Abstract
Temocillin, a 6-α-methoxy derivative of ticarcillin, is a forgotten antibiotic that has recently been rediscovered, and issues about clinical breakpoints and optimal therapeutic regimens are still ongoing. Temocillin spectrum is almost restricted to Enterobacteriaceae. The addition of the α-methoxy moiety on ticarcillin confers resistance to hydrolysis by Ambler classes A and C β-lactamases (extended spectrum β-lactamases, Klebsiella pneumoniae carbapenemase and AmpC hyperproduced enzymes). Temocillin is bactericidal, and the effect of inoculum size on its activity is relatively mild. The proportion of spontaneous resistant mutants in vitro to temocillin is low, as found in vivo. After intravenous infusion, temocillin showed a prolonged elimination half-life of approximately 5 h. The percentage of protein binding of temocillin is high (approximately 80%), and is concentration-dependent. Temocillin clearance is mainly renal, and urinary recovery is high, ranging from 72 to 82% after 24 h. Furthermore, the penetration of temocillin into bile and peritoneal fluid is high, but poor into cerebrospinal fluid. The cumulative percentage of a 24-h period during which the free drug concentration exceeds the minimum inhibitory concentration (fT > MIC) at steady-state pharmacokinetic conditions seems to be the best pharmacokinetic/pharmacodynamic (PK/PD) index correlating with temocillin efficacy. An fT > MIC of 40–50% is associated with antibacterial effect and survival in vivo. Monte Carlo simulations performed in critically ill patients showed that the 2 g every 12 h and 2 g every 8 h regimens provide a 95% probability of target attainment of 40% fT > MIC up to an MIC of 8 mg/L. In less severely ill patients or in specific foci of infection, such as urinary tract infection, a 4 g daily regimen should be adequate for strains with temocillin MIC up to 16 mg/L. Data regarding actual wild-type MIC distribution, clinical efficacy, PK profiling in volunteers or patients, and PD targets are scarce, and further studies are required to support appropriate dosing recommendations and determination of clinical breakpoints.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Temocillin is an old antibiotic that has been rediscovered because of its limited spectrum of activity focused on resistant enterobacteriaceae, which are worrying bacteria worldwide. |
Optimal dosing regimens of temocillin, according to bacterial susceptibility and patient characteristics, are not yet well-defined. |
Additional pharmacokinetic studies are required in infected, non-intensive care unit patients. |
1 Introduction
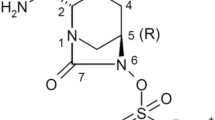
Temocillin (BRL 17421) is a 6-α-methoxy derivative of ticarcillin belonging to the β-lactam family (Fig. 1), marketed as Negaban® (Eumedica, Brussels, Belgium), which contains an R:S epimer ratio of approximately 65:35. Temocillin exhibits a low molecular weight (414 Da) and high water solubility (logD at pH 7.4 = −5.19) [1].
Temocillin was developed in the 1980s but was quickly abandoned due to its narrow spectrum, which, at that time, was perceived as a major drawback. It was mainly used as an orphan drug for the treatment of Burkholderia cepacia infections in patients with cystic fibrosis. As the epidemiologic threat of extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBL-E) has grown, interest in neglected and disused antibiotics, such as colistin, cefoxitin, fosfomycin and temocillin, has increased [2]. Indeed, the singular spectrum of temocillin, almost restricted to Enterobacteriaceae, and its resistance to hydrolysis by numerous β-lactamases are now recognised as an important ecological and bacteriological advantage.
To date, temocillin is available in numerous countries (UK, Belgium, Luxembourg and France) and is recommended for the treatment of septicaemia, urinary tract infection (UTI) and lower respiratory tract infection where susceptible Gram-negative bacilli are suspected or confirmed. French guidelines for the management of acute UTI restricted its use for documented UTI caused by proven susceptible ESBL-E [3].
We reviewed the antimicrobial activity, pharmacodynamics (PD) and pharmacokinetics (PK) of temocillin, and the impact of the temocillin PK/PD profile on its therapeutic use in clinical practice and in specific populations.
2 Antimicrobial Activity
The addition of the α-methoxy moiety on ticarcillin structure has some microbiological consequences.
-
1.
Temocillin has reduced affinity to penicillin-binding protein (PBP)1, PBP2 and PBP3, but binds tightly to PBP5 and PBP6 [4, 5], explaining the restricted spectrum to Enterobacteriaceae and the lack of activity against Gram-positive bacteria and anaerobic bacteria [6,7,8,9,10,11,12,13,14,15,16]. Remarkably, Neisseria gonorrhoea is usually sensitive to temocillin (Table 1) [17].
-
2.
By blocking the entry of a water molecule into a serine-dependent active site of β-lactamase [18], the α-methoxy radical confers resistance to hydrolysis by Ambler classes A and C β-lactamase (i.e ESBL, Klebsiella pneumoniae carbapenemase [KPC] and AmpC hyperproduced enzymes), but not to class B metalloenzymes or some class D enzymes (Table 2) [19,20,21,22,23,24,25,26,27,28].
The MexAB-OprM-driven efflux contributes to the intrinsic resistance of Pseudomonas aeruginosa to temocillin, but some strains (15%) with mutations in mexA or mexB, isolated from cystic fibrosis patients, are sensitive to temocillin, with minimum inhibitory concentrations (MICs) ≤8 mg/L [29, 30].
3 Pharmacodynamics
3.1 Relationship Between Concentrations and Bactericidal Activity
Temocillin is bactericidal, like all β-lactams, with minimal bactericidal concentration (MBC) values being the same or twofold greater than MIC values for Enterobacteriaceae [6, 7]. Bactericidal activity under discontinuous exposure to temocillin concentrations in vitro, simulating serum PKs of 2 g every 12 h in humans, showed a rapid bactericidal effect (<8 h) against Enterobacteriaceae, with an MIC of 2 mg/L [10]. Nevertheless, bactericidal kinetics are slower against Enterobacteriaceae with chromosomic AmpC β-lactamase, despite similar temocillin MICs [8, 10]. The in vitro postantibiotic effect of temocillin has not yet been specifically assessed, but, as for other penicillins, it is expected to be absent against Enterobacteriaceae.
3.2 Inoculum Effect
The effect of inoculum size on temocillin activity is relatively mild and β-lactamase-producing-dependent. Indeed, minimal MIC and MBC changes are observed, with growing inoculum size between 103 and 107 colony-forming units (CFU)/mL for Escherichia coli and Klebsiella pneumoniae, whereas inoculum effect is markedly higher for Enterobacteriaceae with chromosomic AmpC β-lactamase (i.e. Enterobacter cloacae, Serratia marcescens, Morganella moraganii, Providencia stuartii), mostly on MBC, with an 8- to 16-fold increase between 105 and 107 CFU/mL [6]. A modest inoculum effect was observed on MIC with emerging β-lactamase-producing strains (i.e. CTX-M-15, KPC), with a two- to fourfold MIC increase, but data regarding inoculum effect on MBC are lacking for such strains [26, 31].
3.3 Protein-Binding Impact
Since temocillin is highly bound to human serum proteins (≈80%), the impact of protein binding on MIC was assessed in vitro by adding human serum or albumin to the medium. A two- to fourfold increase of MIC was observed in the presence of human serum, but the maximal killing rate (obtained at a concentration of four times the MIC) was not impaired by the addition of human serum, suggesting that the impact of protein binding on temocillin activity in vivo may be limited [6, 7, 31].
3.4 Mutant Selection Frequency
The proportion of spontaneous resistant mutants in vitro to temocillin is low (ranging from 1 × 10−8 to 1 × 10−10), is not affected by β-lactamase production, and appears only after repeated (six to eight) subcultures [7, 26, 31]. These data are consistent with data found in vivo, with no temocillin mutant selection after treatment for 24 h in two animal models with two different inoculum sizes (105 and 107 CFU/mL) [31, 32]. Only one case of breakthrough bacteraemia during temocillin treatment has been reported, occurring in a K. pneumoniae-infected patient with transient renal dysfunction and probable insufficient dosing regimen (1 g once daily) [33]. In such mutants, resistance mechanisms to temocillin are still poorly studied.
3.5 Drug-to-Drug Interaction
3.5.1 Antibiotic Interaction Effect
The in vitro combination effect of temocillin with aminoglycosides produces no synergistic action against Enterobacteriaceae [6, 34], as against Burkholderia cepacia [11].
3.5.2 Temocillin Compatibility with Other Drugs
Temocillin compatibility with other drugs under conditions mimicking their coadministration through the same line of infusion was assessed in one study. The main incompatibilities were with carbapenems, piperacillin/tazobactacam and amoxicillin/clavulanate for chemical incompatibilities, and vancomycin, ciprofloxacin, propofol, and midalozam for physical incompatibilities [35]. These incompatibilities should be kept in mind as the combination of two or three antimicrobial agents through prolonged or continuous infusion is recommended for treating infections by multidrug-resistant, Gram-negative bacteria, especially in critically ill patients [36].
4 Pharmacokinetics (PKs)
4.1 PK Parameters in Healthy Volunteers
No oral formulation of temocillin is available; an o-methyl phenyl ester was studied but is not presently marketed [37]. Temocillin is administrated intramuscularly or intravenously at doses of 1 or 2 g two or three times daily. The percentage of protein binding of temocillin is high (approximately 80%), and is concentration-dependent. Decreased serum binding was observed with increasing doses of temocillin (i.e. 85% after intravenous administration of 500 mg vs. 63% after intravenous administration of 2 g) [7, 38, 39]. Indeed, Overbosch et al. found that there was only one binding site for temocillin on each albumin molecule [38]. Key PK parameters from 10 healthy subjects are reported in Table 3 [39].
As a consequence of protein-binding saturation, the volume of distribution increases with increasing temocillin doses [39, 40]. The urinary concentration of temocillin after 500 mg twice daily is approximately 500 mg/L [7]. The penetration of temocillin in prostate tissue was assessed in 20 patients receiving a 2 g intravenous dose of temocillin prior to prostatectomy. The mean temocillin concentration was 38 and 27 mg/kg in peripheral and central prostate tissue, respectively [41]. Furthermore, the bile concentrations of temocillin were assessed in two studies in patients with biliary tract diseases [42, 43], and were up to eight to ten times the corresponding serum concentrations, but with huge variations in the bile/serum concentration ratios. Two studies focused on the intraperitoneal penetration of temocillin. Mean temocillin concentrations in peritoneal fluid were 46 mg/L 2 h after intravenous administration of 1 g, and 52 mg/L 4 h after intravenous administration of 2 g, corresponding to a tissue/plasma area under the curve over 24 h ratio of 0.6. Remarkably, temocillin accumulation was noted with a tissue/plasma concentration ratio of 1.7, 12 h after infusion [44, 45]. Cowan et al. studied lung tissue penetration of temocillin in eight patients undergoing elective resection of the whole or part of the lung. The serum and lung tissue concentrations of temocillin were determined 30 min after a 2 g intravenous bolus of temocillin. The mean lung tissue concentration was 45 mg/kg, corresponding to a tissue/serum concentration ratio of 0.26 [46]. Temocillin concentration in sputum after administration of 2 g twice daily was approximately 2 mg/L [47]. Brückner et al. assessed the diffusion of temocillin in cerebrospinal fluid (CSF) in four neurosurgical patients with external ventricular drains and four patients with meningitis. The temocillin dose was 2 g twice daily. Temocillin concentration in CSF was low, with a CSF/serum concentration ratio of approximately 10%, albeit this ratio was higher in meningitis patients (15%, versus 8% in patients without meningitis). No CSF temocillin accumulation was observed [48]. Table 4 summarises temocillin concentrations in various tissues and compartments.
After intravenous infusion, temocillin showed a prolonged elimination half-life of approximately 5 h, regardless of dose infusion (i.e. 0.5, 1 or 2 g). Temocillin clearance is mainly renal. Glomerular filtration is more important than tubular excretion as probenecid slightly affects renal clearance of temocillin [38]. Renal clearance of temocillin increases with dose, but, as serum binding decreases, the renal clearance of free temocillin is not affected by dose variation [38]. The urinary recovery of unmetabolised temocillin is high, ranging from 72 to 82% after 24 h [7].
4.2 PKs in Specific Populations
4.2.1 Patients with Renal Impairment
As temocillin is primarily excreted by the renal route, impairment of renal function altered temocillin PKs. Nevertheless, peak serum concentration and distribution volume at steady state are not influenced by renal impairment. Temocillin clearance is found to be linearly correlated with creatinine clearance; therefore, several dosage adjustments have been proposed with dose or interval adjustments. As temocillin is a time-dependent antibacterial effect, and serum concentrations of temocillin in the first 4 h are not affected by renal impairment, interval adjustments should be preferred (Table 5) [40, 49, 50].
4.2.2 Intermittent Haemodialysis Patients
In patients with end-stage renal disease (ESRD), the elimination half-life of temocillin is markedly prolonged—26 h for a 2 g every 48 h schedule. Volume of distribution is not affected in haemodialysis patients as it is in patients with renal impairment, and huge variations are observed in serum albumin and protein binding in ESRD. Temocillin is highly dialysable, with a fraction eliminated by dialysis of approximately 55%. According to these results, a three-times-weekly schedule was proposed by Vandecasteele et al., producing a time during which the free serum concentration remained above the MIC (%fT > MIC) as high as 50–90%, even for MICs of 16 mg/L [51].
4.2.3 Intensive Care Unit Patients
Key PK parameters were evaluated in one study of 10 intensive care unit (ICU) patients. After a 2 g infusion of temocillin, peak serum concentration (147 mg/L), volume of distribution (14.3 L), total clearance (40.7 mL/min), serum half-life (4.3 h), and protein binding (76.3%) were consistent with values observed in healthy volunteers, despite use of two different PK/PD models (i.e. a one-compartment model for critically ill patients, a two-compartment open model for healthy volunteers) [35, 39]. Nevertheless, wide variations of PK parameters (mainly on clearance and volume of distribution) were observed in ICU patients, as reported for other β-lactams in ICU patients [52].
5 PK/PD Relationship and Temocillin Activity
5.1 PK/PD Predictive Parameter
Despite numerous in vivo studies having defined the cumulative percentage of a 24-h period during which the free drug concentration exceeds the MIC (%fT > MIC) at steady-state PK conditions, such as the major PK/PD index correlating with the efficacy of β-lactam antibiotics against Gram-negative bacteria [53], no specific study aimed to define the best PK/PD index correlating with the in vivo efficacy of temocillin. Nevertheless, it could be postulated that temocillin follows the same PK/PD index as other β-lactam antibiotics. Indeed, two experimental studies confirmed this hypothesis. In a murine model of UTI, Soubirou et al. treated mice infected with a well-characterised uropathogenic E. coli with a temocillin MIC of 8 mg/L, with various therapeutic schedules. The authors found that the fT > MIC was well-correlated with the maximum effect (E max) after 24 h of temocillin treatment [31]. Interestingly, they also found that the E max was observed for a fT > MIC of 40%, which is consistent with values for penicillin associated with bacteriostatic effect and survival in animal models with Gram-negative bacteria [54]. In a lethal murine model of infection, Alexandre et al. treated mice infected by isogenic strains with increasing temocillin MIC (MICs from 8 to 256 mg/L) with a humanised therapeutic schedule [32]. Reduction in viable bacteria counts and survival rates after 24 h of treatment with temocillin were correlated with fT > MIC [32]. Together, these data suggest that fT > MIC is the best PK/PD index correlating with temocillin efficacy, and that a fT > MIC of 40–50% is associated with antibacterial effect and survival in vivo.
5.2 Probability of Target Attainment
Due to its singular commercial history, few data are available regarding the temocillin PK/PD index and probability of target attainment (PTA) for various dosing regimens in different patient populations. Indeed, only two studies using Monte Carlo simulations are available, focusing only on ICU patients. The first set of Monte Carlo simulations was performed by De Jongh et al. with PK data from six subjects receiving 30-min infusions of 2 g every 12 h for a mean duration of therapy of 8 days [35]. Population PK modelling was performed using a one-compartment model. The PTA of temocillin was estimated for one dosing regimen (2 g every 12 h) for MIC targets ranging from 0.25 to 128 mg/L (Fig. 2). This Monte Carlo simulation showed that the 2 g every 12 h regimen provides a 95% PTA of 40% fT > MIC up to an MIC of 8 mg/L. The mean fT > MIC values of populations were also simulated for two additional temocillin regimens: 2 g every 24 h and 2 g every 8 h (Table 6). An fT > MIC > 40% was expected to be reached for isolates with an MIC of 4 and 16 mg/L, for the 2 g every 24 h and 2 g every 8 h regimens, respectively. Due to the small subject sample and the singularity of ICU patients, wide variations in Monte Carlo simulation were observed, with 95% confidence intervals (CIs) for reaching MIC, with a fT > MIC of 40% ranging from 8 to 32 mg/L (Fig. 2).

Reproduced from De Jongh et al. [35], with permission
Probabilities of target attainment of temocillin (obtained using Monte Carlo simulation: solid line indicates median value; dotted lines indicate 95% confidence interval) for the 2 g every 12 h schedule, using the pharmacokinetic data of the six patients treated according to this dosage. The abscissa shows the MIC range used for the simulations and the ordinate the fraction of time (as a percentage) during which free serum levels remain above the corresponding MIC. The horizontal dotted line indicates the 40% fT > MIC limit. MIC minimum inhibitory concentration.
The second set of Monte Carlo simulations was performed by Laterre et al. using data from 11 ICU patients receiving temocillin as a 2 g every 8 h regimen (over a 30-min period) [55]. Population PK modelling was performed using a two-compartment model. The PTA of temocillin was estimated for one dosing regimen (2 g every 8 h) for MIC targets ranging from 2 to 256 mg/L. The 2 g every 8 h regimen provides a 95% PTA given at a 40% fT > MIC target up to an MIC of 8 mg/L, according to this Monte Carlo estimation. The mean population fT > MIC for MICs of 8, 16 and 32 mg/L were >90, 80%, and just below 40%, respectively (Fig. 3).

Reproduced from Laterre et al. [55], with permission
Probabilities of target attainment of temocillin (obtained using Monte Carlo simulation) for the 2 g every 8 h schedule. The abscissa shows the MIC range used for the simulations and the ordinate the fraction of time (as a percentage) during which free serum levels remain above the corresponding MIC. The horizontal dotted line indicates the 50% fT > MIC limit. MIC minimum inhibitory concentration.
5.3 Clinical Applications
Despite these two discerning studies, PK/PD data regarding less severely ill patients are lacking. Moreover, epidemiologic data surveillance of temocillin resistance have just began in some countries (i.e. France). This could explain the two major issues regarding temocillin clinical use: (1) the optimal dosing regimen and (2) the lack of international consensus regarding clinical breakpoints.
One retrospective study including 92 patients infected with Enterobacteriaceae (with 52% of strains producing ESBL or dAmpC) treated with temocillin showed that a 1 g every 12 h regimen was associated with a relative risk of clinical and microbiological failure of 2.9 (95% CI 1.1–7.7) and 5.0 (95% CI 1.7–14.6), respectively, compared with a 2 g every 12 h dosing regimen [56]. It is now recognised that a 1 g every 12 h regimen is a suboptimal dosage with a higher risk of failure.
Controversy is still ongoing between the use of a 2 g every 12 h or 2 g every 8 h regimen. Based on their results, Laterre et al. proposed that temocillin 6 g daily was adequate to reach an average %fT > MIC value of 80% for an MIC of 16 mg/L (Table 6) [55]. However, this study was not designed to assess the clinical efficacy of temocillin and included only severely ill patients. In less severely ill patients, or in a specific focus of infection, such as a UTI, a 4 g daily regimen should be adequate, even for strains with temocillin MIC up to 16 mg/L (Table 6; Fig. 2). Indeed, in the study by Balakrishnan et al., strains were defined as susceptible for a temocillin MIC up to 16 mg/L, and no clinical or microbiological cure rate differences were found according to temocillin MICs. Interestingly, 100% of clinical and microbiological cure rates were observed in the subset of patients with UTIs due to ESBL- or dAmpC-producing strains [56]. These data are consistent with recent data found in two animal models where a temocillin regimen simulating a 2 g every 12 h human regimen demonstrated significant activity against strains whose temocillin MIC were up to 16 mg/L [31, 32].
Saturable binding to albumin may have several consequences. First, in hypoalbuminemic patients, such as ICU patients, the unbound active fraction may increase. Second, a short duration perfusion could result in a higher peak concentration and higher unbound active fraction, leading to greater tissue penetration. Nevertheless, these considerations should be taken with caution according to the wide interindividual variability of protein binding.
Finally, in the majority of less severe patients, particularly in patients with UTI, a 2 g every 12 h regimen should be adequate, even against strains with a temocillin MIC up to 16 mg/L. For ICU patients, due to the wide variations in actual antibiotic concentrations and the need for a higher %fT > MIC target, a 2 g every 8 h regimen should be preferred. Moreover, to avoid intraindividual variation of antibiotic concentrations in such patients, temocillin may be administered by continuous infusion as this administration showed a higher probability of reaching the desired PK/PD target than three-times-daily infusion [55]. Nevertheless, no prospective study evaluating temocillin efficacy according to temocillin regimen, foci of infection, patient’s severity and temocillin MICs is available. In addition, current epidemiological data of temocillin resistance focusing on a specific focus of infection, such as a UTI, are lacking. Hence, physicians should follow national guidelines regarding temocillin breakpoints and regimens pending for international consensus about these issues (Table 7).
6 Conclusion and Perspectives
Temocillin is an old ‘revived’ antibiotic with an interesting antimicrobial activity, with both (i) a narrow spectrum almost limited to Enterobacteriaceae, strongly suggesting a reduced impact on human gut microbiota and therefore little propensity for selecting resistance pathogen and a minimal risk of Clostridium difficile infection, as suggested by retrospective studies [33, 56, 57]; and (ii) a resistance to hydrolysis by numerous β-lactamases, including the pandemic ESBL or a worrying threat such as KPC.
As for other disused antibiotics, data regarding clinical efficacy, PK profiling in volunteers or patients, and the PD target are scarce [58]. This is in contrast with the urgent medical need of therapeutic options against emerging, multiresistant, Gram-negative bacteria, supporting the use of old antibiotics despite present-day processes and requirements.
Nevertheless, available PK/PD data provide valuable information. Temocillin exhibits a long serum half-life, suitable for its use in a twice-daily regimen. One gram twice daily in normal renal function patients should be abandoned. Renal clearance is predominant, and high unchanged temocillin concentrations are recovered in urine. Monte Carlo simulations regarding %fT > MIC revealed that a 2 g every 12 h regimen is adequate to treat infection caused by strains with temocillin MIC up to 16 mg/L. In specific populations with altered PK and PD parameters, such as ICU patients, a 2 g every 8 h regimen should be preferred for treating MIC with the same clinical breakpoints. However, based on actual PK/PD data, a clinical breakpoint of 32 mg/L appears difficult to reach, except for the treatment of uncomplicated UTIs, as recommended by the British Society for Antimicrobial Chemotherapy (Table 7).
There is a clear need for redevelopment for old antibiotics. Temocillin studies regarding wild-type MIC distribution (particularly from urine samples), PK data for non-severely ill patients or specific populations such as elderly patients, as well as clinical PK/PD studies, are required to support appropriate dosing recommendations and determination of clinical breakpoints.
References
Miranda Bastos AC, Vandecasteele SJ, Tulkens PM, Spinewine A, Van Bambeke F. Development and validation of a high performance liquid chromatography assay for the determination of temocillin in serum of haemodialysis patients. J Pharm Biomed Anal. 2014;90:192–7.
Livermore DM, Tulkens PM. Temocillin revived. J Antimicrob Chemother. 2008;63:243–5.
SPILF. Diagnostic et antibiothérapie des infections urinaires bactériennes communautaires de l’adulte. 2015. Available at: http://www.infectiologie.com/UserFiles/File/spilf/recos/infections-urinaires-spilf-argumentaire.pdf.
Labia R, Baron P, Masson JM, Hill G, Cole M. Affinity of temocillin for Escherichia coli K-12 penicillin-binding proteins. Antimicrob Agents Chemother. 1984;26:335–8.
Bush K, Smith SA, Ohringer S, Tanaka SK, Bonner DP. Improved sensitivity in assays for binding of novel beta-lactam antibiotics to penicillin-binding proteins of Escherichia coli. Antimicrob Agents Chemother. 1987;31:1271–3.
Jules K, Neu HC. Antibacterial activity and beta-lactamase stability of temocillin. Antimicrob Agents Chemother. 1982;22:453–60.
Slocombe B, Basker MJ, Bentley PH, Clayton JP, Cole M, Comber KR, et al. BRL 17421, a novel beta-lactam antibiotic, highly resistant to beta-lactamases, giving high and prolonged serum levels in humans. Antimicrob Agents Chemother. 1981;20:38–46.
Malottke R, Potel J. Antibacterial activity of temocillin. Drugs. 1985;29(Suppl 5):67–73.
Martinez-Beltran J, Loza E, Gomez-Alferez A, Romero-Vivas J, Bouza E. Temocillin. In vitro activity compared with other antibiotics. Drugs. 1985;29(Suppl 5):91–7.
Bauernfeind A. Bacteriostatic and bactericidal activity of penicillins at constant and variable concentrations. Drugs. 1985;29(Suppl 5):9–14.
Bonacorsi S, Fitoussi F, Lhopital S, Bingen E. Comparative in vitro activities of meropenem, imipenem, temocillin, piperacillin, and ceftazidime in combination with tobramycin, rifampin, or ciprofloxacin against Burkholderia cepacia isolates from patients with cystic fibrosis. Antimicrob Agents Chemother. 1999;43:213–7.
Acker HV, Snick EV, Nelis HJ, Coenye T. In vitro activity of temocillin against planktonic and sessile Burkholderia cepacia complex bacteria. J Cyst Fibros. 2010;9:450–4.
Van Landuyt HW, Boelaert J, Piot P, Verbist L. In vitro activity of temocillin against clinical isolates. Drugs. 1985;29(Suppl 5):1–8.
Jephcott AE, Egglestone SI. In vitro activity of temocillin against Neisseria gonorrhoeae including penicillinase-producing strains. Drugs. 1985;29(Suppl 5):18–23.
Fuchs PC, Barry AL, Jones RN, Thornsberry C. Temocillin: in vitro activity against 734 selected clinical isolates, including beta-lactamase-producing strains. Diagn Microbiol Infect Dis. 1984;2:55–63.
Verbist L. In vitro activity of temocillin (BRL 17421), a novel beta-lactamase-stable penicillin. Antimicrob Agents Chemother. 1982;22:157–61.
Ghathian K, Calum H, Gyssens IC, Frimodt-Møller N. Temocillin in vitro activity against recent clinical isolates of Neisseria gonorrhoeae compared with penicillin, ceftriaxone and ciprofloxacin. J Antimicrob Chemother. 2016;71:1122–3.
Matagne A, Ghuysen M-F, Frère J-M. Interactions between active-site-serine beta-lactamases and mechanism-based inactivators: a kinetic study and an overview. Biochem J. 1993;295:705–11.
Woodford N, Pike R, Meunier D, Loy R, Hill R, Hopkins KL. In vitro activity of temocillin against multidrug-resistant clinical isolates of Escherichia coli, Klebsiella spp. and Enterobacter spp., and evaluation of high-level temocillin resistance as a diagnostic marker for OXA-48 carbapenemase. J Antimicrob Chemother. 2014;69:564–7.
Fournier D, Chirouze C, Leroy J, Cholley P, Talon D, Plésiat P, et al. Alternatives to carbapenems in ESBL-producing Escherichia coli infections. Médecine Mal Infect. 2013;43:62–6.
Mischnik A, Baumert P, Hamprecht A, Rohde A, Peter S, Feihl S, et al. Susceptibility to penicillin derivatives among third-generation cephalosporin-resistant Enterobacteriaceae recovered on hospital admission. Diagn Microbiol Infect Dis. 2017;87(1):71–3.
Rodriguez-Villalobos H, Malaviolle V, Frankard J, de Mendonça R, Nonhoff C, Struelens MJ. In vitro activity of temocillin against extended spectrum β-lactamase-producing Escherichia coli. J Antimicrob Chemother. 2006;57:771–4.
Tärnberg M, Östholm-Balkhed A, Monstein H-J, Hällgren A, Hanberger H, Nilsson LE. In vitro activity of beta-lactam antibiotics against CTX-M-producing Escherichia coli. Eur J Clin Microbiol Infect Dis. 2011;30:981–7.
Livermore DM, Hope R, Fagan EJ, Warner M, Woodford N, Potz N. Activity of temocillin against prevalent ESBL- and AmpC-producing Enterobacteriaceae from south-east England. J Antimicrob Chemother. 2006;57:1012–4.
Glupczynski Y, Huang T-D, Berhin C, Claeys G, Delmée M, Ide L, et al. In vitro activity of temocillin against prevalent extended-spectrum beta-lactamases producing Enterobacteriaceae from Belgian intensive care units. Eur J Clin Microbiol Infect Dis. 2007;26:777–83.
Adams-Haduch JM, Potoski BA, Sidjabat HE, Paterson DL, Doi Y. Activity of temocillin against KPC-Producing Klebsiella pneumoniae and Escherichia coli. Antimicrob Agents Chemother. 2009;53:2700–1.
Mutters NT, Zimmermann S, Kaase M, Mischnik A. Activity of temocillin, mecillinam, ceftazidime, and ceftazidime/avibactam against carbapenem-non-susceptible Enterobacteriaceae without carbapenemase production. Eur J Clin Microbiol Infect Dis. 2015;34:2429–37.
Zykov IN, Sundsfjord A, Småbrekke L, Samuelsen Ø. The antimicrobial activity of mecillinam, nitrofurantoin, temocillin and fosfomycin and comparative analysis of resistance patterns in a nationwide collection of ESBL-producing Escherichia coli in Norway 2010–2011. Infect Dis. 2016;48:99–107.
Buyck JM, Guénard S, Plésiat P, Tulkens PM, Bambeke FV. Role of MexAB-OprM in intrinsic resistance of Pseudomonas aeruginosa to temocillin and impact on the susceptibility of strains isolated from patients suffering from cystic fibrosis. J Antimicrob Chemother. 2012;67:771–5.
Chalhoub H, Pletzer D, Weingart H, Braun Y, Tunney MM, Elborn JS, et al. Mechanisms of intrinsic resistance and acquired susceptibility of Pseudomonas aeruginosa isolated from cystic fibrosis patients to temocillin, a revived antibiotic. Sci Rep. 2017;7:40208.
Soubirou JF, Rossi B, Couffignal C, Ruppé E, Chau F, Massias L, et al. Activity of temocillin in a murine model of urinary tract infection due to Escherichia coli producing or not producing the ESBL CTX-M-15. J Antimicrob Chemother. 2015;70:1466–72.
Alexandre K, Chau F, Guérin F, Massias L, Lefort A, Cattoir V, et al. Activity of temocillin in a lethal murine model of infection of intra-abdominal origin due to KPC-producing Escherichia coli. J Antimicrob Chemother. 2016;71:1899–904.
Gupta ND, Smith RE, Balakrishnan I. Clinical efficacy of temocillin. J Antimicrob Chemother. 2009;64:431–3.
Just HM, Becker C, Gieringer J, Wenz A, Bassler M, Daschner F. In vitro combination-effect of temocillin with ticarcillin and aminoglycosides on gram-negative and gram-positive bacteria. Drugs. 1985;29(Suppl 5):74–7.
De Jongh R, Hens R, Basma V, Mouton JW, Tulkens PM, Carryn S. Continuous versus intermittent infusion of temocillin, a directed spectrum penicillin for intensive care patients with nosocomial pneumonia: stability, compatibility, population pharmacokinetic studies and breakpoint selection. J Antimicrob Chemother. 2008;61:382–8.
Tzouvelekis LS, Markogiannakis A, Piperaki E, Souli M, Daikos GL. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect. 2014;20:862–72.
Basker MJ, Merrikin DJ, Ponsford RJ, Slocombe B, Tasker TCG. BRL 20330, an oral prodrug of temocillin: bioavailability studies in man. J Antimicrob Chemother. 1986;18:399–405.
Overbosch D, van Gulpen C, Mattie H. Renal clearance of temocillin in volunteers. Drugs. 1985;29(Suppl 5):128–34.
Hampel B, Feike M, Koeppe P, Lode H. Pharmacokinetics of temocillin in volunteers. Drugs. 1985;29(Suppl 5):99–102.
Höffler D, Koeppe P. Temocillin pharmacokinetics in normal and impaired renal function. Drugs. 1985;29(Suppl 5):135–9.
Baert L, Aswarie H, Verbist L, Horton R. Penetration of temocillin into prostatic tissue after intravenous dosing. Acta Clin Belg. 1989;44:358–9.
Spelsberg F, Bauernfeind A, Wiest W, Hanser P. Biliary concentrations of temocillin. Drugs. 1985;29(Suppl 5):122–7.
Poston GJ, Greengrass A, Moryson CJ. Biliary concentrations of temocillin. Drugs. 1985;29(Suppl 5):140–5.
Wise R, Donovan IA, Drumm J, Dyas A, Cross C. The intraperitoneal penetration of temocillin. J Antimicrob Chemother. 1983;12:93–6.
Wittke RR, Adam D, Klein HE. Therapeutic results and tissue concentrations of temocillin in surgical patients. Drugs. 1985;29(Suppl 5):221–6.
Cowan W, Baird A, Sleigh JD, Gray JM, Leiper JM, Lawson DH. Lung tissue penetration of temocillin. Drugs. 1985;29(Suppl 5):151–3.
Legge JS, Reid TM, Palmer JB. Clinical efficacy, tolerance and pharmacokinetics of temocillin in patients with respiratory tract infections. Drugs. 1985;29(Suppl 5):118–21.
Brückner O, Trautmann M, Borner K. A study of the penetration of temocillin in the cerebrospinal fluid. Drugs. 1985;29(Suppl 5):162–6.
Leroy A, Humbert G, Fillastre J-P, Borsa F, Godin M. Pharmacokinetics of temocillin (BRL 17421) in subjects with normal and impaired renal function. J Antimicrob Chemother. 1983;12:47–58.
Boelaert J, Daneels R, Schurgers M, Lambert AM, Van Landuyt HW, Mellows G, et al. The pharmacokinetics of temocillin in patients with normal and impaired renal function. J Antimicrob Chemother. 1983;11:349–56.
Vandecasteele SJ, Miranda Bastos AC, Capron A, Spinewine A, Tulkens PM, Van Bambeke F. Thrice-weekly temocillin administered after each dialysis session is appropriate for the treatment of serious Gram-negative infections in haemodialysis patients. Int J Antimicrob Agents. 2015;46:660–5.
Roberts JA, Paul SK, Akova M, Bassetti M, Waele JJD, Dimopoulos G, et al. DALI: defining antibiotic levels in intensive care unit patients: are current β-Lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58:1072–83.
Craig WA. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect Dis Clin N Am. 2003;17:479–501.
Leggett JE, Fantin B, Ebert S, Totsuka K, Vogelman B, Calame W, et al. Comparative antibiotic dose-effect relations at several dosing intervals in murine pneumonitis and thigh-infection models. J Infect Dis. 1989;159:281–92.
Laterre P-F, Wittebole X, Van de Velde S, Muller AE, Mouton JW, Carryn S, et al. Temocillin (6 g daily) in critically ill patients: continuous infusion versus three times daily administration. J Antimicrob Chemother. 2015;70:891–8.
Balakrishnan I, Awad-El-Kariem FM, Aali A, Kumari P, Mulla R, Tan B, et al. Temocillin use in England: clinical and microbiological efficacies in infections caused by extended-spectrum and/or derepressed AmpC-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother. 2011;66:2628–31.
Habayeb H, Sajin B, Patel K, Grundy C, Al-Dujaili A, Van de Velde S. Amoxicillin plus temocillin as an alternative empiric therapy for the treatment of severe hospital-acquired pneumonia: results from a retrospective audit. Eur J Clin Microbiol Infect. 2015;34:1693–9.
Muller AE, Theuretzbacher U, Mouton JW. Use of old antibiotics now and in the future from a pharmacokinetic/pharmacodynamic perspective. Clin Microbiol Infect. 2015;21:881–5.
Bergan T, Olszewski WL, Engeset A. Temocillin penetration of peripheral lymph. Drugs. 1985;29(Suppl 5):114–7.
British Society for Antimicrobial Chemotherapy. BSAC Clinical Breakpoints [Internet]. 2015. Available at: http://www.bsac.org.uk/wp-content/uploads/2012/02/BSAC-Susceptibility-testing-version-143.pdf.
Société Française de Microbiologie. Comité de l’antibiogramme de la Société Française de Microbiologie. 2017. Available at: http://www.sfm-microbiologie.org/UserFiles/files/casfm/CASFMV1_0_MARS_2017.pdf. Accessed 28 Apr 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received in the preparation of this manuscript.
Conflict of interest
Bruno Fantin received funding from Eumedica pharmaceuticals for part of the temocillin experimental studies performed in the laboratory [31, 32]. Dr Kevin Alexandre has no conflicts of interest to declare regarding this manuscript.
Rights and permissions
About this article
Cite this article
Alexandre, K., Fantin, B. Pharmacokinetics and Pharmacodynamics of Temocillin. Clin Pharmacokinet 57, 287–296 (2018). https://doi.org/10.1007/s40262-017-0584-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-017-0584-7