midazolam versus Either Intravenous or Rectal Diazepam
3.2.2.1 Buccal Midazolam versus Intravenous Diazepam: Clinical Seizure Cessation After Drug Administration
Two studies with 212 seizures were included (Fig. 3a). No significant statistical heterogeneity among trials was detected, and there was no statistically significant difference in clinical seizure cessation after drug administration between the buccal midazolam and intravenous diazepam groups (OR 0.68, 95 % CI 0.31–1.53).
Conventional meta-analyses of studies comparing buccal midazolam versus either intravenous or rectal diazepam. a Buccal midazolam versus intravenous diazepam: clinical seizure cessation after drug administration; b buccal midazolam versus intravenous diazepam: serious adverse effects; c buccal midazolam versus rectal diazepam: clinical seizure cessation after drug administration; d buccal midazolam versus rectal diazepam: serious adverse effects. B buccal, CI confidence intervals, df degrees of freedom, DZP diazepam, IV intravenous, MDZ midazolam, M–H Mantel–Haenszel meta-analysis, R rectal
3.2.2.2 Buccal Midazolam versus Intravenous Diazepam: Serious Adverse Effects
Two studies with 212 seizures were included (Fig. 3b). Statistical heterogeneity among trials was not evaluable, and there was no statistically significant difference in serious adverse effects between the buccal midazolam and intravenous diazepam groups (OR 1.01, 95 % CI 0.36–2.86).
3.2.2.3 Buccal Midazolam versus Rectal Diazepam: Clinical Seizure Cessation After Drug Administration
Five studies with 769 seizures were included (Fig. 3c). No significant statistical heterogeneity among trials was detected. Compared with rectal diazepam, buccal midazolam was more effective in seizure cessation after drug administration (OR 1.78, 95 % CI 1.11–2.85).
3.2.2.4 Buccal Midazolam versus Rectal Diazepam: Serious Adverse Effects
Five studies with 769 seizures were included (Fig. 3d). No significant statistical heterogeneity among trials was detected, and there was no statistically significant difference in serious adverse effects between the buccal midazolam and rectal diazepam groups (OR 0.70, 95 % CI 0.27–1.86).
3.2.3 Common Reference-Based Indirect Comparisons by Combining Meta-Analyses of AEDs
3.2.3.1 Intranasal Midazolam versus Buccal Midazolam (Common Comparator: Intravenous Diazepam): Clinical Seizure Cessation After Drug Administration
No difference was observed between buccal midazolam and intranasal midazolam in clinical seizure cessation after drug administration (OR 0.98, 95 % CI 0.32–3.01). After excluding the study conducted in adults by de Haan et al. [35], no difference between intranasal and buccal midazolam in terms of seizure cessation was observed (OR 0.81, 95 % CI 0.43–1.54).
3.2.3.2 Intranasal Midazolam versus Buccal Midazolam (Common Comparator: Intravenous Diazepam): Serious Adverse Effects
ORs were not estimable as no adverse effects were reported.
3.2.3.3 Intranasal Midazolam versus Buccal Midazolam (Common Comparator: Rectal Diazepam): Clinical Seizure Cessation After Drug Administration
No difference was observed between buccal midazolam and intranasal midazolam in clinical seizure cessation after drug administration (OR 0.87, 95 % CI 0.46–1.64).
3.2.3.4 Intranasal Midazolam versus Buccal Midazolam (Common Comparator: Rectal Diazepam): Serious Adverse Effects
No difference was observed between buccal midazolam and intranasal midazolam in occurrence of serious adverse effects (OR 2.81, 95 % CI 0.39–20.12).
4 Discussion
The results of the present meta-analysis suggest that there is no difference in efficacy between intranasal and buccal midazolam used as acute rescue therapy for early SE. Regarding tolerability/safety, it is possible that intranasal midazolam might be associated with higher adverse effects than buccal midazolam.
The results of this meta-analysis should be read with caution, paying attention to the fact that the comparisons between intranasal and buccal midazolam were made indirectly using data generated from individual comparisons versus either intravenous or rectal diazepam. Indirect comparison meta-analyses should not be considered as a substitute for comparative clinical trials comparing two or more AEDs head-to-head, nor for long-term clinical experience. However, as RCTs directly comparing intranasal midazolam with buccal midazolam are not available, the adjusted indirect method may provide some evidence of the relative efficacy and safety of these competing AEDs.
The indirect comparative data on efficacy showed no difference between intranasal and buccal midazolam, both after using intravenous diazepam and after using rectal diazepam as the common comparator (OR 0.98, 95 % CI 0.32–3.01 and OR 0.87, 95 % CI 0.46–1.64, respectively). The presence of similar results after indirect comparisons with two different common comparators may indicate that results of the indirect comparison meta-analyses are accurate. However, it is worth remembering that the absence of evidence of a difference in efficacy between intranasal and buccal midazolam is not synonymous with evidence of no difference as the included studies and their sample sizes may have been underpowered to detect such a difference [43].
Regarding safety/tolerability, the meta-analysis showed no difference between buccal midazolam and intranasal midazolam in occurrence of serious adverse effects as the 95 % CI include the null value of 1. However, due to the small numbers of events this OR is based on, the width of the CIs is extremely high, ranging from 0.39 to 20.12, suggesting that there is a great amount of uncertainty about the true value of the OR that has been estimated. Furthermore, the distribution of the 95 % CI around the obtained value of 2.81 is asymmetrical, with values falling more on the positive (i.e. values >1) than on the negative side. This latter finding suggests that intranasal midazolam might be associated with a higher incidence of adverse effects than buccal midazolam. Consequently, even if the meta-analysis showed no difference, it is still possible that the use of intranasal midazolam is associated with a clinically relevant increase in adverse effects compared with buccal midazolam.
Indirect comparisons have been recommended to evaluate the efficacy and tolerability of alternative interventions in case of lack of direct evidence [15, 44]. Despite initial concerns that indirect comparisons are more prone to bias than direct comparisons [22], a critical appraisal of the literature analysing results of 44 meta-analyses from 28 systematic reviews showed that in most cases (93 %) adjusted indirect comparisons were in agreement with the results of head-to-head RCTs, and in only 2 % of cases the discrepancy between the direct and the adjusted indirect estimate were clinically relevant [15]. The validity of the adjusted indirect comparisons depends on the internal validity and similarity of the RCTs involved [15]. These two aspects should therefore be always carefully considered to investigate potential causes of discrepancy between the direct and the adjusted indirect estimate, which can be due to clinical and methodological heterogeneity between trials. In the absence of head-to-head RCTs between intranasal and buccal midazolam, we were unable to draw conclusions on a possible discrepancy between direct and indirect comparisons. However, all RCTs included in our indirect comparison meta-analysis shared similar methodological and clinical features.
A significant statistical heterogeneity was observed, indicative of inconsistency in the results of included studies in only one meta-analysis, i.e. that comparing intranasal midazolam with rectal diazepam for clinical seizure cessation. The statistical heterogeneity is due to the presence of variability among studies beyond the amount expected due to chance alone, and may hence be explained by differences in clinical features of study participants (clinical heterogeneity) and/or by different study methodologies adopted or different treatment regimens (methodological heterogeneity). Interestingly, in this meta-analysis, all studies except one [35] were conducted in a pediatric population. After repeating this meta-analysis by excluding one study conducted in adults [35], no significant statistical heterogeneity was detected. This finding suggests that age may represent a relevant source of heterogeneity across different trials. However, after repeating the common reference-based indirect comparison meta-analysis by excluding this study [35], no difference between intranasal and buccal midazolam in terms of seizure cessation (OR 0.81, 95 % CI 0.43–1.54, comparator: intravenous diazepam) was again observed. However, it is important to consider that most patients included in our review (1303 of 1331) were younger than 16 years, therefore generalizability of our results is limited to this age.
The similar efficacy of intranasal and buccal midazolam may be explained by the pharmacokinetic properties. The mouth and the nose have surface areas rich in blood supply responsible for rapid absorption into the systemic circulation, avoiding gastrointestinal destruction and first-pass metabolism through liver biotransformation. However, to date no pharmacokinetic study has been conducted to directly compare the velocity and completeness of absorption following intranasal or buccal administration of this drug.
Compared with the intravenous or rectal route of administration, the buccal or intranasal route is likely to result in a significantly shorter time interval from arrival in the Emergency Department, or from arrival of paramedics, to drug administration. This is confirmed by five of seven RCTs included in our review, which showed that, compared with intravenous or rectal diazepam, intranasal or buccal midazolam was administered more rapidly [26, 31, 33, 34, 39]. A previous systematic review also considering intramuscular midazolam showed that, although non-intravenous midazolam (intramuscular, buccal, or intranasal) can be administered more rapidly than intravenous or rectal diazepam, this ‘time gain’ does not necessarily result in higher seizure control [9]. In fact, no significant differences in clinical seizure control were found between non-intravenous midazolam and diazepam by any route [9]. Whether this lack of difference is due to unpowered studies or is real needs to be investigated by further studies. However, the mean difference between non-intravenous midazolam and intravenous or rectal diazepam administration was found to be lower than 4 min, with an upper 95 % CI of 5 min (−3.56 min; 95 % CIs −2.11 to −5.00) [9], suggesting that, although statistically relevant, this ‘time gain’ may not be clinically relevant.
Compared with rectal or intravenous diazepam or lorazepam administered intravenously, intranasal and buccal midazolam have higher social acceptability and ease of use. Not surprisingly, several studies have shown that most patients and caregivers prefer using intranasal or buccal midazolam rather than rectal diazepam [35, 45, 46]. However, compared with intravenous lorazepam, midazolam has a short duration of action, which may result in the re-emergence of seizure activity. Furthermore, both intranasal and buccal routes of administration may not be fully free from concerns. Buccal administration carries the risk of aspiration or inconsistent absorption due to ictal hypersalivation and buccal secretion (however, these risks have not been systematically assessed in clinical trials and hence need to be confirmed). Conversely, intranasal administration of midazolam may be limited by the irritation produced by its acid PH and the relatively large volume to be administered [47, 48]. Furthermore, the intranasal route carries the potential risk for anterior and/or posterior drainage, leading to reduced or erratic bioavailability [49].
5 Conclusions
The direct comparisons carried out in the present review show no significant difference in seizure control following intranasal or buccal midazolam compared with rectal or intravenous diazepam, and suggest a possible higher efficacy of buccal midazolam over rectal diazepam in controlling early SE. Although direct head-to-head RCTs comparing intranasal midazolam with buccal midazolam are required to verify the accuracy of our adjusted indirect comparisons, our review suggests that intranasal and buccal midazolam share similar efficacy in the treatment of early SE in children. Caution should be taken when using intranasal midazolam, which might be associated with a clinically relevant increase in adverse events. Hence, we would suggest only using intranasal midazolam with caution and in a setting where clinical monitoring of vital functions is possible.
Buccal midazolam should be considered as a valid alternative to rectal diazepam or intravenous diazepam or lorazepam as first-line AEDs in early SE, especially in the prehospital setting where positioning an intravenous access may be difficult and rectal drug administration may be seen as socially unacceptable.
References
DeLorenzo RJ, Pellock JM, Towne AR, Boggs JG. Epidemiology of status epilepticus. J Clin Neurophysiol. 1995;12:316–25.
Cascino GD. Generalized convulsive status epilepticus. Mayo Clin Proc. 1996;71:787–92.
Berg AT, Shinnar S, Levy SR, Testa FM. Status epilepticus in children with newly diagnosed epilepsy. Ann Neurol. 1999;45:618–23.
Berg AT, Shinnar S, Testa FM, et al. Status epilepticus after the initial diagnosis of epilepsy in children. Neurology. 2004;63:1027–34.
Silbergleit R, Lowenstein D, Durkalski V, Conwit R, Neurological Emergency Treatment Trials (NETT) Investigators. RAMPART (Rapid Anticonvulsant Medication Prior to Arrival Trial): a double-blind randomized clinical trial of the efficacy of intramuscular midazolam versus intravenous lorazepam in the prehospital treatment of status epilepticus by paramedics. Epilepsia. 2011;52(Suppl 8):45–7.
Silbergleit R, Lowenstein D, Durkalski V, Conwit R, NETT Investigators. Lessons from the RAMPART study—and which is the best route of administration of benzodiazepines in status epilepticus. Epilepsia. 2013;54(Suppl 6):74–7.
Lowenstein DH, Bleck T, Macdonald RL. It’s time to revise the definition of status epilepticus. Epilepsia. 1999;40:120–2.
Wermeling DP, Miller JL, Archer SM, Manaligod JM, Rudy AC. Bioavailability and pharmacokinetics of lorazepam after intranasal, intravenous, and intramuscular administration. J Clin Pharmacol. 2001;41:1225–31.
Brigo F, Nardone R, Tezzon F, Trinka E. Nonintravenous midazolam versus intravenous or rectal diazepam for the treatment of early status epilepticus: a systematic review with meta-analysis. Epilepsy Behav. 2015;49:325–336. doi:10.1016/j.yebeh.2015.02.030.
Prasad M, Krishnan PR, Sequeira R, Al-Roomi K. Anticonvulsant therapy for status epilepticus. Cochrane Database Syst Rev. 2014;9:CD003723.
Brigo F. New anti-epileptic drugs: overcoming the limits of randomised controlled trials. Int J Evid Based Healthc. 2011;9:440–3.
Brigo F, Igwe SC, Nardone R, Tezzon F, Bongiovanni LG, Trinka E. A common reference-based indirect comparison meta-analysis of intravenous valproate versus intravenous phenobarbitone for convulsive status epilepticus. Epileptic Disord. 2013;15:314–23.
Smith CT, Marson AG, Chadwick DW, Williamson PR. Multiple treatment comparisons in epilepsy monotherapy trials. Trials. 2007;5(8):34.
ICWG. Report of the Indirect Comparisons Working Group to the Pharmaceutical Benefits Advisory Committee: assessing indirect comparisons. Canberra (ACT): Australian Government Department of Health and Aging; 2009. http://www.pbs.gov.au/industry/useful-resources/PBAC_feedback_files/ICWG%20Report%20FINAL2.pdf. Accessed 16 Apr 2015.
Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ. 2003;326:472–5.
Higgins JPT, Green S, editors. Handbook for systematic reviews of interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration; 2011.
Deeks JJ. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med. 2002;21:1575–600.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Emerson JD. Combining estimates of the odds ratio: the state of the art. Stat Methods Med Res. 1994;3:157–78.
Glenny AM, Altman DG, Song F, et al. International Stroke Trial Collaborative Group. Indirect comparisons of competing interventions. Health Technol Assess. 2005;9:1–134 (iii–iv).
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis or randomized controlled trials. J Clin Epidemiol. 1997;50:683–91.
Otoul C, Arrigo C, van Rijckevorsel K, French JA. Meta-analysis and indirect comparisons of levetiracetam with other second-generation antiepileptic drugs in partial epilepsy. Clin Neuropharmacol. 2005;28:72–8.
Zaccara G, Giovannelli F, Maratea D, Fadda V, Verrotti A. Neurological adverse events of new generation sodium blocker antiepileptic drugs. Meta-analysis of randomized, double-blinded studies with eslicarbazepine acetate, lacosamide and oxcarbazepine. Seizure. 2013;22:528–36.
Scott RC, Besag FM, Neville BG. Buccal midazolam and rectal diazepam for treatment of prolonged seizures in childhood and adolescence: a randomised trial. Lancet. 1999;353:623–6.
Lahat E, Goldman M, Barr J, Bistritzer T, Berkovitch M. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: prospective randomised study. BMJ. 2000;321:83–6.
Fişgin T, Gurer Y, Teziç T, Senbil N, Zorlu P, Okuyaz C, et al. Effects of intranasal midazolam and rectal diazepam on acute convulsions in children: prospective randomized study. J Child Neurol. 2002;17:123–6.
Mahmoudian T, Zadeh MM. Comparison of intranasal midazolam with intravenous diazepam for treating acute seizures in children. Epilepsy Behav. 2004;5:253–5.
Baysun S, Aydin OF, Atmaca E, Gürer YK. A comparison of buccal midazolam and rectal diazepam for the acute treatment of seizures. Clin Pediatr (Phila). 2005;44:771–6.
McIntyre J, Robertson S, Norris E, Appleton R, Whitehouse WP, Phillips B, et al. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: a randomised controlled trial. Lancet. 2005;366:205–10.
Bhattacharyya M, Kalra V, Gulati S. Intranasal midazolam vs rectal diazepam in acute childhood seizures. Pediatr Neurol. 2006;34:355–9.
Mpimbaza A, Ndeezi G, Staedke S, Rosenthal PJ, Byarugaba J. Comparison of buccal midazolam with rectal diazepam in the treatment of prolonged seizures in Ugandan children: a randomized clinical trial. Pediatrics. 2008;121:e58–64.
Talukdar B, Chakrabarty B. Efficacy of buccal midazolam compared to intravenous diazepam in controlling convulsions in children: a randomized controlled trial. Brain Dev. 2009;31:744–9.
Ashrafi MR, Khosroshahi N, Karimi P, Malamiri RA, Bavarian B, Zarch AV, et al. Efficacy and usability of buccal midazolam in controlling acute prolonged convulsive seizures in children. Eur J Paediatr Neurol. 2010;14:434–8.
de Haan GJ, van der Geest P, Doelman G, Bertram E, Edelbroek P. A comparison of midazolam nasal spray and diazepam rectal solution for the residential treatment of seizure exacerbations. Epilepsia. 2010;51:478–82.
Holsti M, Dudley N, Schunk J, Adelgais K, Greenberg R, Olsen C, et al. Intranasal midazolam vs rectal diazepam for the home treatment of acute seizures in pediatric patients with epilepsy. Arch Pediatr Adolesc Med. 2010;164:747–53.
Javadzadeh M, Sheibani K, Hashemieh M, Saneifard H. Intranasal midazolam compared with intravenous diazepam in patients suffering from acute seizure: a randomized clinical trial. Iran J Pediatr. 2012;22:1–8.
Tonekaboni SH, Shamsabadi FM, Anvari SS, Mazrooei A, Ghofrani M. A comparison of buccal midazolam and intravenous diazepam for the acute treatment of seizures in children. Iran J Pediatr. 2012;22:303–8.
Thakker A, Shanbag P. A randomized controlled trial of intranasal-midazolam versus intravenous-diazepam for acute childhood seizures. J Neurol. 2013;260:470–4.
Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet. 2002;359:696–700.
Ravani P, Parfrey PS, Curtis B, Barrett BJ. Clinical research of kidney diseases 1: researchable questions and valid answers. Nephrol Dial Transplant. 2007;22:3681–90.
Brigo F, Storti M, Del Felice A, Fiaschi A, Bongiovanni LG. IV valproate in generalized convulsive status epilepticus: a systematic review. Eur J Neurol. 2012;19:1180–91.
Guyatt G, Jaeschke R, Heddle N, Cook D, Shannon H, Walter S. Basic statistics for clinicians: 1. Hypothesis testing. CMAJ. 1995;152:27–32.
McAlister FA, Laupacis A, Wells GA, Sackett DL. Users’ Guides to the Medical Literature: XIX. Applying clinical trial results B. Guidelines for determining whether a drug is exerting (more than) a class effect. JAMA. 1999;282(14):1371–7.
Wilson MT, Macleod S, O’Regan ME. Nasal/buccal midazolam use in the community. Arch Dis Child. 2004;89:50–1.
Klimach VJ, Epic Clinical Network. The community use of rescue medication for prolonged epileptic seizures in children. Seizure. 2009;18:343–6.
Armijo JA, Herranz JL, Pena Pardo MA, Adín J. Intranasal and buccal midazolam in the treatment of acute seizures. Rev Neurol. 2004;38:458–68.
Mula M. The safety and tolerability of intranasal midazolam in epilepsy. Expert Rev Neurother. 2014;14:735–40.
Aggarwal R, Cardozo A, Homer JJ. The assessment of topical nasal drug distribution. Clin Otolaryngol Allied Sci. 2004;29:201–5.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(6):e1000097.
Acknowledgments
We are indebted to Annalisa Cuzzoni (Medical Library of Bolzano) for developing and implementing the search strategies, and we thank Piero Bonzano and Vincenzo De Simone (Accord Healthcare), as well as Alberto Liuti (ViroPharma), for seeking information on unpublished randomized controlled trials on buccal midazolam or studies in progress.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received in relation to the preparation of this article.
Conflicts of interest
Francesco Brigo has received speakers’ honoraria from UCB Pharma.
Eugen Trinka has acted as a paid consultant to Bial, Biogen Idec, Eisai, Ever Neuropharma, Medtronics, Takeda, Upsher-Smith, and UCB; has received speakers’ honoraria from Bial, Boehringer, Eisai, GL Lannacher, and UCB Pharma; and has received research funding from Biogen Idec, Merck, Novartis, Red Bull, UCB Pharma, the European Union, FWF (Österreichischer Fond zur Wissenschaftsförderung), and Bundesministerium für Wissenschaft und Forschung. Raffaele Nardone and Frediano Tezzon declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brigo, F., Nardone, R., Tezzon, F. et al. A Common Reference-Based Indirect Comparison Meta-Analysis of Buccal versus Intranasal Midazolam for Early Status Epilepticus. CNS Drugs 29, 741–757 (2015). https://doi.org/10.1007/s40263-015-0271-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-015-0271-x