Abstract
We comprehensively reviewed published literature to determine whether it supported the link between corrected QT (QTc) interval prolongation and torsade de pointes (TdP) for the 11 second-generation antipsychotics and seven second-generation antidepressants commonly implicated in these complications. Using PubMed and EMBASE, we identified four thorough QT studies (one each for iloperidone, ziprasidone, citalopram, and escitalopram), 40 studies specifically designed to assess QTc interval prolongation or TdP, 58 publications based on data from efficacy and safety trials, 18 toxicology studies, and 102 case reports. Thorough QT studies, QTc prolongation-specific studies, and studies based on efficacy and safety trials did not link drug-associated QTc interval prolongation with TdP. They only showed that the drugs reviewed caused varying degrees of QTc interval prolongation, and even that information was not clear and consistent enough to stratify individual drugs for this risk. The few toxicology studies provided valuable information but their findings are pertinent only to situations of drug overdose. Case reports were most informative about the drug–QTc interval prolongation–TdP link. At least one additional well established risk factor for QTc prolongation was present in 92.2 % of case reports. Of the 28 cases of TdP, six (21.4 %) experienced it with QTc interval <500 ms; 75 % of TdP cases occurred at therapeutic doses. There is little evidence that drug-associated QTc interval prolongation by itself is sufficient to predict TdP. Future research needs to improve its precision and broaden its scope to better understand the factors that facilitate or attenuate progression of drug-associated QTc interval prolongation to TdP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Current literature does not provide sufficient and consistent information to stratify second-generation antipsychotics and antidepressants for their potential to prolong the corrected QT (QTc) interval and/or cause torsade de pointes (TdP). |
QTc interval prolongation associated with second-generation antipsychotics and antidepressants is by itself not sufficient to cause TdP. |
TdP can occur at therapeutic doses of second-generation antipsychotics and antidepressants and with a QTc interval <500 ms. |
Future research needs to improve its precision and broaden its scope to better understand the factors that facilitate or attenuate progression of drug-associated QTc interval prolongation to TdP. |
1 Introduction
Torsade de pointes (TdP) is a potential complication of several psychotropic medications but is difficult to study because of its rarity. Prolonged corrected QT (QTc) interval is often associated with TdP. Regulatory agencies such as the USA Federal Drug Administration (FDA) use the QTc prolonging effect of marketed drugs as a surrogate marker to monitor their safety and take necessary action if concerning data emerge.
We have written on the topic of psychotropic drug-associated QTc interval prolongation and TdP for the last several years [1, 2]. In our recent publications [3–7], mostly triggered by the recent FDA-issued warnings for citalopram [8] and quetiapine [9], we showed that, at therapeutic doses, QTc interval prolongation by itself was a poor predictor of TdP. We asserted that the current approach to studying psychotropic drug-associated TdP over-emphasizes QTc interval prolongation as a risk factor and ignores other risk factors that are concurrently present in most cases of psychotropic drug-associated TdP. While preparing these manuscripts, we observed that current literature lacked rigor and clarity to reliably categorize individual psychotropic drugs for their risk to prolong QTc interval or cause TdP. To study this observation further, we decided to comprehensively review the literature on second-generation antipsychotics (SGAPs) and second-generation antidepressants (SGADs) commonly implicated in QTc interval prolongation and TdP.
2 Methods
2.1 Identification of Second-Generation Antipsychotics (SGAPs) and Antidepressants (SGADs) of Interest
We used the CredibleMeds website [10] to identify SGAPs and SGADs for this review. CredibleMeds is a component of the Arizona University-Based Center for Education and Research on Therapeutics (AZCERT) with a special focus on drugs that prolong the QT interval and cause sudden death. CredibleMeds specifies four categories of risk. Three of these categories are described in Table 1. The drugs in these three categories, along with certain cardiac drugs, make the fourth list of drugs that should be avoided in patients with inherited long QT syndrome. All nine SGAPs (amisulpride, clozapine, iloperidone, olanzapine, paliperidone, quetiapine, risperidone, sertindole, and ziprasidone) and seven SGADs (citalopram, escitalopram, fluoxetine, mirtazapine, paroxetine, sertraline, and venlafaxine) listed in various CredibleMeds drug categories as of November 2013 were included in this review (Table 1).
2.2 Method of Literature Search
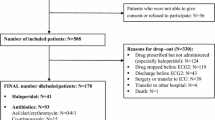
We searched PubMed and EMBASE (including MEDLINE) in November 2013 for English language literature without setting any other limits. Our search terms included a combination of the names of medications included in this review with (QT OR QT prolongation OR QTc OR QTc prolongation OR torsades de pointes OR torsade OR TdP OR sudden cardiac death OR SCD). From the 598 results in PubMed and 324 results in EMBASE, we excluded reviews (127 in PubMed and 147 in EMBASE) and animal studies (45 in PubMed and 15 in EMBASE). We then merged the two sets of results and removed 42 duplications. The remaining 546 publications formed the core of our literature search. We also identified a few additional studies from the reference lists of the selected publications.
2.3 Literature Summary and Synthesis
We reviewed abstracts, or full-text articles when abstracts were not available, of all our search results to determine how best to present the literature. The publications were heterogeneous in methodology, scope, method used to measure QTc interval, definition of QTc interval prolongation, and quality. Setting arbitrary exclusion or inclusion criteria to limit the number of publications would have compromised the comprehensive scope of this review. We decided to summarize all the studies that provided clear information about QTc prolongation with values and/or TdP.
We summarized the literature under two major sections: one each for SGAPs (Sect. 4) and SGADs (Sect. 5). Within each of these sections, we summarized literature pertinent to individual SGAPs and SGADs under five categories described below in Sect. 3. At the end of the SGAPs and SGADs sections, we appraised the literature to determine whether it allowed us to rank individual SGAPs and SGADs for their risk to prolong QTc interval and/or cause TdP.
3 Categories of Studies of SGAPs and SGADs Associated with the Risk of QTc Interval Prolongation and/or Torsade de Pointes (TdP)
3.1 Studies Using the Approach Suggested for a Thorough QT (TQT) Study
The International Conference on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use provides guidance on the clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs [11, 12]. Studies designed based on the guidance are referred to as thorough QT (TQT) studies. A TQT is typically conducted during the early phase of drug development in healthy individuals to determine the effect of the study drug on cardiac repolarisation, as measured by QT/QTc prolongation, with and without metabolic inhibition of the study drug. It has high sensitivity to detect small changes in QTc interval. A positive control (i.e., a drug known to prolong the QTc interval) is employed for assay sensitivity. The positive control should have an effect on the mean QT/QTc interval of about 5 ms (i.e., an effect that is close to the QT/QTc effect that represents the threshold of regulatory concern, around 5 ms). A TQT study is considered positive if the upper bound of the one-sided 95 % confidence interval for time-matched and placebo-adjusted increase in QTc interval from baseline exceeds 10 ms at any time during the study. Study drugs exceeding the regulatory threshold of interest may be subject to further electrocardiographic (ECG) follow-up studies during later stages of development. Studies that generally followed this design were categorized as ‘TQT studies’ in this review.
3.2 QTc Prolongation-Specific Studies
Studies that specifically assessed QTc prolongation (or TdP) but were not TQT studies or toxicology studies are categorized as ‘QTc prolongation-specific studies’. Most of these studies followed an open-label, non-randomized design, had a small sample size, and can be broadly categorized as observational or cross-sectional studies.
3.3 Toxicology Studies with Information on QTc Prolongation
Studies assessing the effects of toxic ingestion of SGAP(s) or SGAD(s) on QTc interval or TdP were categorized as ‘toxicology studies’. Overall, these studies followed a very similar design—analysis of ECGs of patients presenting with an overdose of the specified drug to determine changes in the QTc interval.
3.4 Efficacy and/or Safety Studies with Information on QTc Prolongation
Efficacy and safety studies that specifically reported QTc prolonging effect(s) of the drug(s) were categorized as ‘efficacy and safety studies’. Most of these studies were randomized and placebo-controlled, many were double-blinded, and several had an active comparator. Most of the studies excluded patients with cardiovascular or other medical comorbidities. Studies separately analyzing data from safety and efficacy trials for the effect of drug(s) on QTc interval were included in this category instead of the ‘QTc prolonging-specific studies’ category.
3.5 Case Reports
Case reports were included if information about the drug(s) implicated and values for QTc interval prolongation and/or information about TdP was provided. We identified additional well established risk factors for QTc prolongation in the case reports [8, 13–15]. Specifically, these were (1) age >60 years, (2) female sex, (3) drug overdose, (4) metabolic inhibition of the drug by another drug (only well known interactions were counted), (5) hepatic impairment, (6) concurrent use of another QTc prolonging drug, (7) hypokalemia, (8) hypomagnesemia, (9) presence of cardiac disease (coronary heart disease, cardiac conduction or structural problems, or heart failure), and (10) congenital long QT syndrome or documentation of prolonged QTc before exposure to the culprit drug.
The number of studies for SGAPs and SGADs grouped into the above-mentioned categories are listed in Supplementary Tables 1 and 2, respectively. These studies are summarized in several tables and supplementary tables.
4 Studies of QTc Prolongation for Individual SGAPs
4.1 Amisulpride
We did not find a TQT study of amisulpride. In the only QTc prolongation-specific study including amisulpride [16] (Table 2, study 13), amisulpride did not significantly prolong QTc interval.
We found two amisulpride toxicology studies (Table 3, studies 1 and 2) [17, 18]. In a study [17] of 83 cases of amisulpride overdose with 440 ECGs, abnormal QT heart rate (HR) pair on QT nomogram occurred in 61 (73 %) cases. TdP occurred in six (7 %) patients; all had an abnormal QT-HR pair. The only case of citalopram co-ingestion (560 mg) experienced an abnormal QT-HR pair but did not experience TdP. The second amisulpride toxicology study [18] seemingly drew data from the first one [17] and found that prolonged QTc interval defined as Bazett’s formula-corrected QTc interval (QTcB) >500 ms, Fridericia’s formula-corrected QTc interval (QTcF) >500 ms or an abnormal QT-HR pair, all predicted TdP.
In the only efficacy and safety study of amisulpride (Supplementary Table 3) [19], data were pooled from 11 clinical studies of amisulpride. The incidence of prolonged QTc interval (>450 ms in men and >470 ms in women) was comparable for amisulpride (3.5 %), risperidone (3 %), and haloperidol groups (1 %). An increase in QTc interval ≥60 ms was observed in 1 % of amisulpride-treated patients but in none in the other two groups.
In ten case reports of amisulpride-related QTc prolongation (Supplementary Table 4 and Table 4) [20–25], nine (90 %) cases had at least one additional well established risk factor. A QTc interval ≥500 ms was observed in seven (70 %) cases. TdP occurred in three cases (30 %); in one case with a QTc interval <500 ms, who had tolerated thioridazine for several years without such a complication.
4.2 Clozapine
We did not find a TQT study of clozapine; we found five QTc prolongation-specific studies for it (Table 2, studies 1, 2, 13, 18, and 19) [16, 26–29]. In a study [26] of 82 adult patients switched from other SGAPs to clozapine, there was no difference in mean QTcB interval or in number of patients with prolonged QTcB interval before or 18 weeks after clozapine treatment. The QTcB interval of one patient previously taking quetiapine decreased from 550 to 453 ms. Kang et al. [27] monitored ECGs in 61 adult patients and found a correlation between the dose of clozapine and QTc interval. Two patients showed prolonged QTc interval on clozapine; in one of them, it decreased from 533 to 430 ms despite ongoing clozapine treatment. In a study [16] of 13 adult patients, clozapine prolonged QTc interval from baseline but not significantly. In another study [28] of 56 patients, the mean QTcB interval was >500 ms in each of the three treatment groups (clozapine, haloperidol, and olanzapine) and significantly longer than the mean QTc interval (345.2 ms) of the healthy control group. In a large cross-sectional study [29], the mean QTcB interval in clozapine-treated group (397 ms) was significantly longer than the risperidone (389 ms; P < 0.009) and the first-generation antipsychotic (392 ms; P < 0.04) groups. QTc was more common in women (7.3 %) than in men (3.2 %) and in patients with (10 %) than without cardiovascular disease (3.9 %).
The only clozapine toxicology study [30] we found (Table 3, study 3) observed QTc prolongation (defined as QTc >390 ms for males and QTc >440 ms for females) in 8.2 % of the cases.
We found six efficacy and safety studies including clozapine as a treatment (Supplementary Table 3, studies 2–7) [31–36]. Five of these studies [31–35] involved the addition of another antipsychotic to ongoing clozapine treatment and did not provide information specific to clozapine itself. The sixth study [36] compared ziprasidone versus clozapine. The mean change in QTcB was comparable in both treatment groups. The incidence of QTcB ≥450 ms was higher in the clozapine group (14.1 %) than in the ziprasidone group (4.5 %).
We found five published case reports of clozapine-associated QTc prolongation (Supplementary Table 5 and Table 4) [37–41]. None of these cases were of clozapine overdose and none experienced TdP. In two cases, QTc prolongation occurred after a cardiac event. In two other cases, QTc prolongation was also observed with haloperidol and olanzapine.
4.3 Iloperidone
The TQT study for iloperidone [42] was a randomized, open-label, parallel-group study. Adults with schizophrenia or schizoaffective disorder with normal ECG (QTc interval ≤460 ms) at baseline and without significant medical illness were randomized to receive iloperidone 8 mg twice daily (bid) (N = 28), 12 mg bid (N = 34), and 24 mg once daily (od) (N = 31), ziprasidone 80 mg bid (N = 32), or quetiapine 375 mg bid (N = 33) after a 10-day washout period. During treatment period 1, the patients followed fixed titration and dosing regimens to achieve the target dose of the medication. Metabolic inhibitors were added in study period 2 (paroxetine to iloperidone groups and ketoconazole to the other two groups). During study period 3, ketoconazole was added to paroxetine in the iloperidone group. Patients received several ECGs at baseline and during the treatment phases. The QTc correction was based on the formula QTc = QT/RRc, where c denoted the correction factor. Four corrections (Bazett, Fridericia, FDA, and baseline) were used to calculate QTc intervals. QTcF was used as the primary correction method. Mean QTcF changes from baseline to steady state at t max (the time after administration of a drug when the maximum plasma concentration is reached) in treatment period 1 were 8.5 ms with iloperidone 8 mg bid, 9.0 ms with iloperidone 12 mg bid, 15.4 ms with iloperidone 24 mg od, 9.6 ms with ziprasidone, and 1.3 ms with quetiapine. The mean QTc changes at t max followed similar patterns with all four correction factors. During treatment periods 2 and 3, the mean changes in QTcF from baseline to steady state t max were numerically higher in all treatment groups than the values for treatment period 1. Ten patients experienced QTc changes ≥60 ms from baseline in any treatment period, all of whom received iloperidone: three patients received 8 mg bid, five patients received 12 mg bid, and two patients received 24 mg od. These events occurred in treatment period 1 (three patients), treatment period 2 (two patients), and treatment period 3 (five patients). No patients in any treatment group had QTc intervals ≥500 ms at any time during the study using any QT correction formula. QTc intervals tended to increase with increasing concentrations of iloperidone (P < 0.02 for concentration effects during treatment period 2); this effect was not seen for ziprasidone or quetiapine. For all treatment groups, a longer baseline QTc interval was associated with a smaller change during the treatment period.
We did not find any QTc prolongation-specific or QTc prolongation-related toxicology studies or case reports for iloperidone.
We found three efficacy and safety studies for iloperidone (Supplementary Table 3, studies 8–10) [43–45]. In a pooled analysis of three studies [43] comparing iloperidone (N = 371) and haloperidol (N = 118), treatment with iloperidone and haloperidol produced similar changes in QTcF from baseline. In a study comparing iloperidone with ziprasidone [44], both drugs caused a comparable increase in QTcF, which was significantly greater than placebo. The third study [45] was a pooled analysis of data from three short-term studies. Active treatments included three doses of iloperidone (4–8 mg/day, 10–16 mg/day, and 20–24 mg/day), haloperidol, and risperidone. The mean QTcF interval increased with all treatments, which was significant for iloperidone and haloperidol but not risperidone.
4.4 Olanzapine
No TQT study has been conducted for olanzapine itself but it was included in the study conducted for ziprasidone. In this study [46, 47], olanzapine treatment caused a marginal increase in QTcB interval from baseline both without (6.4 ms) and with (5.3 ms) metabolic inhibition. Of the individuals in the olanzapine group, 4 % experienced increases ≥60 ms in QTcB interval during the steady-state without metabolic inhibition, which was the same as seen in the risperidone and haloperidol groups and lower than that of the quetiapine (11 %), ziprasidone (24 %), and thioridazine (39 %) groups.
The 11 QTc prolongation-specific studies of olanzapine [16, 28, 48–56] (Table 2, studies 3, 13–16, 18, 20 and 23–26) mostly had a small sample size. Agelink et al. [16] noted an insignificant increase in QTcB interval with olanzapine. There were four studies by Suzuki et al. In one study [49], an increase in the dose of olanzapine significantly increased mean QTcB interval by 8 ms but none of the patients exceeded the defined threshold of 430 ms for males and 450 ms for females. In another study [50], QTcB interval decreased significantly from 403.8 to 390.7 ms after patients were switched from olanzapine to aripiprazole. In their third study [51], the QTcB interval increased after patients were switched from olanzapine to quetiapine, but not significantly. Lastly, they observed that the QTcB interval of olanzapine-treated patients was significantly longer than risperidone-treated patients [48]. Watanabe et al. [52] noted that the mean night-time QTcF of the risperidone group was significantly longer than that of the olanzapine and control groups, but the mean daytime QTcF intervals were similar.
Huang et al. [53] cross-switched 12 patients each from olanzapine to risperidone and vice versa. The QTcB interval increased significantly after the switch to risperidone, and decreased insignificantly after the switch to olanzapine. The QTcB interval was significantly longer in the risperidone group than in the olanzapine group. The findings of a small study by Cohen et al. [28] are unusual. The mean QTcB for all treatment groups (clozapine, haloperidol, and olanzapine) was >500 ms and significantly longer than that of the control group (345.2 ms). de Castro et al. [54] did not observe any significant effect of olanzapine treatment on the QTc interval in youth. Similarly, Ozeki et al. [55] did not notice an effect of olanzapine (and several other SGAPs) on QTcB interval in a large sample of adult patients in a ‘real-world’ setting. Meyer-Massetti et al. [56] found 489 cases of olanzapine-associated QT prolongation, TdP, and/or cardiac arrest (vs. 365 haloperidol-associated and 520 quetiapine-associated cases) in the World Health Organization (WHO) Global Individual Case Safety Report database. Based on 75 cases with relevant information, the daily dose of olanzapine ranged from 1.25 to 700 mg.
We did not find any QTc prolongation-related toxicology study for olanzapine. In the 12 efficacy and safety publications of olanzapine [57–68] (Supplementary Table 3, studies 11–22), ziprasidone was the active comparator in four studies [57–60]. Overall, ziprasidone treatment was associated with a greater numerical increase in QTc interval than olanzapine treatment, but the difference between treatments was statistically significant in only one study [57]. No patient had a QTc interval ≥500 ms with either treatment. In the study comparing olanzapine with sertindole [61], sertindole prolonged the QTc interval significantly while olanzapine did not. Czekalla et al. [62] performed a pooled analysis of four efficacy studies. A similar number of olanzapine-treated patients had a maximum QTc interval increase (17.9 %) and decrease (17.8 %) of ≥30 ms. Four out of 1,424 patients had consistent post-baseline and endpoint values ≥450 ms, with the greatest value being 462 ms. The marginal decrease in QTc interval with ongoing olanzapine treatment observed by Street et al. [63] is unreliable because the ‘baseline’ was not truly olanzapine free.
Three publications [64–66] compared intramuscular (IM) olanzapine with IM haloperidol in acutely agitated patients. One [64] did not observe significant changes in QTc interval from baseline to 24 h after treatment with either drug or between-group (including the placebo group) differences. The other study [66] observed that the incidence of prolonged QTc interval (defined as QTcB ≥430 ms for males and QTcB ≥450 ms for females) was higher in the placebo group than in the olanzapine group. The third publication [65] performed a pooled analysis of several similar studies and concluded that the incidences of prolonged (endpoint ≥99th percentile of healthy adults or ≥500 ms) or lengthened (increase ≥60 ms) QTc intervals during treatment with IM olanzapine were never significantly greater than with the comparators. Similarly, the effect of IM olanzapine on QTc interval was comparable to that of IM lorazepam and IM placebo in two studies [67, 68]. In one of these studies of patients with dementia [68], a few patients in each group had a QTc interval ≥500 ms during the 24-h period.
Only one of six published case reports of olanzapine-associated QTc prolongation (Supplementary Table 6 and Table 4) [69–74] involved an overdose. All cases had additional risk factors for QTc interval prolongation besides olanzapine. None of the cases experienced TdP. In the only case with a QTc interval >500 ms, the prolongation was triggered by initiation of ciprofloxacin. One case had a history of QTc prolongation with sulpiride and clozapine; another experienced it with low-dose risperidone.
4.5 Paliperidone
We did not find any TQT study, toxicology study, or case report for paliperidone.
We found two QTc prolongation-specific studies for paliperidone (Table 2, studies 27 and 29) [75, 76]. Hough et al. [75] compared paliperidone extended-release (ER) and quetiapine in a randomized, double-blind, placebo-controlled study. On the basis of a pre-specified 10-ms non-inferiority margin, paliperidone ER was found to be non-inferior to quetiapine. The second study [76] evaluated the effect of risperidone and its 9-hydroxy metabolite, paliperidone on the QTcB interval in 61 adult patients who had been on a stable dose of risperidone for at least 4 weeks. Plasma levels of paliperidone, but not of risperidone, correlated positively with the QTcB interval.
Both efficacy and safety publications of paliperidone ER were pooled analyses (Supplementary Table 3, studies 23 and 24) [77, 78]. Meltzer et al. [77] performed a pooled analysis of three similarly designed 6-week, multicenter, double-blind, randomized, fixed-dose, placebo-controlled studies of paliperidone ER in 1,326 adult patients with acute schizophrenia. There were no clinically relevant differences between the proportions of patients who had a normal QTc (<450 ms) at baseline and a maximum post-baseline QTc value of >450 ms and <480 ms. Gopal et al. [78] performed a post hoc analysis of the risperidone and paliperidone clinical trials database (Johnson & Johnson®-sponsored 64 placebo- or active-controlled studies of 11,096 patients) to estimate the risk of sudden death, cardiovascular events, and cerebrovascular events during treatment. The incidence of QTcF prolongation/TdP was 1.8 % in the risperidone/paliperidone group, 0.9 % in the placebo group, and 1.9 % in the active control group. In an analysis of maximum QTcF increase from baseline, more risperidone- and paliperidone-treated patients showed increases of 30–60 ms, versus placebo, in both the younger than 30 and older than 74 years age groups, and more risperidone-treated patients aged 30–74 years showed increases greater than 60 ms versus placebo.
4.6 Quetiapine
Quetiapine was an active treatment in the TQT study conducted for ziprasidone [46]. Quetiapine prolonged QTcB interval from baseline both without (14.5 ms) and with (19.7 ms) metabolic inhibition. Of the individuals in the quetiapine group, 11 % experienced a ≥60 ms increase in QTcB interval from baseline during the steady state without metabolic inhibition, which was higher than that of the haloperidol, olanzapine, and risperidone groups (4 % in each group), and lower than that of the ziprasidone (24 %) and thioridazine (39 %) groups. Quetiapine was also a comparator drug along with ziprasidone in the TQT study of iloperidone [42]. Quetiapine caused marginal and insignificant increases in the QTc interval, which was numerically the least of the three study drugs. The mean change in QTc interval varied by the method used to correct the QT interval. For quetiapine without metabolic inhibition, mean change in QTcF from baseline was 1.3 ms and mean change in QTcB was 12.6 ms. All ten patients who experienced a QTc change ≥60 ms from baseline were receiving iloperidone.
We found six QTc prolongation-specific studies for quetiapine (Table 2, studies 15, 16, 20, 23, 26 and 27) [48, 51, 54–56, 75]. Suzuki et al. [51] observed an increase in QTcB interval after patients were switched from aripiprazole (N = 11), olanzapine (N = 6), or risperidone (N = 3) to quetiapine. The increase was statistically significant for all 20 patients and for patients switched from aripiprazole. In a cross-sectional study of 222 patients, Suzuki et al. [48] found that the mean QTcB interval of the quetiapine group was significantly longer than that of the risperidone and aripiprazole groups. In a cross-sectional analysis of 1,017 patients with schizophrenia taking various psychotropic medications, Ozeki et al. [55] did not find an association between use of quetiapine and QTcB interval prolongation. de Castro et al. [54] compared youth taking olanzapine (N = 12), quetiapine (N = 8), or risperidone (N = 20) with healthy controls (N = 40) and found that the QTcB interval of both groups was comparable. Using quetiapine as a control, Hough et al. [75] found that paliperidone ER was non-inferior to quetiapine with regards to its QTc-prolonging effect. Meyer-Massetti et al. [56] found 520 cases of quetiapine-associated QT prolongation, TdP, and/or cardiac arrest (vs. 365 haloperidol-associated and 489 olanzapine-associated cases) in the WHO Global Individual Case Safety Report database based on 89 cases with relevant information; the daily dose of quetiapine ranged from 25 to 4,500 mg.
Three toxicology studies provided information on the QTc-prolonging effect of quetiapine (Table 3, studies 4, 5, and 6) [79–81]. Eyer et al. [80] retrospectively identified 20 cases of “predominantly” quetiapine overdose, of which six were quetiapine mono-intoxications. Based on QT-HR nomogram, the QT interval was normal in 11 patients, at borderline risk in eight patients, and abnormal in one patient. Fatal complications (possible arrhythmia) occurred in one patient who had co-ingested an unknown amount of citalopram. Isbister and Duffull [81] retrospectively analyzed data from 176 patients presenting with 286 incidents of quetiapine overdose. At least one ECG was available for 260 incidents. “At risk for TdP” QT-HR pair was observed in 24 incidents (8.4 %). All these occurred at HR >100 beats/minute. No arrhythmias were reported from the available continuous telemetry data. Lastly, Balit et al. [79] reviewed 40 cases of quetiapine overdose. For ten patients for whom ECGs were available and who had ingested no other cardiotoxic drugs, the mean QTcB interval was prolonged to 487 ms. One patient with 24 g quetiapine ingestion had a QTcB of 535 ms. There were no arrhythmias and no deaths in this case series.
In the only efficacy and safety study of quetiapine we found (Supplementary Table 3, study 25) [82], adult intensive care unit patients with delirium were randomized to receive quetiapine (N = 18) or placebo (N = 18). QTc interval changes were comparable between groups. The findings are confounded by the fact that both groups were allowed to receive IM haloperidol on as needed basis.
We found 16 case reports of quetiapine-associated QTc prolongation (Supplementary Table 7 and Table 4) [24, 83–96]. The majority of cases (81 %) experienced a QTc interval ≥500 ms. In just over half of the case reports (53 %), QTc prolongation occurred with therapeutic doses of quetiapine. TdP was reported in four cases, all at therapeutic doses and with a QTc interval ≥500 ms. One of the cases had a history of QTc prolongation with therapeutic ziprasidone, another experienced it with switch to aripiprazole, and another had a history of ventricular ectopics with amisulpride.
4.7 Risperidone
In the TQT study primarily conducted for ziprasidone [46], risperidone prolonged QTcB interval by 10 ms from baseline without metabolic inhibition and 3.2 ms after metabolic inhibition. Of the individuals in the risperidone group, 4 % experienced a ≥60 ms increase in QTcB interval from baseline during steady state without metabolic inhibition, which was similar to that seen for haloperidol and olanzapine groups, and lower than that of the quetiapine (11 %), ziprasidone (24 %), and thioridazine (39 %) groups.
We found 14 QTc prolongation-specific studies for risperidone (Table 2, studies 4–7, 15–17, 19, 23–26, 28 and 29) [29, 48, 51–55, 76, 97–102]. Ranjbar et al. [97] observed that the QTcB interval was significantly longer in 112 patients receiving risperidone for the first time than in patients not receiving risperidone. Similarly, Yerrabolu et al. [98] observed a significant increase in QTc interval after risperidone treatment in 20 patients. Conversely, Chiu et al. [99] did not observe a significant change in QTcB interval of 72 patients after risperidone treatment. Llerena et al. [100] did not find a correlation between risperidone or 9-OH-risperidone (paliperidone) levels and QTc interval. However, in the study by Suzuki et al. [76], QTcB interval correlated positively with paliperidone level but not with risperidone level.
The remaining nine studies [29, 48, 51–55, 101, 102] included antipsychotics in addition to risperidone. In one study [51], three patients who switched from risperidone to quetiapine experienced an insignificant increase in QTcB interval. In another study of 222 patients [48], the mean QTcB interval of the risperidone group was significantly shorter than that of the quetiapine and olanzapine groups. Germano et al. [101] observed in 60 youth that risperidone treatment was associated with a significant increase in mean QTcB but aripiprazole treatment was not. Yang et al. [29] obtained ECGs on 1,006 patients and found that the mean QTc interval was significantly shorter in the risperidone group (389 ms) than in the clozapine group (397 ms) but comparable to the first-generation antipsychotic (FGA) group (393 ms). Ozeki et al. [55] observed that risperidone (and olanzapine, quetiapine, and zotepine) did not cause a clinically significant prolongation of QTcB interval, which was defined as >470 ms for men and >480 ms for women. In a study of 24 patients [53], the mean QTcB increased significantly after switch from olanzapine (393 ms) to risperidone (421.6 ms) and decreased significantly after switch from risperidone (413 ms) to olanzapine (407.7 ms). Watanabe et al. [52] found that the mean QTcF interval of patients taking risperidone was significantly longer than patients taking olanzapine at night time but comparable during the daytime. de Castro et al. [54] observed that the mean change in QTcB with risperidone and olanzapine treatment was comparable to that of the control group. Lastly, a small study of youth [102] did not observe any significant changes in QTc interval with risperidone.
The only toxicology study of risperidone-associated QTc prolongation [103] (Table 3, study 7) evaluated the ECGs of 38 patients with risperidone mono-intoxication. Tachycardia (HR >100 beats/min) was noted on 58 % of occasions, which did not correlate with dose. An abnormal QT-HR pair on QT nomogram was observed for four of the 41 ECGs available, but all except one were associated with an HR >110 beats/min.
We found nine efficacy and safety studies of risperidone (Supplementary Table 3, studies 6, 7, 10, 24 and 26–30) [33, 34, 45, 78, 104–108]. A small study of risperidone-alone treatment [104] and another small study of risperidone add-on treatment to clozapine [34] did not notice any statistically or clinically significant effects of risperidone on QTcB interval. Another study [105] comparing risperidone monotherapy with risperidone-haloperidol combination therapy also did not notice any significant QTcB interval changes in either group. Kane et al. [106] observed a significantly greater effect of sertindole on QTcF interval than risperidone. Another study [107] using QTcB interval noted the same. In a small augmentation study of clozapine-treated patients [33], there was a statistically significant increase in QTcF after clozapine-ziprasidone treatment but not after clozapine-risperidone treatment. The increase was not clinically significant in either group. In a study comparing risperidone and aripiprazole [108], both treatments had comparable and insignificant effects on QTcB interval. Two studies performed a pooled analysis [45, 78]. One of these studies [78] combined data from 64 trials of risperidone and paliperidone. Incidence of QTcF prolongation/TdP was 1.8 % in the risperidone/paliperidone group, 0.9 % in the placebo group, and 1.9 % in the active control group. In the other pooled analysis [45], all active treatments (haloperidol, iloperidone, and risperidone) increased the mean QTcF interval, which was not significant for the risperidone group.
We found 13 case reports of risperidone-associated QTc prolongation (Supplementary Table 8 and Table 4) [1, 70, 85, 109–118], of which only two involved risperidone overdose. The QTc interval was <500 ms in seven cases. TdP occurred in five cases, in three with a QTc interval <500 ms. One of the cases showed QTc prolongation with a low dose of risperidone but tolerated haloperidol and clozapine well. Another case tolerated quetiapine without QTc prolongation.
4.8 Sertindole
We did not find any TQT study, toxicology study, or case report for sertindole.
We found three QTc prolongation-specific studies for sertindole (Table 2, studies 8, 9 and 13) [16, 119, 120]. Nielsen et al. [120] obtained ECGs at baseline and at steady-state in 37 patients switched to sertindole. The mean QTcF interval prolonged significantly by 20 ms after the switch. Atmaca et al. [119] obtained ECGs on 21 patients before and 3 and 6 months after initiating sertindole. The QTc interval was significantly longer at 6 months than at baseline. At any evaluation point, only one female (451 ms) and one male (433 ms) had borderline prolongation, both at 3 months. In a study of 51 patients, Agelink et al. [16] noted that all treatments (amisulpride, olanzapine, sertindole, or clozapine) prolonged the mean QTcB interval but the increase was significant only for sertindole. One patient from the sertindole group had a QTcB interval of 503 ms.
We found five efficacy and safety studies of sertindole with information specific to QTc prolongation (Supplementary Table 3, studies 4, 15, 28, 29 and 31) [31, 61, 106, 107, 121]. In a study comparing sertindole with olanzapine [61], the incidence of QTcF prolongation was significantly higher in the sertindole group (26.5 %) than in the olanzapine group (5.7 %). The mean QTcF change from baseline to the last assessment was 24.7 ms in the sertindole group and 3.5 ms in the olanzapine group. The incidence of QTcF >500 ms was 2.6 and 0 % for the sertindole and olanzapine groups, respectively. In a study comparing sertindole with risperidone [107], sertindole treatment was associated with a significantly greater increase in QTc than risperidone treatment. The incidence of QTc >500 ms was 5.1 and 0 % for the sertindole and risperidone groups, respectively. Another study of over 300 patients also observed a greater effect of sertindole on QTc interval than risperidone [106].
In a study of patients with limited response to clozapine [31], sertindole or placebo was added to clozapine. Treatment with sertindole caused a significant increase in the electronically assigned mean QTcB interval (12 ms) versus placebo (0 ms), but the manual QTcF assessment did not reveal any statistically significant changes. Using sertindole surveillance data, Pezawas et al. [121] analyzed ECGs of all 34 comorbid and co-medicated sertindole-treated patients. Sertindole treatment was associated with a significant prolongation of QTcB and QTcF intervals from baseline. For 25 pairs of ECGs, both QTcB and QTcF intervals were significantly longer during sertindole treatment than during treatment with another antipsychotic.
4.9 Ziprasidone
The TQT study of ziprasidone (the Pfizer 054 study) [46, 47] was a multi-center, randomized, open-label, parallel-group study of patients with psychotic disorder who had been completely withdrawn from their previous treatment and had normal baseline ECGs (QTc <450 ms). Patients received escalating doses of ziprasidone (N = 35, 25 male; maximum dose 160 mg/day), risperidone (N = 28, 22 male; maximum dose 16 mg/day), olanzapine (N = 28, 20 male; maximum dose 20 mg/day), quetiapine (N = 29, 22 male; maximum dose 750 mg/day), thioridazine (N = 31, 25 male; maximum dose 300 mg/day), or haloperidol (N = 32, 25 male; maximum dose 15 mg/day) over 10 days. After the maximum dose of randomized therapy was achieved, the metabolic inhibitor (ketoconazole, paroxetine, and fluvoxamine) appropriate for each drug was administered. Drug levels were monitored at drug steady states both with and without metabolic inhibition. ECGs were obtained at baseline and at times estimated to correspond with the mean t max for each study drug. The QTc interval was calculated using Bazett’s formula. Increase in the mean QTcB interval from baseline to the steady state of maximum dose was 20.6 ms for ziprasidone, 10 ms for risperidone, 6.4 ms for olanzapine, 14.5 ms for quetiapine, 35.8 ms for thioridazine, and 4.7 ms for haloperidol. The respective increase after metabolic inhibition was 20.4, 3.2, 5.3, 19.7, 28, and 8.9 ms. The percentage of patients with ≥60 ms increase in QTcB interval from baseline during the steady state without metabolic inhibition was 24, 4, 4, 11, 39, and 4 % for the ziprasidone, risperidone, olanzapine, quetiapine, thioridazine, and haloperidol groups, respectively. No patient in any treatment group experienced a QTcB interval ≥500 ms.
Ziprasidone was also a comparator drug along with quetiapine in the TQT study of iloperidone [42]. Overall, the mean QTc changes caused by ziprasidone were numerically comparable or less than those caused by iloperidone and higher than those caused by quetiapine. Ten patients in the iloperidone group but none in the ziprasidone (or quetiapine) group experienced QTc changes ≥60 ms from baseline during any treatment period. A concentration-dependent effect on QTc interval was not noted for ziprasidone.
We found five QTc prolongation-specific studies for ziprasidone (Table 2, studies 10–12, 21 and 22) [122–126]. In a study of 20 youth [122], ziprasidone treatment was associated with a significant increase in QTcB from baseline. Eight patients experienced a QTcB interval >440 ms, the highest being 470 ms. Emul et al. [123] did not notice any significant effects of IM ziprasidone on QTc interval in 11 adult inpatients. In a retrospective case series of 15 patients receiving high doses (≥240 mg/day) of ziprasidone [124], the mean pre- and post-treatment QTcB intervals were similar. Maximum post-treatment QTc was 452 ms. Miceli et al. [125] observed that both IM haloperidol and IM ziprasidone caused QTc prolongation in a concentration-dependent manner. None of the patients had a QTc interval ≥480 ms. Two patients in the ziprasidone group (none in the haloperidol group) had an increase in QTc ≥60 ms relative to the time-matched baseline values. Miceli et al. [126] also noted a concentration-dependent prolongation of QTc interval with oral haloperidol and oral ziprasidone.
The only ziprasidone toxicology study [127] (Table 3, study 8) analysed data on 56 cases of ziprasidone overdose in which ziprasidone was either ingested alone or co-ingested with drugs not associated with QTc prolongation. A QTc interval between 450 and 500 ms was observed in seven (12.5 %) cases and >500 ms in one (1.8 %) case.
Of a total of 28 efficacy and safety studies of ziprasidone, in 15 publications (Supplementary Table 3, studies 32–44, 46 and 47) [128–142] ziprasidone was the only studied drug, in three publications [32, 35, 143] (Supplementary Table 3, studies 3, 5 and 45) ziprasidone was added to existing antipsychotic or antidepressant treatment, in nine publications [33, 36, 44, 57–60, 144, 145] (Supplementary Table 3, studies 2, 6, 9, 11–14, 48 and 49) ziprasidone was compared with another drug, and one publication [146] (Supplementary Table 2, study 50) analyzed pooled data from several studies.
Of the 15 ziprasidone-alone studies, seven [128–134] were of children and adolescents. The QTc interval increased with ziprasidone treatment, which was reported as statistically significant in two studies [129, 133]. There were occasional cases of clinically significantly prolonged QTc interval (QTc interval ≥450 ms), though some studies [128, 131, 132] used a slightly higher threshold to define clinical significance. One study [131] reported a QTcF interval increase of ≥60 ms in two of 193 patients receiving ziprasidone. No patients developed a QTc interval ≥500 ms. Overall, similar findings were reported in studies of adult patients [135–140]. From ECGs of 149 adult patients, Mencacci [138] found that ziprasidone treatment caused mild QTc interval prolongation (450–470 ms) in 12 patients (8.1 %) and moderate prolongation (>480 ms) in one patient (0.6 %). Two small studies of IM ziprasidone in patients with dementia [141, 142] did not notice a significant change in mean QTc interval from pre- to post-treatment. In one of these studies [141], one of the 14 patients (7.1 %) had a QTc interval >500 ms and a 25 % increase from baseline.
Ziprasidone add-on treatment to clozapine increased the QTcB interval significantly at week 16 in one study [35]. In another study of patients receiving olanzapine or clozapine [32], the addition of ziprasidone prolonged the QTc interval significantly at week 2 but not at week 6. The addition of ziprasidone to treatment with selective serotonin reuptake inhibitors (SSRIs) was not associated with a significant increase in the mean QTc interval from baseline, but an increase in QTc interval ≥30 ms was noted in two of 13 patients [143].
Four studies with an active comparator involved olanzapine; the QTc interval increased significantly in the ziprasidone group compared with the olanzapine group in one of these [57] but not in the other three studies [58–60]. No patient in these studies had a QTc interval ≥500 ms. Sacchetti et al. [36] observed that clozapine and ziprasidone caused comparable changes in mean QTcB interval but the incidence of new-onset QTcB interval ≥450 ms was higher in the clozapine group (14.1 %) than in the ziprasidone group (4.5 %). Potkin et al. [144] noted that lurasidone and ziprasidone caused comparable changes in the QTcF interval from baseline. Another study [44] also noted comparable increases in QTcF interval with iloperidone and ziprasidone treatments, which was significantly greater than with placebo for both treatments. One patient in each treatment group had a >60 ms increase in QTcF interval but none experienced a QTcF >500 ms. Zink et al. [33] found that the addition of ziprasidone to clozapine was associated with a significant increase in QTcF interval but the addition of risperidone was not. A study [145] comparing ziprasidone and chlorpromazine did not find any significant effect of either drug on QTc interval.
Camm et al. [146] analysed QTc interval changes associated with ziprasidone based on data from Pfizer®-sponsored phase II–IV randomized controlled trials (RCTs); evaluable data were available for 4,306 adult patients). Incidences of QTc interval >450 ms (0.8 %) or QTc interval >480 ms (0.023 %) were rare. QTc prolongation >30 ms was observed in 9.0 %, >60 ms in 0.7 %, and >75 ms in 0.3 % of patients receiving ziprasidone. In the placebo-controlled studies, the mean change in QTc interval from baseline to end of study was 3.6 ms in the ziprasidone group and –0.3 ms in the placebo group. Data from IM ziprasidone studies, and bipolar studies in which ziprasidone was used adjunctively with lithium, valproate, or lamotrigine, demonstrated similar QTc effects. An increase in the QTc interval correlated with serum ziprasidone concentration.
We found 13 case reports [147–157] (14 incidents) of ziprasidone-associated QTc interval prolongation (Supplementary Table 9 and Table 4), six (46.1 %) of which involved ziprasidone overdose. All except one incident had risk factors in addition to therapeutic ziprasidone. The QTc interval was ≥500 ms in 12 of 14 incidents. TdP occurred on two occasions (14.3 %) and, on both occasions, the QTC interval was ≥500 ms. QTc prolongation was observed after a single injection of ziprasidone in two cases. In two cases, the QTc interval normalized after a decrease in the dose of ziprasidone from 240 to 160 mg/day.
4.10 Summary of Risk of QTc Prolongation and TdP with Individual SGAPs
While it is clear that all nine SGAPs reviewed above have the potential to prolong QTc interval, it is difficult to rank individual SGAPs for this risk (i.e., QTc prolongation). There is no consensus definition of ‘clinically significant’ prolongation of QTc interval, but a QTc interval ≥450 ms and/or an increase in QTc interval ≥30 ms from baseline are commonly used minimal values [11]. The literature on sertindole is limited, but it consistently shows that sertindole causes statistically and clinically significant prolongation of the QTc interval. Similarly, limited but emerging data suggest that iloperidone may cause statistically and clinically significant prolongation of the QTc interval. Abundant data on ziprasidone suggest that it causes statistically significant prolongation of the QTc interval, and clinically significant prolongation may be observed but QTc interval ≥480 ms or an increase in QTc interval ≥60 ms from baseline is infrequent. The effect of therapeutic clozapine and quetiapine on the QTc interval appears to be similar to that of ziprasidone, except that the dose-response effect is better established for ziprasidone than for clozapine or quetiapine. Clozapine can cause tachycardia, and drawing from studies using Bazett’s correction likely over-estimates its risk to prolong the QTc interval. Olanzapine and risperidone (and paliperidone based on very limited literature and from indirect evidence as a metabolite of risperidone) appear to have a modest effect on the QTc interval when used in therapeutic doses. Data are too limited to categorize therapeutic amisulpride with regards to its potential to prolong the QTc interval.
Currently, it is not possible to categorize individual SGAPs for their risk to cause TdP. TdP was not reported for any of these drugs in studies employing therapeutic doses. Toxicology studies (Table 3) suggest that TdP is more likely after overdose with amisulpride than it is after overdose with clozapine, quetiapine, risperidone, or ziprasidone. Case-report material (Table 4) identifies TdP as a rare complication that can happen even with SGAPs associated with modest increases in the QTc interval, at therapeutic doses of SGAPs, and with a QTc interval <500 ms. Case-report material also signifies individual vulnerability and the presence of other risk factors as important factors for SGAP-associated QTc prolongation and TdP.
5 Studies of QTc Prolongation for Individual SGADs
5.1 Citalopram
The TQT study for citalopram [8] was a randomized, double-blind, placebo-controlled, crossover study in which 119 subjects received citalopram 20 and 60 mg/day, moxifloxacin 400 mg/day (active control), and placebo. Citalopram caused a dose-dependent increase in the QTc interval. Change in the mean QTc interval from baseline was 8.5, 18.5, and 13.4 ms with citalopram 20 mg/day, citalopram 60 mg/day, and moxifloxacin 400 mg/day, respectively.
We found two QTc prolongation-specific studies [158, 159] for citalopram and one study [13] commenting on its potential to cause TdP (Table 5, studies 1, 9, and 10). Castro et al. [158] performed a cross-sectional study of several antidepressants. Methadone was included as a measure of assay sensitivity. Patients with an ECG in the exposure period (14–90 days) were older with greater medical comorbidity than those who did not have an ECG. The proportion of patients with abnormal (451–500 ms for men, 471–500 ms for women) or high (>500 ms) QTc values was similar across treatment groups. Dose was a significant predictor of QTc for citalopram, escitalopram, and amitriptyline (and methadone). Of patients who started taking citalopram with a QTc in the normal range, 13.1 % shifted to an abnormal range after the dose increase.
Using the public version of the FDA adverse event reporting system, Astrom-Lilja et al. [13] evaluated spontaneously reported cases of TdP. Among a total of 61,788 adverse drug reactions, 88 cases of TdP were identified. Citalopram was the third most common suspected drug in the reports (10 %; 9/88). Authors wondered why TdP was not listed in citalopram product summary (then). Dubnov-Raz et al. [159] retrieved data on 52 neonates with a gestational age ≥35 weeks born to women receiving treatment with citalopram, paroxetine, fluoxetine, fluvoxamine, sertraline, or venlafaxine. The mean QTcB was significantly longer in the group of newborns exposed to antidepressants than in control subjects.
Seven toxicology studies [160–166] inform us about the effect of citalopram on the QTc interval (Table 6, studies 1, 2, 5–9). Grundemar et al. [160] reviewed five cases of citalopram overdose. QTc interval was prolonged (>440 ms) in all. In a retrospective review of 24 cases of citalopram overdose [161], the QTcB interval was ≥450 ms in eight cases (33 %). Two of these cases had toxic co-ingestion of quetiapine and one of mirtazapine. Hayes et al. [162] reviewed 374 cases of citalopram-alone and 421 cases of escitalopram-alone overdose. QTc prolongation (>440 ms for males and >460 ms for females) was observed in 14 (3.7 %) cases of citalopram overdose and seven (1.7 %) cases of escitalopram overdose. The difference between groups was not significant. In another review of 316 citalopram and 63 escitalopram overdoses [163], the incidence of mild (>390 ms for males and >440 ms for females; citalopram 7.9 % and escitalopram 6.3 %) and moderate QTc prolongation (>430 ms for males and >485 ms for females; citalopram 0.6 % and escitalopram 1.6 %) was statistically similar.
Isbister et al. [164] reviewed the ECGs of 297 cases of single SSRI (citalopram, fluoxetine, fluvoxamine, paroxetine, or sertraline) toxic ingestion. The median QTcB interval of the SSRI groups was significantly greater than the median QTcB interval of the comparison group consisting of 318 patients ingesting non-cardiotoxic medications. A total of 68 % of citalopram overdoses had a QTcB >440 ms, and 12 % had a QTcB >500 ms, which was significantly greater than other SSRIs. Kelly et al. [165] reviewed 225 consecutive cases of overdose with citalopram, mirtazapine, nefazodone, or venlafaxine. Of 12 patients with a QTcB >450 ms without co-ingestion of another cardiotoxic drug, one patient had ingested mirtazapine, four venlafaxine, and seven citalopram. In the study by Waring et al. [166], citalopram conferred a significantly greater likelihood of higher-than nomogram QT interval than ingestion of mirtazapine or venlafaxine. A significantly higher proportion of patients in the citalopram group (32 %) had a QTcB ≥440 ms than the venlafaxine (18 %) and mirtazapine groups (16 %), but not with regards to a QTcB ≥500 ms.
In the only efficacy and safety publication of citalopram (Supplementary Table 10, study 1), Rasmussen et al. [167] (401) performed a pooled analysis of the data available up until 1999. In the only randomized, double-blind study of healthy volunteers, pooled data from three prospective studies and a retrospective analysis of data from the placebo-controlled studies, citalopram was comparable to placebo with regards to its effect on QTc interval. Review of 9 years of post-marketing surveillance data identified 17 cases of citalopram-associated QTc prolongation and/or TdP. Most of these cases had risk factors for QTc prolongation in addition to therapeutic citalopram.
We found 16 case reports of citalopram-induced QTc prolongation and/or TdP (Supplementary Table 11 and Table 7) [69, 87, 112, 168–180]. All patients had at least one additional risk factor for QTc prolongation besides therapeutic doses of citalopram. TdP occurred in nine cases, seven (77.8 %) of whom were receiving therapeutic doses (up to 60 mg/day), one was receiving 80 mg/day, and one presented with an overdose.
5.2 Escitalopram
The TQT study for escitalopram [8] was a randomized, double-blind, placebo-controlled, crossover study. 113 subjects received escitalopram 10 and 30 mg/day, moxifloxacin 400 mg/day (active control), and placebo. Escitalopram caused a dose-dependent increase in the QTc interval. Change in the mean QTc interval from baseline was 4.5, 10.7, and 9.0 ms with escitalopram 10 mg/day, escitalopram 30 mg/day, and moxifloxacin 400 mg/day, respectively.
We did not find any QTc prolongation-specific study for escitalopram. Three studies assessed QTc prolongation associated with overdose of escitalopram (Table 6, studies 3, 5, and 6) [162, 163, 181]. van Gorp et al. [181] reviewed ECGs from 78 presentations of escitalopram overdose involving 68 patients. QT-HR plotted on QT nomogram identified 11 presentations (ten patients; 14.7 %) at risk of TdP. The other two toxicology studies [162, 163] included citalopram besides escitalopram and are described in greater detail in Sect. 5.1 above. In both these studies, the QTc-prolonging effect of citalopram and escitalopram was comparable.
Two efficacy and safety publications provided information about escitalopram-induced QTc prolongation (Supplementary Table 10, studies 2 and 3) [182, 183]. In one of these [182], treatment with escitalopram 10 mg/day for 1 year was compared with placebo for prophylaxis of depression in patients with acute coronary syndrome. Escitalopram use was not associated with an increase in QTcB interval or an increase in the incidence of QTcB interval >450 ms. The second publication [183] performed a pooled analysis of data from all randomized placebo-controlled studies sponsored by H. Lundbeck A/S or Forest Laboratories, Inc. Both in 14 short-term (8–12 weeks) and three long-term (24 weeks) studies, the difference to placebo in the mean change in the QTcF interval between escitalopram10 mg and 20 mg was not significant at end of study or at last assessment. One of 2,407 escitalopram patients had a QTcF interval >500 ms and a change from baseline >60 ms.
In the six case reports for escitalopram (Supplementary Table 12 and Table 7) [184–189], four involved an overdose. All patients had risk factors for QTc prolongation besides therapeutic escitalopram. No case experienced TdP.
5.3 Fluoxetine
We did not find a TQT study or any pertinent efficacy and safety study of fluoxetine.
We found four QTc prolongation-specific studies for fluoxetine (Table 5, studies 2, 9, 10 and 11) [158, 159, 190, 191]. In an open-label study of 12 healthy volunteers, Zhao et al. [190] observed that co-administration of fluoxetine with cisapride significantly decreased cisapride plasma concentrations. There were no clinically significant changes in QTcB intervals (QTcB ≥450 ms or an increase in QTcB of ≥15 % from baseline) during administration of cisapride alone or with fluoxetine. In the study by Dubnov-Raz et al. [159] already discussed in Sect. 5.1 above, neonatal exposure to all included antidepressants was associated with QTcB interval prolongation. Of the five neonates with a QTcB interval ≥460 ms, two were exposed to fluoxetine and three to paroxetine. In the cross-sectional study based on data from electronic health records [158], antidepressant use was associated with QTc prolongation. Analysis for individual antidepressants was not offered. Dose of fluoxetine did not predict QTc interval. Baker et al. [191] compared the ECG effects of fluoxetine and doxepin in patients with major depression over a period of 6 weeks. Unlike doxepin, fluoxetine had no affect on QTc interval.
In the toxicology study by Isbister et al. [164] (Table 6, study 7), 42 of the 297 single SSRI overdoses were of fluoxetine. Citalopram had a significantly greater effect on QTc interval than other included SSRIs. In patients with fluoxetine overdose, 36 % had a QTcB interval >440 ms and 10 % had a QTcB interval >500 ms.
We found nine case reports for fluoxetine (Supplementary Table 13 and Table 7) [192–200]. Seven cases had at least one additional risk factor besides therapeutic fluoxetine. TdP occurred in three cases, in all with a QTc interval <500 ms.
5.4 Mirtazapine
We did not find a TQT study, any pertinent efficacy and safety studies, or case reports of mirtazapine. The only QTc prolongation-specific study for mirtazapine [158] (Table 5, study 9) included several antidepressants and was discussed in Sect. 5.1. In this cross-sectional study, 20.4 % of individuals had abnormal (451–500 ms for men, 471–500 ms for women) or high (>500 ms) QTc values without any significant difference between treatment (amitriptyline, bupropion, citalopram, duloxetine, escitalopram, fluoxetine, mirtazapine, nortriptyline, paroxetine, sertraline, and venlafaxine) groups. Dose of mirtazapine was not a predictor of QTc interval.
We found two toxicology studies of mirtazapine (Table 6, studies 8 and 9) [165, 166]. Kelly et al. [165] reviewed data on 225 consecutive patients presenting with citalopram, mirtazapine, nefazodone, and venlafaxine overdose. The mean QTcB interval for the mirtazapine group (423.9 ms) was comparable with those for the other groups. One of the 12 patients with a QTcB >450 ms and without co-ingestion of another cardiotoxic drug had ingested mirtazapine. In the second study, Waring et al. [166] assessed the QTcB interval and plotted a QT interval versus HR nomogram on patients presenting with overdose of citalopram, mirtazapine, or venlafaxine. Overall, QT interval was on or above the nomogram for 2.4 % of the patients. For the mirtazapine group, 16 % of patients had a QTcB ≥440 ms and none had a QTcB ≥500 ms.
5.5 Paroxetine
We did not find a TQT study or any case report for paroxetine.
We found five QTc prolongation-specific studies for paroxetine (Table 5, studies 3, 4, 5, 9 and 10) [158, 159, 201–203]. Martin et al. [201] investigated the effects of paroxetine on the pharmacodynamic and pharmacokinetic profile of terfenadine in 12 healthy adults. Paroxetine co-administration did not affect terfenadine pharmacokinetics. The QTc interval changed slightly from terfenadine-alone administration (404 ms) to paroxetine co-administration (405 ms). In a study of 40 adult patients, Kuhs and Rudolf [202] noted that amitriptyline altered the QTcB interval while paroxetine did not. Edwards et al. [203] also did not observe any significant effects of paroxetine on QTc interval in a small study of depressed patients. In the study of neonates exposed to antidepressants through maternal use [159], three of the five neonates with a QTcB interval ≥460 ms were exposed to paroxetine. Lastly, in the cross-sectional study [158] discussed earlier in Sect. 5.1, the dose of paroxetine did not predict QTc interval.
In the only toxicology study including paroxetine (Table 6, study 7) [164], 78 of the 297 single SSRI overdoses were of paroxetine. Citalopram had a significantly greater effect on QTc interval than other included SSRIs. In patients with paroxetine overdose, 40 % had a QTcB interval >440 ms and 1 % had a QTcB interval >500 ms.
Both efficacy and safety studies of paroxetine were pooled analyses (Supplementary Table 10, studies 4 and 5) [204, 205]. Krulewicz et al. [205] pooled data from three 8- to 10-week, randomized, placebo-controlled, double-blind trials of paroxetine in patients aged 7–18 years. A total of 1,451 ECGs from 449 patients receiving placebo (N = 207), paroxetine (N = 200), or imipramine (N = 42) were analyzed. Treatment with placebo or paroxetine did not significantly change mean QTcB or QTcF values from baseline. A similar proportion of placebo- and paroxetine-treated patients had an increase in QTcB or QTcF interval of >60 ms or 30–60 ms from baseline. None of the treatment groups were associated with a QTcB or QTcF value of >500 ms. Nelson et al. [204] pooled data from four 8-week randomized, double-blind, placebo- (N = 371) and paroxetine-controlled (N = 359) studies of duloxetine (N = 736) for major depression in adults. Neither treatment altered baseline QTc interval. No treatment-emergent QTc prolongation, defined as ≥30 ms change from baseline, was observed.
5.6 Sertraline
We did not find a TQ study for sertraline. There were three QTc prolongation-specific studies of sertraline (Table 5, studies 6, 9 and 10) [158, 159, 206]. Alderman [206] studied the pharmacokinetic and ECG effects of sertraline on cisapride and pimozide in 30 adult healthy volunteers. Co-administration of sertraline significantly decreased the concentration of cisapride and significantly increased the concentration of pimozide than when these drugs were administered alone. No subject exhibited a prolonged QTc interval (defined as ≥15 % increase) with co-administration of sertraline with cisapride or pimozide. In the study showing that neonatal exposure to antidepressants through maternal use [159] prolongs QTc interval (discussed in Sect. 5.1), none of the five neonates with a QTcB interval ≥460 ms were exposed to sertraline. Lastly, in the cross-sectional study [158] also discussed in Sect. 5.1, the included antidepressants had a comparable effect on QTc interval. Dose of sertraline did not predict QTc interval.
In the toxicology study by Isbister et al. [164] (Table 6, study 7), 103 of the 297 single SSRI overdoses were of sertraline. Citalopram had a significantly greater effect on QTc interval than other included SSRIs. In patients with sertraline overdose, 40 % had a QTcB interval >440 ms and 6 % had a QTcB interval >500 ms.
We found three efficacy and safety studies of sertraline with information on its effect on QTc interval (Supplementary Table 10, studies 6–8) [207–209]. In a double-blind study [207], depressed patients hospitalized for myocardial infarction were randomized to receive sertraline or placebo for 24 weeks. Mean QTc interval as well as number of patients with a QTc interval >450 ms decreased from baseline to week 16 for both groups without any differences between the groups. Wilens et al. [208] compared sertraline with placebo for obsessive-compulsive disorder in youth over a period of 12 weeks. Neither treatment group experienced a significant increase in mean QTcB interval from baseline. Lastly, Guy and Silke [209] analysed 2,500 ECGs from four studies in which sertraline (dose range 50–400 mg/day) was compared with placebo and/or amitriptyline. Amitriptyline treatment was associated with a significant prolongation of QTc interval from baseline, but sertraline treatment was not.
We found only two case reports for sertraline-related QTc interval prolongation and/or TdP (Supplementary Table 14 and Table 7) [210, 211]. TdP occurred at a therapeutic dose of sertraline in one of these cases.
5.7 Venlafaxine
We did not find a TQT study or any pertinent efficacy and safety study of venlafaxine.
We found three QTc prolongation-specific studies of venlafaxine (Table 5, studies 7, 8, 9) [158, 212, 213]. Mbaya et al. [212] studied ECGs of 37 depressed adult patients treated with high doses of venlafaxine for at least 1 year. QTc was normal (defined as <440 ms in men and <470 ms in women) in all patients except one female patient who had a QTc of 476 ms. There was no association between the dose of venlafaxine ER and QTc interval. Johnson et al. [213] studied ECGs of patients ≥60 years of age treated with venlafaxine ER for up to 12 weeks. For 40 patients with a baseline and follow-up ECG, mean QTcB interval at follow-up was not significantly different from baseline. One patient experienced an increase in QTc interval from 403 to 456 ms and another patient experienced an increase from 433 to 469 ms. Venlafaxine was among the antidepressants in the study by Castro et al. [158] showing 20.4 % of individuals having abnormal (451–500 ms for men, 471–500 ms for women) or high (>500 ms) QTc values. There was no significant difference between treatment (amitriptyline, bupropion, citalopram, duloxetine, escitalopram, fluoxetine, mirtazapine, nortriptyline, paroxetine, sertraline, and venlafaxine) groups. Dose of venlafaxine did not predict QTc interval.
We found four toxicology studies of venlafaxine (Table 6, studies 4 and 8–10) [165, 166, 214, 215]. Isbister [214] studied 369 incidents of venlafaxine overdoses in 273 patients by plotting the QT interval versus HR on a QT nomogram. In 22 admissions (6 %), the QT-HR pair was above the ‘at-risk for TdP’ line; these cases were more likely to have larger ingestions or co-ingestion of other drugs known to affect the QT interval. No arrhythmias were observed on continuous telemetry. Chan et al. [215] compared the QT-HR nomogram of 36 cases of venlafaxine overdose with 44 randomly selected cases of SSRI overdose. For three cases in each group, the QT interval was marginally above the ‘at risk’ for TdP line on the nomogram. In the study by Kelly et al. [165], 96 of the 225 cases were of venlafaxine overdose. The mean QTcB interval of the venlafaxine group (420.2 ms) was comparable with those of other groups. Four of the 12 patients with a QTcB >450 ms and without co-ingestion of another cardiotoxic drug had ingested venlafaxine. Cases of venlafaxine overdoses were also included in the study by Waring et al. [166]. For the venlafaxine group, 18 % of patients had a QTcB ≥440 ms and one had a QTcB ≥500 ms.
We found five case reports of venlafaxine-related QTc interval prolongation and/or TdP (Supplementary Table 15 and Table 7) [87, 94, 216–218]. QTc interval was >500 ms for all cases. All cases had risk factors for QTc prolongation besides therapeutic venlafaxine. The only case of TdP was on the therapeutic dose of venlafaxine. Two cases without TdP died of other cardiac complications.
5.8 Summary of Risk of QTc Prolongation and TdP with Individual SGADs
It is obvious that all the SGADs reviewed above are associated with varying degrees of QTc interval prolongation. There is a reasonable amount of fairly consistent literature suggesting that citalopram prolongs QTc interval in a dose-dependent manner, which may be statistically and clinically significant in some situations. Current literature does not allow definitive stratification of the risk of QTc interval prolongation with other SGADs. It is even harder to categorize the risk of TdP with individual SGADs. Citalopram does seem to standout for this particular risk in toxicology studies and case reports, but occasional cases of TdP have been reported for some other SGADs as well. Further complicating literature interpretation is the fact that most cases of SGAD-associated TdP occurred at therapeutic doses of these drugs.
6 Discussion
This comprehensive review highlights the limitations in the literature on SGAP- and SGAD-associated QTc interval prolongation and/or TdP. Foremost among the limitations is the paucity of well designed studies specifically assessing drug-induced QTc interval prolongation. The bulk of the literature on this topic derives from efficacy and safety trials. Such studies almost always exclude patients with medical and cardiovascular comorbidity and their findings have limited relevance to at-risk patients seen in clinical practice [8, 14, 29]. The few QTc interval-specific studies mostly had a small sample size and employed a retrospective, cross-sectional, or naturalistic observation design without control of confounding factors. Toxicology studies provided valuable information but they are few in number, and their findings are mostly pertinent to situations of drug overdose. Case reports provide unique information, especially about the factors that may link QTc interval prolongation to TdP, but the information does not allow comparison between drugs and cannot be generalized to clinical practice.
Several well established methods exist to correct QT interval for HR [219, 220]. Overall, the machine-read QTcB interval was the most common method used in the studies we reviewed. Toxicology studies mostly employed the QT nomogram method to report their findings. Fridericia’s method was used fairly often, but population- or individual-based correction methods were used rarely. The method used to calculate the QTc interval can significantly impact on the findings of a study. For example, use of Bazett’s formula for drugs likely to cause tachycardia, such as clozapine, will over-estimate the risk. Nielsen et al. [31] noted that sertindole augmentation of clozapine treatment caused a statistically significant prolongation in the machine-calculated QTcB interval versus placebo, but the difference was not significant with manually calculated QTcF. In another study, the change in mean QTc interval after treatment with quetiapine was 12.6 ms using Bazett’s formula but only 1.3 ms with Fridericia’s formula. In a study of 207 patients being treated for schizophrenia and 207 age- and gender-matched healthy controls, Hingorani et al. [221] noted that mean QTcB interval was significantly longer in patients (412 ms) than in controls (402 ms) but mean QTcF intervals were comparable (398 ms in patients vs. 401 ms in controls). The method used to calculate the QTc interval can also have clinical implications as noted in the interesting discussion by Dhillon et al. [31, 38], Nielsen [222], and Law et al [223].
Lack of a consistent sex- and extent-based definition of ‘prolonged QTc interval’ further complicates any attempts to compare or consolidate studies. Values to define ‘prolonged QTc interval’ ranged from >390 ms for males and >440 ms for females [30] to >470 ms for males and >480 ms for females [55, 163]. Some studies (e.g., Kane et al. [145]) only reported a QTc interval prolongation as ≥500 ms. Only a few studies (e.g., Mencacci [138, 163] and Yilmaz et al. [138, 163]), set distinct sex-based values for QTc interval prolongation or made a distinction between mild and moderate QTc prolongation. Similarly, reporting of the extent of prolongation in QTc interval from baseline was inconsistent. Furthermore, studies rarely controlled for confounding variables such as timing of ECGs in relation to drug administration or concurrent presence of other risk factors for QTc interval prolongation.
Besides the methodological limitations described above, there is one serious conceptual limitation in the current literature—emphasis on drug-associated QTc prolongation per se over-shadows other factors that link psychotropic drugs to TdP. In a comprehensive review of 102 publications, Bednar et al. [14] observed that 108 of 116 (92.2 %) cases of non-cardiac drug-associated TdP occurred at a QTc interval ≥500 ms, and the remaining 7.8 % occurred with a QTc interval <500 ms. Authors emphasized that both the magnitude of QTc prolongation and characteristics of the population were the most important determinants of risk for TdP. They identified heart disease, female sex, bradycardia, hypokalemia, and other electrolyte abnormalities as important risk factors. Viskin et al. [15] reviewed data from 229 published cases of drug-associated TdP. A total of 96 % of cases had one or more risk factors for QTc interval prolongation/TdP in addition to the drug. The major risk factors (cases) included female sex (71 %), significant cardiac disease (42 %), concurrent use of more than one QTc-prolonging drug (39 %), concurrent use of a metabolic inhibitor of a QTc interval-prolonging drug (38 %), hypokalemia (28 %), toxic ingestion of the drug (20 %), and pre-treatment prolonged QTc interval, history of a familial long QT syndrome or a previous episode of drug-induced TdP (18 %). Aström-Lilja et al. [13] reviewed 88 spontaneously reported cases of TdP in Sweden. In addition to drug treatment, two or more established risk factors were present in 85 % of the cases. Heart disease (90 %) was the most common risk factor, followed by age over 65 years (72 %) and female gender (70 %).
The case report material we reviewed (Tables 4, 7, and Supplementary Tables 4–9 and 11–15) mirrors the findings of the above-mentioned studies [13–15]. Overall, there were 102 reports (101 cases) of QTc interval prolongation involving six SGAPs and five SGADs. TdP occurred in 28 (27.5 %) reports; the QTc interval was <500 ms in six (21.4 %) and ≥500 ms in the remaining 22 (78.6 %). At least one additional well established risk factor for QTc prolongation (see Sect. 3.5 for specific risk factors counted) was noted in 94 (92.2 %) of the 102 reports. It is also important to note that in nine (64.3 %) of 14 reports of SGAP-associated TdP and 12 (85.7 %) of 14 reports of SGAD-associated TdP, TdP occurred at therapeutic doses of these agents.
Our review has several limitations. We focused only on published, English-language literature available through two leading indexing sites. We intentionally did not seek unpublished data from the manufacturers. Manufacturers are not obligated to release all the pertinent data, and in some cases may not even respond to such requests [224]. Selective release of data creates bias [225]. Furthermore, had there been any significant data with the manufacturers, they would have either been published or have come to the attention of agencies like the FDA. We did not include meta-analyses in this review because their findings are limited by what is (and is not) included in the analysis. Broad-based efficacy and safety meta-analysis [226] may not rigorously control for factors pertinent to the QTc interval, while meta-analysis using stringent study selection criteria to control for such factors [224] may be left with only a handful of studies for analysis. Furthermore, meta-analyses are mostly based on data from clinical trials of relatively healthy patients, and their finding cannot be generalized to real-life clinical practice. Case report material cannot be used to compare between drugs as it does not control for prescription/exposure rates and reporting bias, such as, an interest to report generated by regulatory concerns. Discussion on non-clinical in vitro human ether-a-go-go-related gene (hERG) channel affinity studies and the in vivo QT assay studies was beyond the scope of this review. Such studies are few, are not available for all drugs included in this review, and do not provide comparison between drugs. Based on our familiarity with this literature [4–7], we know its inclusion in this review would not have made a meaningful difference in our findings.
7 Directions for Future Clinical Research
Investigational agents from drug classes known to prolong the QTc interval undergo non-clinical in vitro and in vivo studies. Discussion pertaining to such research is beyond the scope of this review. Interested readers are referred to the ICH S7B document [227] and the publication by Farkas and Nattel [228]. It is important to remember that the target point of interest is TdP. ‘Prolonged QTc interval’ is only a surrogate marker of TdP and has its limitations [229, 230]. It is essential to research both sides of the drug–QTc interval prolongation–TdP link to best understand the whole process.
The precision of drug-associated QTc interval prolongation studies depends greatly upon the method used to measure the QT interval and the formula used to correct it for HR. Fixed-correction methods are easy to use but less precise than the population-based or individual-based (most precise) correction methods [219, 220, 231]. Unfortunately, the fixed-correction method used most often (Bazett’s correction) overestimates QTc interval prolongation over a broad range of HRs [232]. We are pleased that ICH has now recognized it “an inferior method” and recommended using Fridericia’s correction (or another suitable method) instead [12]. It is also well known that automated calculations lack precision [12]. Manual measurement involves measuring the QT interval in lead II or one of the leads from V3-V6 (usually V5) over 3–5 cycles and calculating the mean. The tangent method defines the end of the T-wave most reliably [233, 234]. In this method, the intercept between the isoelectric line and the tangent of the steepest part of the T-wave defines the end of the T-wave. In situations of T-wave morphology alterations, such as presence of U-waves, biphasic T-waves, or flat T-waves, manual reading by an experienced ECG reader is often appropriate. We hope that Fridericia’s correction using manual or manual adjudication method becomes the basic standard in all future QTc interval-related studies and case reports. Other correction method(s) can be used in addition to this method; such studies will allow comparison between the methods [166, 235]. More precise population- or individual-based correction methods would be desired in TQT and other rigorously designed QTc prolongation-specific studies. Readers interested in the topic of how to assess a QTc interval are referred to a publication by Nielsen et al. [233].
To improve consistency across studies and to prevent reporting bias, there is a need to predefine QTc interval prolongation. Currently used values are QTcB interval-based [236] but are generalized to other methods of correction. Normal QTc interval values are ≤430 ms for males and ≤450 ms for females. A QTc interval ≥500 ms is a strong predictor of a drug’s risk to cause TdP in both sexes. Increase in QTc interval from baseline of ≥30 ms, ≥60 ms, or ≥15 % are also valuable predictors of a drug’s risk to cause TdP. When possible, all drug-associated QTc interval prolongation/TdP studies should aim at reporting the results based on these values. Additional sex-based values may be used.
It is also important to adjust for the possible drug concentration-dependent variations in QTc interval. If study design allows, ECGs should be obtained during drug steady state at t max. t max can be estimated from the established pharmacokinetics of the drug. Rigorous studies may need to calculate study-specific t max and monitor serum levels of the study drug as well as any of its metabolites with possible effects on the QTc interval. Implications of such monitoring are reflected in a study [76] noticing that the serum level of the 9-hydroxy metabolite of risperidone but not risperidone correlated positively with QTcB interval, and another study [46] observing a decrease in the QTc-prolonging effect of risperidone after metabolic inhibition.
Future studies should also attempt to identify potentially confounding factors and control them whenever possible. While this may be of little relevance to efficacy and safety studies of relatively healthy patients, it can have significant implications for studies based on clinical patients (e.g., naturalistic observational and pharmaco-vigilance studies), in whom multiple risk factors for QTc interval prolongation are not uncommon [158, 167, 207, 237, 238]. The QTc interval varies tremendously between individuals [239]. This necessitates establishing the baseline QTc interval of the study population. For psychiatric patients, it would be important to establish a drug-free baseline based on pharmacokinetic principles to eliminate any existing psychotropic drug effect. Establishing a drug-free baseline is also important considering that factors associated with markers of ventricular repolarisation, such as cigarette smoking, obesity, and metabolic syndrome, are highly prevalent in this patient population [240–242]. It would also be important to control for drugs that may lessen QTc interval prolongation [243, 244]. When a control group is used, it must be age- and sex- as well as risk factor-matched. Otherwise, the sample size should be large enough to allow analyses after controlling for the confounding variables.
There is a tremendous need for research that focuses on the link between the drug and TdP. SGAP- and SGAD-associated QTc interval prolongation by itself is not sufficient to link a drug with TdP. The extreme rarity of SGAP- and SGAD-associated TdP limits us from researching TdP as an endpoint through well designed prospective studies. In this scenario, an approach of ‘reverse research’ of patients with drug-induced TdP may be the only viable option. Toxicology studies, pharmaco-vigilance data, and case reports provide important information in this regard. Information from case reports of patents experiencing QTc interval prolongation and/or TdP during therapeutic drug use can be particularly insightful and may help develop ‘selected vulnerability’ hypotheses (e.g., role of genetic factors [245, 246]) for further exploration.
Ventricular repolarisation is the most vulnerable period for a possible arrhythmia, and several ECG descriptors of this phase have been studied as predictors of cardiac outcome [247]. Potential markers that have been explored in psychotropic drug studies include two markers of ventricular repolarisation dispersion [98, 120, 129], namely QT dispersion (the difference between the longest and shortest QT interval in the 12 leads) and T-peak to T-end (TpTe; the interval from the peak of the T-wave to the end of the T-wave), and T-wave morphology changes (e.g., flat, asymmetric, or notched T-wave) [248, 249]. There is a need to further study these and other potential markers in the context of drug use to determine if any of them is easier to study and can predict ventricular repolarisation defect better than QTc interval prolongation.
There are several other important aspects of drug-QTc interval prolongation-TdP research. We only highlighted a few points to initiate the discussion. We hope that experts from the clinical and preclinical sciences, pharmaceutical industry, and regulatory agencies will advance this endeavour.
8 Conclusion
While all the nine SGAPs and seven SGADs we reviewed are associated with TdP, current literature does not allow categorization of individual agents for this risk. Clumping of QTc interval prolongation with TdP has transformed the ‘TdP-marker’ to ‘TdP-synonymous’. In reality, evidence suggests that drug-associated QTc interval prolongation may predict TdP, but by itself (i.e., in the absence of other risk factors) rarely leads to TdP. The association between drug-associated QTc interval prolongation and TdP is not a simple one like that between clouds and rain (or snow). It is a complex one, like that between clouds and hail, with many factors in play to create the right environment for it to happen. Future research needs to improve its precision and broaden its scope to better understand the factors that facilitate or attenuate progression of drug-associated QTc interval prolongation to TdP.
References
Vieweg WV. New generation antipsychotic drugs and QTc interval prolongation. Prim Care Companion J Clin Psychiatry. 2003;5:205–15.
Vieweg WV, Wood MA, Fernandez A, Beatty-Brooks M, Hasnain M, Pandurangi AK. Proarrhythmic risk with antipsychotic and antidepressant drugs: implications in the elderly. Drugs Aging. 2009;26:997–1012.
Hasnain M, Vieweg WV, Breden Crous EL, Hancox JC. Methadone and torsade de pointes: how can we better understand the association? Am J Med. 2013;126:757–8.
Hasnain M, Vieweg WV, Howland RH, Kogut C, Breden Crouse EL, Koneru JN, et al. Quetiapine and the need for a thorough QT/QTc study. J Clin Psychopharmacol. 2014;34:3–6.
Vieweg WV, Hasnain M, Howland RH, Hettema JM, Kogut C, Wood MA, et al. Citalopram, QTc interval prolongation, and torsade de pointes. How should we apply the recent FDA ruling? Am J Med. 2012;125:859–68.
Vieweg WV, Hasnain M, Hancox JC, Baranchuk A, Digby GC, Kogut C, et al. Risperidone, QTc interval prolongation, and torsade de pointes: a systematic review of case reports. Psychopharmacology (Berl). 2013;228:515–24.
Hasnain M, Vieweg WV, Howland R.H., Kogut C, Crouse E.L.B., Koneru JN, et al. Quetiapine, QTc interval prolongation, and torsade de pointes: a review of case reports. Ther Adv Psychopharmacol. 2014;4(3):130-8.
FDA. FDA Drug Safety Communication: Revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. http://www.fda.gov/drugs/drugsafety/ucm297391.htm. Accessed 29 July 2014.
AstraZeneca. Quetiapine (Seroquel) Prescribing Information. http://www1.astrazeneca-us.com/pi/Seroquel.pdf. Accessed 29 July 2014.
Arizona University-Based Center for Education and Research on Therapeutics (AZCERT). CredibleMeds—A Trusted Partner Providing Reliable Information on Medicines. http://www.crediblemeds.org/everyone/. Accessed 29 July 2014.
International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. The clinical evaluation of Qt/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs (E14). http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Guideline.pdf. Accessed 29 July 2014.
E14 Implementation Working Group. ICH E14 Guideline: the clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. Questions & Answers (R2). http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_QAs_R2_Step4.pdf. Accessed 29 July 2014.
Astrom-Lilja C, Odeberg JM, Ekman E, Hagg S. Drug-induced torsades de pointes: a review of the Swedish pharmacovigilance database. Pharmacoepidemiol Drug Saf. 2008;17:587–92.
Bednar MM, Harrigan EP, Anziano RJ, Camm AJ, Ruskin JN. The QT interval. Prog Cardiovasc Dis. 2001;43:1–45.
Viskin S, Justo D, Halkin A, Zeltser D. Long QT syndrome caused by noncardiac drugs. Prog Cardiovasc Dis. 2003;45:415–27.
Agelink MW, Majewski T, Wurthmann C, Lukas K, Ullrich H, Linka T, et al. Effects of newer atypical antipsychotics on autonomic neurocardiac function: a comparison between amisulpride, olanzapine, sertindole, and clozapine. J Clin Psychopharmacol. 2001;21:8–13.
Isbister GK, Balit CR, Macleod D, Duffull SB. Amisulpride overdose is frequently associated with QT prolongation and torsades de pointes. J Clin Psychopharmacol. 2010;30:391–5.
Joy JP, Coulter CV, Duffull SB, Isbister GK. Prediction of torsade de pointes from the QT interval: analysis of a case series of amisulpride overdoses. Clin Pharmacol Ther. 2011;90:243–5.
Coulouvrat C, Dondey-Nouvel L. Safety of amisulpride (Solian): a review of 11 clinical studies. Int Clin Psychopharmacol. 1999;14:209–18.
Stevenson RJ. Amisulpride overdose: suggested management of prolonged QTc. Emerg Med J. 2010;27:565.
Chung AK, Chua SE. Torsade de pointes associated with low-dose amisulpride: a case report. J Psychopharmacol. 2010;24:433–5.
Isbister GK, Murray L, John S, Hackett LP, Haider T, O’Mullane P, et al. Amisulpride deliberate self-poisoning causing severe cardiac toxicity including QT prolongation and torsades de pointes. Med J Aust. 2006;184:354–6.
Ward DI. Two cases of amisulpride overdose: a cause for prolonged QT syndrome. Emerg Med Australas. 2005;17:274–6.
O’Shea M, Sazhin V, Collins A. Ventricular ectopics during crossover of atypical antipsychotics. Aust N Z J Psychiatry. 2003;37:773–4.
Pedrosa GF, Grohmann R, Ruther E. Asymptomatic bradycardia associated with amisulpride. Pharmacopsychiatry. 2001;34:259–61.
Grande I, Pons A, Baeza I, Torras A, Bernardo M. QTc prolongation: is clozapine safe? Study of 82 cases before and after clozapine treatment. Hum Psychopharmacol. 2011;26:397–403.
Kang UG, Kwon JS, Ahn YM, Chung SJ, Ha JH, Koo YJ, et al. Electrocardiographic abnormalities in patients treated with clozapine. J Clin Psychiatry. 2000;61:441–6.
Cohen H, Loewenthal U, Matar M, Kotler M. Association of autonomic dysfunction and clozapine. Heart rate variability and risk for sudden death in patients with schizophrenia on long-term psychotropic medication. Br J Psychiatry. 2001;179:167–71.
Yang FD, Wang XQ, Liu XP, Zhao KX, Fu WH, Hao XR, et al. Sex difference in QTc prolongation in chronic institutionalized patients with schizophrenia on long-term treatment with typical and atypical antipsychotics. Psychopharmacology (Berl). 2011;216:9–16.
Kramer I, Rauber-Luthy C, Kupferschmidt H, Krahenbuhl S, Ceschi A. Minimal dose for severe poisoning and influencing factors in acute human clozapine intoxication: a 13-year retrospective study. Clin Neuropharmacol. 2010;33:230–4.
Nielsen J, Emborg C, Gydesen S, Dybbro J, Aagaard J, Haderup K, et al. Augmenting clozapine with sertindole: a double-blind, randomized, placebo-controlled study. J Clin Psychopharmacol. 2012;32:173–8.
Henderson DC, Fan X, Copeland PM, Sharma B, Borba CP, Forstbauer SI, et al. Ziprasidone as an adjuvant for clozapine- or olanzapine-associated medical morbidity in chronic schizophrenia. Hum Psychopharmacol. 2009;24:225–32.
Zink M, Kuwilsky A, Krumm B, Dressing H. Efficacy and tolerability of ziprasidone versus risperidone as augmentation in patients partially responsive to clozapine: a randomised controlled clinical trial. J Psychopharmacol. 2009;23:305–14.
Anil Yagcioglu AE, Kivircik Akdede BB, Turgut TI, Tumuklu M, Yazici MK, Alptekin K, et al. A double-blind controlled study of adjunctive treatment with risperidone in schizophrenic patients partially responsive to clozapine: efficacy and safety. J Clin Psychiatry. 2005;66:63–72.
Muscatello MR, Pandolfo G, Mico U, Lamberti CE, Abenavoli E, Scimeca G, et al. Augmentation of clozapine with ziprasidone in refractory schizophrenia: a double-blind, placebo-controlled study. J Clin Psychopharmacol. 2014;34:129–33.
Sacchetti E, Galluzzo A, Valsecchi P, Romeo F, Gorini B, Warrington L. Ziprasidone vs clozapine in schizophrenia patients refractory to multiple antipsychotic treatments: the MOZART study. Schizophr Res. 2009;110:80–9.
Sharma TR, Chahil R. Dose dependent, new onset QTc prolongation in a patient with paranoid schizophrenia receiving clozapine. Asian J Psychiatr. 2011;4:221–2.
Dhillon R, Bastiampillai T, Tee K, Vanlint A. Clozapine and associated QTc prolongation. Aust N Z J Psychiatry. 2011;45:1098–9.
Tanner MA, Culling W. Clozapine associated dilated cardiomyopathy. Postgrad Med J. 2003;79:412–3.
Cohen H, Loewenthal U, Matar MA, Kotler M. Reversal of pathologic cardiac parameters after transition from clozapine to olanzapine treatment: a case report. Clin Neuropharmacol. 2001;24:106–8.
Dewan V, Roth BA. Antipsychotic-induced QTc interval prolongation. Can J Psychiatry. 2004;49:646.
Potkin SG, Preskorn S, Hochfeld M, Meng X. A thorough QTc study of 3 doses of iloperidone including metabolic inhibition via CYP2D6 and/or CYP3A4 and a comparison to quetiapine and ziprasidone. J Clin Psychopharmacol. 2013;33:3–10.
Kane JM, Lauriello J, Laska E, Di MM, Wolfgang CD. Long-term efficacy and safety of iloperidone: results from 3 clinical trials for the treatment of schizophrenia. J Clin Psychopharmacol. 2008;28:S29–35.
Cutler AJ, Kalali AH, Weiden PJ, Hamilton J, Wolfgang CD. Four-week, double-blind, placebo- and ziprasidone-controlled trial of iloperidone in patients with acute exacerbations of schizophrenia. J Clin Psychopharmacol. 2008;28:S20–8.
Weiden PJ, Cutler AJ, Polymeropoulos MH, Wolfgang CD. Safety profile of iloperidone: a pooled analysis of 6-week acute-phase pivotal trials. J Clin Psychopharmacol. 2008;28:S12–9.
FDA PsychoPharmacological Drugs Advisory Committee. FDA Background on ZeldoxTM (ziprasidone hydrochloride capsules) Pfizer, Inc. http://www.fda.gov/ohrms/dockets/ac/00/backgrd/3619b1b.pdf. Accessed 29 July 2014.
Harrigan EP, Miceli JJ, Anziano R, Watsky E, Reeves KR, Cutler NR, et al. A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J Clin Psychopharmacol. 2004;24:62–9.
Suzuki Y, Sugai T, Fukui N, Watanabe J, Ono S, Tsuneyama N, et al. Sex differences in the effect of four second-generation antipsychotics on QTc interval in patients with schizophrenia. Hum Psychopharmacol. 2013;28:215–9.
Suzuki Y, Ono S, Sugai T, Fukui N, Watanabe J, Tsuneyama N, et al. Dose-dependent effects of olanzapine on QT intervals and plasma prolactin levels in Japanese patients with stable schizophrenia. Hum Psychopharmacol. 2011;26:440–3.
Suzuki Y, Sugai T, Ono S, Sawamura K, Fukui N, Watanabe J, et al. Changes in the metabolic parameters and QTc interval after switching from olanzapine to aripiprazole in Japanese patients with stable schizophrenia. J Clin Psychopharmacol. 2011;31:526–8.
Suzuki Y, Sugai T, Fukui N, Watanabe J, Ono S, Tsuneyama N, et al. Changes in QT interval after switching to quetiapine in Japanese patients with schizophrenia. Hum Psychopharmacol. 2013;28:94–6.
Watanabe J, Suzuki Y, Fukui N, Ono S, Sugai T, Tsuneyama N, et al. Increased risk of antipsychotic-related QT prolongation during night time: a 24-h holter electrocardiogram recording study. J Clin Psychopharmacol. 2012;32:18–22.
Huang CL, Su KP, Hsu HB, Pariante CM. A pilot observational crossover study of QTc interval changes associated with switching between olanzapine and risperidone. J Clin Psychiatry. 2007;68:803–5.
de Castro MJ, Fraguas D, Laita P, Moreno D, Parellada M, Pascual D, et al. QTc changes after 6 months of second-generation antipsychotic treatment in children and adolescents. J Child Adolesc Psychopharmacol. 2008;18:381–3.
Ozeki Y, Fujii K, Kurimoto N, Yamada N, Okawa M, Aoki T, et al. QTc prolongation and antipsychotic medications in a sample of 1017 patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:401–5.
Meyer-Massetti C, Vaerini S, Ratz Bravo AE, Meier CR, Guglielmo BJ. Comparative safety of antipsychotics in the WHO pharmacovigilance database: the haloperidol case. Int J Clin Pharm. 2011;33:806–14.
Ou JJ, Xu Y, Chen HH, Fan X, Gao K, Wang J, et al. Comparison of metabolic effects of ziprasidone versus olanzapine treatment in patients with first-episode schizophrenia. Psychopharmacology (Berl). 2013;225:627–35.
Simpson GM, Weiden P, Pigott T, Murray S, Siu CO, Romano SJ. Six-month, blinded, multicenter continuation study of ziprasidone versus olanzapine in schizophrenia. Am J Psychiatry. 2005;162:1535–8.
Simpson GM, Glick ID, Weiden PJ, Romano SJ, Siu CO. Randomized, controlled, double-blind multicenter comparison of the efficacy and tolerability of ziprasidone and olanzapine in acutely ill in patients with schizophrenia or schizoaffective disorder. Am J Psychiatry. 2004;161:1837–47.
Brown RR, Estoup MW. Comparison of the metabolic effects observed in patients treated with ziprasidone versus olanzapine. Int Clin Psychopharmacol. 2005;20:105–12.
Kwon JS, Mittoux A, Hwang JY, Ong A, Cai ZJ, Su TP. The efficacy and safety of 12 weeks of treatment with sertindole or olanzapine in patients with chronic schizophrenia who did not respond successfully to their previous treatments: a randomized, double-blind, parallel-group, flexible-dose study. Int Clin Psychopharmacol. 2012;27:326–35.
Czekalla J, Beasley CM Jr, Dellva MA, Berg PH, Grundy S. Analysis of the QTc interval during olanzapine treatment of patients with schizophrenia and related psychosis. J Clin Psychiatry. 2001;62:191–8.
Street JS, Clark WS, Kadam DL, Mitan SJ, Juliar BE, Feldman PD, et al. Long-term efficacy of olanzapine in the control of psychotic and behavioral symptoms in nursing home patients with Alzheimer’s dementia. Int J Geriatr Psychiatry. 2001;16(Suppl 1):S62–70.
Wright P, Birkett M, David SR, Meehan K, Ferchland I, Alaka KJ, et al. Double-blind, placebo-controlled comparison of intramuscular olanzapine and intramuscular haloperidol in the treatment of acute agitation in schizophrenia. Am J Psychiatry. 2001;158:1149–51.
Lindborg SR, Beasley CM, Alaka K, Taylor CC. Effects of intramuscular olanzapine vs. haloperidol and placebo on QTc intervals in acutely agitated patients. Psychiatry Res. 2003;119:113–23.
Breier A, Meehan K, Birkett M, David S, Ferchland I, Sutton V, et al. A double-blind, placebo-controlled dose-response comparison of intramuscular olanzapine and haloperidol in the treatment of acute agitation in schizophrenia. Arch Gen Psychiatry. 2002;59:441–8.
Meehan K, Zhang F, David S, Tohen M, Janicak P, Small J, et al. A double-blind, randomized comparison of the efficacy and safety of intramuscular injections of olanzapine, lorazepam, or placebo in treating acutely agitated patients diagnosed with bipolar mania. J Clin Psychopharmacol. 2001;21:389–97.
Meehan KM, Wang H, David SR, Nisivoccia JR, Jones B, Beasley CM Jr, et al. Comparison of rapidly acting intramuscular olanzapine, lorazepam, and placebo: a double-blind, randomized study in acutely agitated patients with dementia. Neuropsychopharmacology. 2002;26:494–504.
Lung DD, Wu AH, Gerona RR. Cardiotoxicity in a citalopram and olanzapine overdose. J Emerg Med. 2013;45:554–8.
Ritchie B, Norris ML. QTc prolongation associated with atypical antipsychotic use in the treatment of adolescent-onset anorexia nervosa. J Can Acad Child Adolesc Psychiatry. 2009;18:60–3.
Letsas KP, Sideris A, Kounas SP, Efremidis M, Korantzopoulos P, Kardaras F. Drug-induced QT interval prolongation after ciprofloxacin administration in a patient receiving olanzapine. Int J Cardiol. 2006;109:273–4.
Su KP, Lane HY, Chuang CL, Chen KP, Shen WW. Olanzapine-induced QTc prolongation in a patient with Wolff–Parkinson–White syndrome. Schizophr Res. 2004;66:191–2.
Imran N, Rampes H, Rosen S. Antipsychotic induced prolongation of QTc interval treated with magnesium. J Psychopharmacol. 2003;17:346–9.
Dineen S, Withrow K, Voronovitch L, Munshi F, Nawbary MW, Lippmann S. QTc prolongation and high-dose olanzapine. Psychosomatics. 2003;44:174–5.
Hough DW, Natarajan J, Vandebosch A, Rossenu S, Kramer M, Eerdekens M. Evaluation of the effect of paliperidone extended release and quetiapine on corrected QT intervals: a randomized, double-blind, placebo-controlled study. Int Clin Psychopharmacol. 2011;26:25–34.
Suzuki Y, Fukui N, Watanabe J, Ono S, Sugai T, Tsuneyama N, et al. QT prolongation of the antipsychotic risperidone is predominantly related to its 9-hydroxy metabolite paliperidone. Hum Psychopharmacol. 2012;27:39–42.
Meltzer HY, Bobo WV, Nuamah IF, Lane R, Hough D, Kramer M, et al. Efficacy and tolerability of oral paliperidone extended-release tablets in the treatment of acute schizophrenia: pooled data from three 6-week, placebo-controlled studies. J Clin Psychiatry. 2008;69:817–29.
Gopal S, Hough D, Karcher K, Nuamah I, Palumbo J, Berlin JA, et al. Risk of cardiovascular morbidity with risperidone or paliperidone treatment: analysis of 64 randomized, double-blind trials. J Clin Psychopharmacol. 2013;33:157–61.
Balit CR, Isbister GK, Hackett LP, Whyte IM. Quetiapine poisoning: a case series. Ann Emerg Med. 2003;42:751–8.
Eyer F, Pfab R, Felgenhauer N, Strubel T, Saugel B, Zilker T. Clinical and analytical features of severe suicidal quetiapine overdoses–a retrospective cohort study. Clin Toxicol (Phila). 2011;49:846–53.
Isbister GK, Duffull SB. Quetiapine overdose: predicting intubation, duration of ventilation, cardiac monitoring and the effect of activated charcoal. Int Clin Psychopharmacol. 2009;24:174–80.
Devlin JW, Roberts RJ, Fong JJ, Skrobik Y, Riker RR, Hill NS, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38:419–27.
Aghaienia N, Brahm NC, Lussier KM, Washington NB. Probable quetiapine-mediated prolongation of the QT interval. J Pharm Pract. 2011;24:506–12.
Barker MJ, Benitez JG, Ternullo S, Juhl GA. Acute oxcarbazepine and atomoxetine overdose with quetiapine. Vet Hum Toxicol. 2004;46:130–2.
Beelen AP, Yeo KT, Lewis LD. Asymptomatic QTc prolongation associated with quetiapine fumarate overdose in a patient being treated with risperidone. Hum Exp Toxicol. 2001;20:215–9.
Bodmer M, Burkard T, Kummer O, Beyrau R, Krahenbuhl S, Haschke M. Pharmacokinetics and pharmacodynamics of quetiapine in a patient with a massive overdose. Ther Drug Monit. 2008;30:553–6.
Digby G, Machaalany J, Malik P, Methot M, Simpson CS, Redfearn D, et al. Multifactorial QT interval prolongation. Cardiol J. 2010;17:184–8.
Digby GC, Perez Riera AR, Barbosa BR, Simpson CS, Redfearn DP, Methot M, et al. Acquired long QT interval: a case series of multifactorial QT prolongation. Clin Cardiol. 2011;34:577–82.
Furst BA, Champion KM, Pierre JM, Wirshing DA, Wirshing WC. Possible association of QTc interval prolongation with co-administration of quetiapine and lovastatin. Biol Psychiatry. 2002;51:264–5.
Gajwani P, Pozuelo L, Tesar GE. QT interval prolongation associated with quetiapine (Seroquel) overdose. Psychosomatics. 2000;41:63–5.
Gupta S, Nienhaus K, Shah SA. Quetiapine and QTc issues: a case report. J Clin Psychiatry. 2003;64:612–3.
Kurth J, Maguire G. Pediatric case report of quetiapine overdose and QTc prolongation. Ann Clin Psychiatry. 2004;16:229–31.
Nelson S, Leung JG. Torsades de pointes after administration of low-dose aripiprazole. Ann Pharmacother. 2013;47:e11.
Precourt A, Dunewicz M, Gregoire G, Williamson DR. Multiple complications and withdrawal syndrome associated with quetiapine/venlafaxine intoxication. Ann Pharmacother. 2005;39:153–6.
Strachan PM, Benoff BA. Mental status change, myoclonus, electrocardiographic changes, and acute respiratory distress syndrome induced by quetiapine overdose. Pharmacotherapy. 2006;26:578–82.
Vieweg WV, Schneider RK, Wood MA. Torsade de pointes in a patient with complex medical and psychiatric conditions receiving low-dose quetiapine. Acta Psychiatr Scand. 2005;112:318–22.
Ranjbar F, Akbarzadeh F, Ahmadi NM, Abbasnejhad M. Risperidone and corrected QT-interval prolongation in surface electrocardiogram. Pak J Biol Sci. 2012;15:496–500.
Yerrabolu M, Prabhudesai S, Tawam M, Winter L, Kamalesh M. Effect of risperidone on QT interval and QT dispersion in the elderly. Heart Dis. 2000;2:10–2.
Chiu CC, Chang WH, Huang MC, Chiu YW, Lane HY. Regular-dose risperidone on QTc intervals. J Clin Psychopharmacol. 2005;25:391–3.
Llerena A, Berecz R, Dorado P, de la Rubia A. QTc interval, CYP2D6 and CYP2C9 genotypes and risperidone plasma concentrations. J Psychopharmacol. 2004;18:189–93.
Germano E, Italiano D, Lamberti M, Guerriero L, Privitera C, D’Amico G, et al. ECG parameters in children and adolescents treated with aripiprazole and risperidone. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:23–7.
Nahshoni E, Spitzer S, Berant M, Shoval G, Zalsman G, Weizman A. QT interval and dispersion in very young children treated with antipsychotic drugs: a retrospective chart review. J Child Adolesc Psychopharmacol. 2007;17:187–94.
Page CB, Calver LA, Isbister GK. Risperidone overdose causes extrapyramidal effects but not cardiac toxicity. J Clin Psychopharmacol. 2010;30:387–90.
Margari L, Matera E, Craig F, Petruzzelli MG, Palmieri VO, Pastore A, et al. Tolerability and safety profile of risperidone in a sample of children and adolescents. Int Clin Psychopharmacol. 2013;28:177–83.
Lin CH, Kuo CC, Chou LS, Chen YH, Chen CC, Huang KH, et al. A randomized, double-blind comparison of risperidone versus low-dose risperidone plus low-dose haloperidol in treating schizophrenia. J Clin Psychopharmacol. 2010;30:518–25.
Kane JM, Potkin SG, Daniel DG, Buckley PF. A double-blind, randomized study comparing the efficacy and safety of sertindole and risperidone in patients with treatment-resistant schizophrenia. J Clin Psychiatry. 2011;72:194–204.
Azorin JM, Strub N, Loft H. A double-blind, controlled study of sertindole versus risperidone in the treatment of moderate-to-severe schizophrenia. Int Clin Psychopharmacol. 2006;21:49–56.
Chan HY, Lin WW, Lin SK, Hwang TJ, Su TP, Chiang SC, et al. Efficacy and safety of aripiprazole in the acute treatment of schizophrenia in Chinese patients with risperidone as an active control: a randomized trial. J Clin Psychiatry. 2007;68:29–36.
Lazarczyk MJ, Bhuiyan ZA, Perrin N, Giannakopoulos P. Selective acquired long QT syndrome (saLQTS) upon risperidone treatment. BMC Psychiatry. 2012;12:220.
Rajabi F, Hajsheikholeslami F, Beyraghi N. Sudden cardiac death after treatment with low dose risperidone in combination with cotrimoxazole. Asian J Psychiatr. 2011;4:218–20.
Pollak PT, Verjee ZH, Lyon AW. Risperidone-induced QT prolongation following overdose correlates with serum drug concentration and resolves rapidly with no evidence of altered pharmacokinetics. J Clin Pharmacol. 2011;51:1112–5.
Blaschke D, Parwani AS, Huemer M, Rolf S, Boldt LH, Dietz R, et al. Torsade de pointes during combined treatment with risperidone and citalopram. Pharmacopsychiatry. 2007;40:294–5.
Ravina T, Ravina P, Gutierrez J. Acquired long QT syndrome: risperidone-facilitated triggered activity and Torsades de Pointes during complete AV block. I. Int J Cardiol. 2007;116:416–20.
Tei Y, Morita T, Inoue S, Miyata H. Torsades de pointes caused by a small dose of risperidone in a terminally ill cancer patient. Psychosomatics. 2004;45:450–1.
Nandagopal JJ, Craig JM, Lippmann S. QTc prolongation: possible association with risperidone and/or haloperidol. Psychosomatics. 2003;44:521.
Ravin DS, Levenson JW. Fatal cardiac event following initiation of risperidone therapy. Ann Pharmacother. 1997;31:867–70.
Brown K, Levy H, Brenner C, Leffler S, Hamburg EL. Overdose of risperidone. Ann Emerg Med. 1993;22:1908–10.
Posey DJ, Walsh KH, Wilson GA, McDougle CJ. Risperidone in the treatment of two very young children with autism. J Child Adolesc Psychopharmacol. 1999;9:273–6.
Atmaca M, Yavuzkir M, Mermi O, Topuz M, Kanmaz E, Tezcan E. Effect of sertindole on QTc interval in patients with schizophrenia. Neurosci Lett. 2008;442:1–3.
Nielsen J, Andersen MP, Graff C, Kanters JK, Hardahl T, Dybbro J, et al. The effect of sertindole on QTD and TPTE. Acta Psychiatr Scand. 2010;121:385–8.
Pezawas L, Quiner S, Moertl D, Tauscher J, Barnas C, Kufferle B, et al. Efficacy, cardiac safety and tolerability of sertindole: a drug surveillance. Int Clin Psychopharmacol. 2000;15:207–14.
Blair J, Scahill L, State M, Martin A. Electrocardiographic changes in children and adolescents treated with ziprasidone: a prospective study. J Am Acad Child Adolesc Psychiatry. 2005;44:73–9.
Emul M, Dalkiran M, Coskun O, Yavuz R, Tosun M, Duran A, et al. P wave and QT changes among inpatients with schizophrenia after parenteral ziprasidone administration. Pharmacol Res. 2009;60:369–72.
Levy WO, Robichaux-Keene NR, Nunez C. No significant QTc interval changes with high-dose ziprasidone: a case series. J Psychiatr Pract. 2004;10:227–32.
Miceli JJ, Tensfeldt TG, Shiovitz T, Anziano RJ, O’Gorman C, Harrigan RH. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32:472–91.
Miceli JJ, Tensfeldt TG, Shiovitz T, Anziano R, O’Gorman C, Harrigan RH. Effects of oral ziprasidone and oral haloperidol on QTc interval in patients with Schizophrenia or schizoaffective disorder. Pharmacotherapy. 2010;30:127–35.
Klein-Schwartz W, Lofton AL, Benson BE, Spiller HA, Crouch BI. Prospective observational multi-poison center study of ziprasidone exposures. Clin Toxicol (Phila). 2007;45:782–6.
Biederman J, Mick E, Spencer T, Dougherty M, Aleardi M, Wozniak J. A prospective open-label treatment trial of ziprasidone monotherapy in children and adolescents with bipolar disorder. Bipolar Disord. 2007;9:888–94.
Correll CU, Lops JD, Figen V, Malhotra AK, Kane JM, Manu P. QT interval duration and dispersion in children and adolescents treated with ziprasidone. J Clin Psychiatry. 2011;72:854–60.
Delbello MP, Versavel M, Ice K, Keller D, Miceli J. Tolerability of oral ziprasidone in children and adolescents with bipolar mania, schizophrenia, or schizoaffective disorder. J Child Adolesc Psychopharmacol. 2008;18:491–9.
Findling RL, Cavus I, Pappadopulos E, Vanderburg DG, Schwartz JH, Gundapaneni BK, et al. Ziprasidone in adolescents with schizophrenia: results from a placebo-controlled efficacy and long-term open-extension study. J Child Adolesc Psychopharmacol. 2013;23:531–44.
Findling RL, Cavus I, Pappadopulos E, Vanderburg DG, Schwartz JH, Gundapaneni BK, et al. Efficacy, long-term safety, and tolerability of ziprasidone in children and adolescents with bipolar disorder. J Child Adolesc Psychopharmacol. 2013;23:545–57.
Malone RP, Delaney MA, Hyman SB, Cater JR. Ziprasidone in adolescents with autism: an open-label pilot study. J Child Adolesc Psychopharmacol. 2007;17:779–90.
Sallee FR, Miceli JJ, Tensfeldt T, Robarge L, Wilner K, Patel NC. Single-dose pharmacokinetics and safety of ziprasidone in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2006;45:720–8.
Centorrino F, MacLean E, Salvatore P, Kidwell JE, Fogarty KV, Berry JM, et al. Ziprasidone: first year experience in a hospital setting. J Psychiatr Pract. 2004;10:361–7.
Goff DC, McEvoy JP, Citrome L, Mech AW, Bustillo JR, Gil R, et al. High-dose oral ziprasidone versus conventional dosing in schizophrenia patients with residual symptoms: the ZEBRAS study. J Clin Psychopharmacol. 2013;33:485–90.
Kudla D, Lambert M, Domin S, Kasper S, Naber D. Effectiveness, tolerability, and safety of ziprasidone in patients with schizophrenia or schizoaffective disorder: results of a multi-centre observational trial. Eur Psychiatry. 2007;22:195–202.
Mencacci C. Efficacy and tolerability of switching to ziprasidone in italian patients with acute exacerbation of schizophrenia: an open-label trial. Pharmacopsychiatry. 2012;45:236–40.
Preval H, Klotz SG, Southard R, Francis A. Rapid-acting IM ziprasidone in a psychiatric emergency service: a naturalistic study. Gen Hosp Psychiatry. 2005;27:140–4.
Zhao T, Park TW, Yang JC, Huang GB, Kim MG, Lee KH, et al. Efficacy and safety of ziprasidone in the treatment of first-episode psychosis: an 8-week, open-label, multicenter trial. Int Clin Psychopharmacol. 2012;27:184–90.
Greco KE, Tune LE, Brown FW, Van Horn WA. A retrospective study of the safety of intramuscular ziprasidone in agitated elderly patients. J Clin Psychiatry. 2005;66:928–9.
Rais AR, Williams K, Rais T, Singh T, Tamburrino M. Use of intramuscular ziprasidone for the control of acute psychosis or agitation in an inpatient geriatric population: an open-label study. Psychiatry (Edgmont). 2010;7:17–24.
Papakostas GI, Petersen TJ, Nierenberg AA, Murakami JL, Alpert JE, Rosenbaum JF, et al. Ziprasidone augmentation of selective serotonin reuptake inhibitors (SSRIs) for SSRI-resistant major depressive disorder. J Clin Psychiatry. 2004;65:217–21.
Potkin SG, Ogasa M, Cucchiaro J, Loebel A. Double-blind comparison of the safety and efficacy of lurasidone and ziprasidone in clinically stable outpatients with schizophrenia or schizoaffective disorder. Schizophr Res. 2011;132:101–7.
Kane JM, Khanna S, Rajadhyaksha S, Giller E. Efficacy and tolerability of ziprasidone in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol. 2006;21:21–8.
Camm AJ, Karayal ON, Meltzer H, Kolluri S, O’Gorman C, Miceli J, et al. Ziprasidone and the corrected QT interval: a comprehensive summary of clinical data. CNS Drugs. 2012;26:351–65.
Berling I, Isbister GK, Calver L, Clunas S. Digital Holter measurement of QT prolongation in ziprasidone overdose. Clin Toxicol (Phila). 2011;49:694–6.
Witsil JC, Zell-Kanter M, Mycyk MB. Single-dose ziprasidone associated with QT interval prolongation. Am J Emerg Med. 2012;30:837.e1–2.
Alipour A, Cruz R, Lott RS. Torsade de pointes after ziprasidone overdose with coingestants. J Clin Psychopharmacol. 2010;30:76–7.
Oldham MA, Catalano G, Catalano MC, Ross MM. QTc prolongation associated with ziprasidone overdose. CNS Spectr. 2008;13:E1.
Eker SS, Sarandol A, Akkaya C, Sivrioglu EY, Kirli S. The potential relationship between QTc interval prolongation and ziprasidone treatment: three cases. J Psychopharmacol. 2009;23:993–6.
Bentley ML, Biscardi FH, Butcher C, Levitov A. Inadvertent administration of intravenous ziprasidone leading to bradycardia and QT interval prolongation. Ann Pharmacother. 2008;42:902–3.
Manini AF, Raspberry D, Hoffman RS, Nelson LS. QT prolongation and Torsades de Pointes following overdose of ziprasidone and amantadine. J Med Toxicol. 2007;3:178–81.
Heinrich TW, Biblo LA, Schneider J. Torsades de pointes associated with ziprasidone. Psychosomatics. 2006;47:264–8.
Simpson BR, Albanese RP Jr. Ziprasidone and hypokalemia: a case of 2 predisposing factors for QTc prolongation without development of torsades de pointes. Prim Care Companion J Clin Psychiatry. 2005;7:134–6.
Jaffe R, Leavitt R, Wind T. QTc prolongation in multiple drug overdose. J Clin Psychopharmacol. 2004;24:348–50.
Biswas AK, Zabrocki LA, Mayes KL, Morris-Kukoski CL. Cardiotoxicity associated with intentional ziprasidone and bupropion overdose. J Toxicol Clin Toxicol. 2003;41:101–4.
Castro VM, Clements CC, Murphy SN, Gainer VS, Fava M, Weilburg JB, et al. QT interval and antidepressant use: a cross sectional study of electronic health records. BMJ. 2013;346:f288.
Dubnov-Raz G, Juurlink DN, Fogelman R, Merlob P, Ito S, Koren G, et al. Antenatal use of selective serotonin-reuptake inhibitors and QT interval prolongation in newborns. Pediatrics. 2008;122:e710–5.
Grundemar L, Wohlfart B, Lagerstedt C, Bengtsson F, Eklundh G. Symptoms and signs of severe citalopram overdose. Lancet. 1997;349:1602.
Jimmink A, Caminada K, Hunfeld NG, Touw DJ. Clinical toxicology of citalopram after acute intoxication with the sole drug or in combination with other drugs: overview of 26 cases. Ther Drug Monit. 2008;30:365–71.
Hayes BD, Klein-Schwartz W, Clark RF, Muller AA, Miloradovich JE. Comparison of toxicity of acute overdoses with citalopram and escitalopram. J Emerg Med. 2010;39:44–8.
Yilmaz Z, Ceschi A, Rauber-Luthy C, Sauer O, Stedtler U, Prasa D, et al. Escitalopram causes fewer seizures in human overdose than citalopram. Clin Toxicol (Phila). 2010;48:207–12.
Isbister GK, Bowe SJ, Dawson A, Whyte IM. Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose. J Toxicol Clin Toxicol. 2004;42:277–85.
Kelly CA, Dhaun N, Laing WJ, Strachan FE, Good AM, Bateman DN. Comparative toxicity of citalopram and the newer antidepressants after overdose. J Toxicol Clin Toxicol. 2004;42:67–71.
Waring WS, Graham A, Gray J, Wilson AD, Howell C, Bateman DN. Evaluation of a QT nomogram for risk assessment after antidepressant overdose. Br J Clin Pharmacol. 2010;70:881–5.
Rasmussen SL, Overo KF, Tanghoj P. Cardiac safety of citalopram: prospective trials and retrospective analyses. J Clin Psychopharmacol. 1999;19:407–15.
Agosti S, Casalino L, Bertero G, Burrone A, Brunelli C, Morelloni S. Citalopram and levosulpiride: a dangerous drug combination for QT prolongation. Am J Emerg Med. 2013;31:1624.e1–2.
Deshmukh A, Ulveling K, Alla V, Abuissa H, Airey K. Prolonged QTc interval and torsades de pointes induced by citalopram. Tex Heart Inst J. 2012;39:68–70.
Unterecker S, Warrings B, Deckert J, Pfuhlmann B. Correlation of QTc interval prolongation and serum level of citalopram after intoxication–a case report. Pharmacopsychiatry. 2012;45:30–4.
Liotier J, Coudore F. Drug monitoring of a case of citalopram overdosage. Drug Chem Toxicol. 2011;34:420–3.
Fayssoil A, Issi J, Guerbaa M, Raynaud JC, Heroguelle V. Torsade de pointes induced by citalopram and amiodarone. Ann Cardiol Angeiol (Paris). 2011;60:165–8.
de Gregorio C, Morabito G, Cerrito M, Dattilo G, Oreto G. Citalopram-induced long QT syndrome and torsade de pointes: role for concomitant therapy and disease. Int J Cardiol. 2011;148:226–8.
Venkatraman N, O’Neil D, Hall AP. Life-threatening overdose with lamotrigine, citalopram, and chlorpheniramine. J Postgrad Med. 2008;54:316–7.
Tarabar AF, Hoffman RS, Nelson L. Citalopram overdose: late presentation of torsades de pointes (TdP) with cardiac arrest. J Med Toxicol. 2008;4:101–5.
Kanjanauthai S, Kanluen T, Chareonthaitawee P. Citalopram induced torsade de pointes, a rare life threatening side effect. Int J Cardiol. 2008;131:e33–4.
Engebretsen KM, Harris CR, Wood JE. Cardiotoxicity and late onset seizures with citalopram overdose. J Emerg Med. 2003;25:163–6.
Catalano G, Catalano MC, Epstein MA, Tsambiras PE. QTc interval prolongation associated with citalopram overdose: a case report and literature review. Clin Neuropharmacol. 2001;24:158–62.
Bucklin MH, Gorodetsky RM, Wiegand TJ. Prolonged lipemia and pancreatitis due to extended infusion of lipid emulsion in bupropion overdose. Clin Toxicol (Phila). 2013;51:896–8.
Altin T, Ozcan O, Turhan S, Ozdemir AO, Akyurek O, Karaoguz R, et al. Torsade de pointes associated with moxifloxacin: a rare but potentially fatal adverse event. Can J Cardiol. 2007;23:907–8.
van Gorp F, Duffull S, Hackett LP, Isbister GK. Population pharmacokinetics and pharmacodynamics of escitalopram in overdose and the effect of activated charcoal. Br J Clin Pharmacol. 2012;73:402–10.
Hanash JA, Hansen BH, Hansen JF, Nielsen OW, Rasmussen A, Birket-Smith M. Cardiovascular safety of one-year escitalopram therapy in clinically nondepressed patients with acute coronary syndrome: results from the DEpression in patients with Coronary ARtery Disease (DECARD) trial. J Cardiovasc Pharmacol. 2012;60:397–405.
Thase ME, Larsen KG, Reines E, Kennedy SH. The cardiovascular safety profile of escitalopram. Eur Neuropsychopharmacol. 2013;23:1391–400.
Mohammed R, Norton J, Geraci SA, Newman DB, Koch CA. Prolonged QTc interval due to escitalopram overdose. J Miss State Med Assoc. 2010;51:350–3.
Baranchuk A, Simpson CS, Methot M, Gibson K, Strum D. Corrected QT interval prolongation after an overdose of escitalopram, morphine, oxycodone, zopiclone and benzodiazepines. Can J Cardiol. 2008;24:e38–40.
Scharko AM, Schumacher J. Prolonged QTc interval in a 14-year-old girl with escitalopram overdose. J Child Adolesc Psychopharmacol. 2008;18:297–8.
Tseng PT, Lee Y, Lin YE, Lin PY. Low-dose escitalopram for 2 days associated with corrected QT interval prolongation in a middle-aged woman: a case report and literature review. Gen Hosp Psychiatry. 2012;34:210–5.
Beyenburg S, Schonegger K. Severe bradycardia in a stroke patient caused by a single low dose of escitalopram. Eur Neurol. 2007;57:50–1.
Schreffler SM, Marraffa JM, Stork CM, Mackey J. Sodium channel blockade with QRS widening after an escitalopram overdose. Pediatr Emerg Care. 2013;29:998–1001.
Zhao Q, Wojcik MA, Parier JL, Pesco-Koplowitz L. Influence of coadministration of fluoxetine on cisapride pharmacokinetics and QTc intervals in healthy volunteers. Pharmacotherapy. 2001;21:149–57.
Baker B, Dorian P, Sandor P, Shapiro C, Schell C, Mitchell J, et al. Electrocardiographic effects of fluoxetine and doxepin in patients with major depressive disorder. J Clin Psychopharmacol. 1997;17:15–21.
Adetunji B, Basil B, Mathews M, Osinowo T. Should the Physician’s Desk Reference contraindicate the use of chlorpromazine-fluoxetine combination? A case report. J Clin Psychopharmacol. 2006;26:438.
Wilting I, Smals OM, Holwerda NJ, Meyboom RH, de Bruin ML, Egberts TC. QTc prolongation and torsades de pointes in an elderly woman taking fluoxetine. Am J Psychiatry. 2006;163:325.
Dubnov G, Fogelman R, Merlob P. Prolonged QT interval in an infant of a fluoxetine treated mother. Arch Dis Child. 2005;90:972–3.
Suchard JR. Fluoxetine overdose-induced seizure. West J Emerg Med. 2008;9:154–6.
Varriale P. Fluoxetine (Prozac) as a cause of QT prolongation. Arch Intern Med. 2001;161:612.
Nykamp DL, Blackmon CL, Schmidt PE, Roberson AG. QTc prolongation associated with combination therapy of levofloxacin, imipramine, and fluoxetine. Ann Pharmacother. 2005;39:543–6.
Appleby M, Mbewu A, Clarke B. Fluoxetine and ventricular torsade–is there a link? Int J Cardiol. 1995;49:178–80.
Michalets EL, Smith LK, Van Tassel ED. Torsade de pointes resulting from the addition of droperidol to an existing cytochrome P450 drug interaction. Ann Pharmacother. 1998;32:761–5.
Graudins A, Vossler C, Wang R. Fluoxetine-induced cardiotoxicity with response to bicarbonate therapy. Am J Emerg Med. 1997;15:501–3.
Martin DE, Zussman BD, Everitt DE, Benincosa LJ, Etheredge RC, Jorkasky DK. Paroxetine does not affect the cardiac safety and pharmacokinetics of terfenadine in healthy adult men. J Clin Psychopharmacol. 1997;17:451–9.
Kuhs H, Rudolf GA. Cardiovascular effects of paroxetine. Psychopharmacology (Berl). 1990;102:379–82.
Edwards JG, Goldie A, Papayanni-Papasthatis S. Effect of paroxetine on the electrocardiogram. Psychopharmacology (Berl). 1989;97:96–8.
Nelson JC, Lu PY, Martynov O, Yu JY, Mallinckrodt CH, Detke MJ. The safety and tolerability of duloxetine compared with paroxetine and placebo: a pooled analysis of 4 clinical trials. Prim Care Companion J Clin Psychiatry. 2006;8:212–9.
Krulewicz S, Carpenter DJ, Fong R, Horrigan JP, Lipschitz A, Perera P, et al. Analysis of electrocardiographic data following use of paroxetine in pediatric depression and obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2006;45:422–30.
Alderman J. Coadministration of sertraline with cisapride or pimozide: an open-label, nonrandomized examination of pharmacokinetics and corrected QT intervals in healthy adult volunteers. Clin Ther. 2005;27:1050–63.
Glassman AH, O’Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT Jr, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701–9.
Wilens TE, Biederman J, March JS, Wolkow R, Fine CS, Millstein RB, et al. Absence of cardiovascular adverse effects of sertraline in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1999;38:573–7.
Guy S, Silke B. The electrocardiogram as a tool for therapeutic monitoring: a critical analysis. J Clin Psychiatry. 1990;51(Suppl B):37–9.
Patane S, Marte F, Di BG. QT interval prolongation and torsade de pointes. Int J Cardiol. 2009;131:e51–3.
de Boer RA, van Dijk TH, Holman ND, van Melle JP. QT interval prolongation after sertraline overdose: a case report. BMC Emerg Med. 2005;5:5.
Mbaya P, Alam F, Ashim S, Bennett D. Cardiovascular effects of high dose venlafaxine XL in patients with major depressive disorder. Hum Psychopharmacol. 2007;22:129–33.
Johnson EM, Whyte E, Mulsant BH, Pollock BG, Weber E, Begley AE, et al. Cardiovascular changes associated with venlafaxine in the treatment of late-life depression. Am J Geriatr Psychiatry. 2006;14:796–802.
Isbister GK. Electrocardiogram changes and arrhythmias in venlafaxine overdose. Br J Clin Pharmacol. 2009;67:572–6.
Chan AN, Gunja N, Ryan CJ. A comparison of venlafaxine and SSRIs in deliberate self-poisoning. J Med Toxicol. 2010;6:116–21.
Batista M, Dugernier T, Simon M, Haufroid V, Capron A, Fonseca S, et al. The spectrum of acute heart failure after venlafaxine overdose. Clin Toxicol (Phila). 2013;51:92–5.
Bosse GM, Spiller HA, Collins AM. A fatal case of venlafaxine overdose. J Med Toxicol. 2008;4:18–20.
Letsas K, Korantzopoulos P, Pappas L, Evangelou D, Efremidis M, Kardaras F. QT interval prolongation associated with venlafaxine administration. Int J Cardiol. 2006;109:116–7.
Wang Y, Pan G, Balch A. Bias and variance evaluation of QT interval correction methods. J Biopharm Stat. 2008;18:427–50.
Malik M. Problems of heart rate correction in assessment of drug-induced QT interval prolongation. J Cardiovasc Electrophysiol. 2001;12:411–20.
Hingorani P, Karnad DR, Natekar M, Kothari S, Narula D, Lokhandwala Y. QTc interval and its variability in patients with schizophrenia and healthy subjects: implications for a thorough QT study. Int J Neuropsychopharmacol. 2012;15:1535–40.
Nielsen J. QTc prolongation and clozapine: fact or artefact? Aust N Z J Psychiatry. 2012;46:793–4.
Law D, Mohan T, Bastiampillai T, Dhillon R. Clozapine rechallenge following QTc prolongation. Aust N Z J Psychiatry. 2013.
Chung AK, Chua SE. Effects on prolongation of Bazett’s corrected QT interval of seven second-generation antipsychotics in the treatment of schizophrenia: a meta-analysis. J Psychopharmacol. 2011;25:646–66.
van Driel ML, De SA, De MJ, Christiaens T. Searching for unpublished trials in Cochrane reviews may not be worth the effort. J Clin Epidemiol. 2009;62:838–44.
Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–62.
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. The non-clinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals (S7B). http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S7B/Step4/S7B_Guideline.pdf. Accessed 29 July 2014.
Farkas AS, Nattel S. Minimizing repolarization-related proarrhythmic risk in drug development and clinical practice. Drugs. 2010;70:573–603.
Panikkath R, Reinier K, Uy-Evanado A, Teodorescu C, Hattenhauer J, Mariani R, et al. Prolonged Tpeak-to-Tend interval on the resting ECG is associated with increased risk of sudden cardiac death. Circ Arrhythm Electrophysiol. 2011;4:441–7.
Hondeghem LM. QT prolongation is an unreliable predictor of ventricular arrhythmia. Heart Rhythm. 2008;5:1210–2.
Davey P. How to correct the QT interval for the effects of heart rate in clinical studies. J Pharmacol Toxicol Methods. 2002;48:3–9.
Luo S, Michler K, Johnston P, Macfarlane PW. A comparison of commonly used QT correction formulae: the effect of heart rate on the QTc of normal ECGs. J Electrocardiol. 2004;37 Suppl:81–90.
Nielsen J, Graff C, Kanters JK, Toft E, Taylor D, Meyer JM. Assessing QT interval prolongation and its associated risks with antipsychotics. CNS Drugs. 2011;25:473–90.
Postema PG, De Jong JS, Van der Bilt IA, Wilde AA. Accurate electrocardiographic assessment of the QT interval: teach the tangent. Heart Rhythm. 2008;5:1015–8.
Chan A, Isbister GK, Kirkpatrick CM, Dufful SB. Drug-induced QT prolongation and torsades de pointes: evaluation of a QT nomogram. QJM. 2007;100:609–15.
Straus SM, Kors JA, de Bruin ML, van der Hooft CS, Hofman A, Heeringa J, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–7.
Allen LaPointe NM, Curtis LH, Chan KA, Kramer JM, Lafata JE, Gurwitz JH et al. Frequency of high-risk use of QT-prolonging medications. Pharmacoepidemiol Drug Saf 2006; 15:361-368.
Curtis LH, Ostbye T, Sendersky V, Hutchison S, Allen LaPointe NM, Al-Khatib SM, et al. Prescription of QT-prolonging drugs in a cohort of about 5 million outpatients. Am J Med. 2003;114:135–41.
Mason JW, Ramseth DJ, Chanter DO, Moon TE, Goodman DB, Mendzelevski B. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J Electrocardiol. 2007;40:228–34.
Carella MJ, Mantz SL, Rovner DR, Willis PW III, Gossain VV, Bouknight RR, et al. Obesity, adiposity, and lengthening of the QT interval: improvement after weight loss. Int J Obes Relat Metab Disord. 1996;20:938–42.
Soydinc S, Davutoglu V, Akcay M. Uncomplicated metabolic syndrome is associated with prolonged electrocardiographic QTc interval and QTc dispersion. Ann Noninvasive Electrocardiol. 2006;11:313–7.
Tasolar H, Balli M, Bayramoglu A, Otlu YO, Cetin M, Altun B, et al. Effect of Smoking on Tp-e interval, Tp-e/QT and Tp-e/QTc ratios as indices of ventricular arrhythmogenesis. Heart Lung Circ. 2014.
Erbas O, Yilmaz M. Metoprolol and diltiazem ameliorate ziprasidone-induced prolonged corrected QT interval in rats. Toxicol Ind Health. 2013.
Ng TM, Olsen KM, McCartan MA, Puumala SE, Speidel KM, Miller MA, et al. Drug-induced QTc-interval prolongation in the intensive care unit: incidence and predictors. J Pharm Pract. 2010;23:19–24.
Mahida S, Hogarth AJ, Cowan C, Tayebjee MH, Graham LN, Pepper CB. Genetics of congenital and drug-induced long QT syndromes: current evidence and future research perspectives. J Interv Card Electrophysiol. 2013;37:9–19.
Gouas L, Nicaud V, Berthet M, Forhan A, Tiret L, Balkau B, et al. Association of KCNQ1, KCNE1, KCNH2 and SCN5A polymorphisms with QTc interval length in a healthy population. Eur J Hum Genet. 2005;13:1213–22.
Al-Zaiti SS, Fallavollita JA, Wu YW, Tomita MR, Carey MG. Electrocardiogram-based predictors of clinical outcomes: A meta-analysis of the prognostic value of ventricular repolarization. Heart Lung. 2014.
Nielsen J, Graff C, Hardahl T, Andersen MP, Kristoffersen J, Struijk JJ, et al. Sertindole causes distinct electrocardiographic T-wave morphology changes. Eur Neuropsychopharmacol. 2009;19:702–7.
Axelsson R, Aspenstrom G. Electrocardiographic changes and serum concentrations in thioridazine-treated patients. J Clin Psychiatry. 1982;43:332–5.
Conflict of interest
The authors do not have any conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
W. Victor Vieweg, MD, an author on this article, died on 7 October 2013, in Charlottesville, VA, USA. Dr Vieweg, who began his career as a cardiologist, later specialized in psychiatry. He was a prolific researcher, especially in the interface of psychiatry and medicine and often ahead of the times in conceptualizing disease and treatment models. In his death, both psychiatry and medicine have lost a great scholar and teacher.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hasnain, M., Vieweg, W.V.R. QTc Interval Prolongation and Torsade de Pointes Associated with Second-Generation Antipsychotics and Antidepressants: A Comprehensive Review. CNS Drugs 28, 887–920 (2014). https://doi.org/10.1007/s40263-014-0196-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-014-0196-9