Abstract
Paclitaxel is an anticancer agent efficacious in the treatment of ovarian, breast, and lung cancer. Due to a strong link between the pharmacokinetics and therapeutic efficacy of paclitaxel, we reviewed the literature on paclitaxel pharmacokinetics. Systematic data mining was performed to extract the maximum concentration (C max), clearance (CL), and time of paclitaxel plasma concentration above 0.05 µmol/L (T > 0.05 µmol/L) following monotherapy of both the widely used cremophor-diluted paclitaxel and nanoparticle albumin-bound (nab-)paclitaxel. We identified a total of 53 studies yielding 121 aggregated pharmacokinetic profiles for paclitaxel monotherapy and extracted reported mean and median estimates of pharmacokinetic parameters. Paclitaxel has been studied formally at doses of 15–825 mg/m2 and infused over 0.5–96 h; included studies examined both weekly and every 3-weeks dosing cycles. The most widely used dose of cremophor-diluted paclitaxel, 175 mg/m2 given as a 3-h infusion, leads to an interstudy median C max of 5.1 µmol/L [interquartile range (IQR) 4.5–5.7], CL of 12.0 L/h/m2 (IQR 10.9–12.9), and T > 0.05 µmol/L of 23.8 h (IQR 21.5–26.8). Importantly, the significant interindividual variation widely reported in the literature is not reflected in these interstudy estimates of pharmacokinetic parameters. Cremophor-diluted paclitaxel pharmacokinetics are non-linear following short (<6 h) but not long (>24 h) infusions. A similar pattern of non-linearity was observed for nab-paclitaxel, although the number of studies was limited. The pharmacokinetics of paclitaxel monotherapy have been widely studied at numerous dose levels of the Cremophor EL® formulation, but are less well-characterized for the newer nab-paclitaxel formulation. In conclusion, paclitaxel pharmacokinetics are non-linear for short infusion times but not for longer infusions. Whether a similar conclusion can be drawn for nab-paclitaxel formulations requires further study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The time above a threshold paclitaxel plasma concentration (0.05 µmol/L) is important for the efficacy and toxicity of the drug. |
Paclitaxel is administered mainly as two formulations: Cremophor EL® diluted or nanoparticle albumin bound. The cremophor-diluted formulation has been widely tested at different doses and infusion times; data are more limited for the nanoparticle formulation. |
The plasma concentrations of paclitaxel do not follow linear pharmacokinetics for short infusions. This is particularly evident for cremophor-based paclitaxel. |
1 Background
Paclitaxel is a widely used drug in the treatment of breast [1], ovarian [2], and lung cancer [3]. Paclitaxel binds to and promotes the assembly of tubulin into dysfunctional microtubules, which leads to chromosome missegregation on multipolar spindles at clinically observed concentrations [4]. The consequence of microtubule dysfunction is inhibition of mitosis and cell proliferation, resulting in the death of rapidly proliferating tumor cells.
Paclitaxel is a high molecular weight drug (853.9 g/mol) with a very low solubility in water (0.7 mg/mL) [5]. It is metabolized primarily by cytochrome P450 (CYP) 2C8 to the largely inactive metabolite 6-hydroxypaclitaxel and to a lesser degree by CYP3A4 to 3′-phenyl-hydroxypaclitaxel [6]. Paclitaxel is a substrate for ATP-binding cassette (ABC) efflux transporters, including multidrug resistance protein 1 [MDR1/P-glycoprotein (P-gp), ABCB1] [7, 8], breast cancer resistance protein (BCRP, ABCG2) [9], and multidrug resistance-associated proteins 1/2 (MRP1/2, ABCC1/C2) [10, 11]. ABC transporter-mediated efflux of paclitaxel back into the intestinal lumen accounts for its very low oral bioavailability and requirement for intravenous administration. The influx transporter organic anion transporter (OAT) polypeptide 1B3 (OATP1B3, SLCO1B3) has been shown to be involved in the hepatic uptake of paclitaxel [12, 13]; paclitaxel is also a substrate for the renal OAT2 (SLC22A7) [14]. Activity of these hepatic and renal transporters may play an important role in the distribution and elimination of paclitaxel and may contribute to variability in the pharmacokinetics of the drug. Paclitaxel activates pregnane X receptor (PXR) [15, 16], leading to upregulation of key drug-metabolizing enzymes such as CYP3A4 [16, 17] and transporters such as ABCB1 [15]. However, the administration of paclitaxel every 1, 2, or 3 weeks has not been associated with altered metabolism over time [18,19,20,21], suggesting that autoinduction is minimal during standard dosing conditions.
Due to the hydrophobic nature of paclitaxel, it was originally diluted in the solvent Cremophor EL®, a poly-oxy-ethylated oil mixed 1:1 with ethanol. This formulation solves one problem, but is associated with hypersensitivity reactions to Cremophor EL®. This was initially circumvented by using longer infusions times. However, the addition of pretreatment with prophylactic antihistamines (both histamine H1 and H2 receptor antagonists) and glucocorticoids has made it possible to reduce infusion times and achieve similarly low rates of hypersensitivity reactions [22]. Recently, a nanoparticle albumin-bound formulation of paclitaxel (nab-paclitaxel) was developed that does not cause infusion hypersensitivity reactions and thus eliminates the need for prophylactic treatment. Paclitaxel is highly bound (90%) to plasma proteins [23], and the free fraction of paclitaxel inversely correlates with Cremophor EL® concentrations [24, 25].
The paclitaxel response rate differs significantly between cancers, with ovarian cancer generally more sensitive than breast cancer [26, 27]. Efficacy and toxicity also depends on combination with other chemotherapeutic agents and even the sequence of chemotherapy administration. For example, in one study the clearance (CL) of paclitaxel was lower when administered after cisplatin compared to administration before the platinum agent [28], though this effect was not replicated in another study [29].
Significant interpatient variability is also observed for paclitaxel adverse events, although the frequency and severity is quite similar across cancer types. The most common and problematic adverse event is peripheral sensory neuropathy, with significant neutropenia also observed. Both peripheral neuropathy and neutropenia are dose limiting and lead to reduced response rates to paclitaxel. Based on a database of 812 patients with various solid tumors treated with single-agent paclitaxel, severe neutropenia (<500 cells/cm3) occurred in 52% of the patients. Peripheral neuropathy was reported for 60% of the patients treated with mixed doses (135–300 mg/m2) and infusion times (3 or 24 h), with 3% reporting severe (grade 3 or higher) neuropathy [30]; these frequencies are known to vary significantly between populations. Clinical symptoms of neuropathy range from numbness and tingling in fingers and hands to cold or heat intolerance and burning pain. Normally, neuropathy symptoms are reversible, but some patients continue to experience neuropathy up to 2 years after drug cessation, significantly impairing quality of life [31].
The reasons for variability in paclitaxel response and toxicity are multifaceted. Some studies have suggested that polymorphisms in CYP2C8 or ABCB1 cause pharmacokinetic variation, while others show no effect. Even when a pharmacogenetic difference in paclitaxel pharmacokinetics has been demonstrated, such as for CYP2C8*3 (and *4), clinical relevance is limited because of the small effect size [32]. Recent genome-wide association studies (GWAS) have found a multitude of genetic variants associated with risk of peripheral neuropathy during treatment with paclitaxel [33,34,35]. Some findings are biologically plausible, such as variants in genes involved in neuronal repair, while others require further investigation to fully understand their relevance. These hypothesis-generating studies are of merit as they might provide new insight into the molecular mechanisms underlying the toxicities; however, substantial validation in multiple cohorts is required before their final interpretation and potential translation into clinical practice.
Drug–drug interactions (DDIs) affecting the pharmacokinetics of paclitaxel have not been systematically studied, likely because of ethical considerations of testing potentially harmful DDIs in cancer patients. Clinicians often extrapolate from case observations, in vitro data, and limited epidemiological studies to optimally manage polytherapy during cancer treatment. For example, a metabolite of clopidogrel that inhibits CYP2C8 in vitro was linked to a very low CL of paclitaxel and increased risk of neuropathy in an ovarian cancer patient [36]. This was later supported by a small case series in which seven out of eight patients treated with clopidogrel and paclitaxel experienced grade 3 neutropenia [37]. More recently, 48 patients treated with paclitaxel and clopidogrel were found to have increased rates of neuropathy compared with a control group of 88 patients using low-dose aspirin in place of clopidogrel. The study concluded that the risk of peripheral neuropathy is approximately two-fold higher in patients using clopidogrel and paclitaxel in doses of 135 mg/m2 or greater [38].
The pharmacokinetics of paclitaxel are known to correlate with treatment response [39, 40] and adverse effects [41,42,43,44]. Thus, a comprehensive understanding may lead to improved treatment outcomes. The pharmacokinetics of paclitaxel were comprehensively reviewed by Sonnichsen and Relling [45] in 1994. However, a large number of paclitaxel pharmacokinetic studies have been published since and a new formulation, nab-paclitaxel, has been introduced to the market. These updates are captured in the current literature review, along with a systematic analysis of paclitaxel CL, maximum plasma concentration (C max), and time of paclitaxel plasma concentration above 0.05 µmol/L (T > 0.05 µmol/L) for a range of doses of cremophor-diluted and albumin-bound paclitaxel given as single-agent therapy.
2 Methods
PubMed was searched with the following sequence: (Paclitaxel or Taxol) AND pharmacokinetics [(MESH) or (All fields)] and restricted to clinical trials in English with human subjects. The search was performed on 21 June 2016 and gave a total of 608 hits. Titles and abstracts for studies with any formulation of paclitaxel were evaluated twice by two independent reviewers and identified 322 publications for further consideration. Papers without abstracts were only included if it was clear from the title that they would be relevant. Full texts were then read by at least one reviewer and 182 publications were identified that described a minimum of 6 h of pharmacokinetic sampling for paclitaxel or nab-paclitaxel. After exclusion of studies where paclitaxel was not given as monotherapy or pharmacokinetic parameters were not available, 53 publications remained [19, 21, 46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96] and serve as the basis for this review (Fig. 1).
The following information was extracted from all publications: number of patients, duration of infusion (h), formulation of paclitaxel (cremophor-diluted or nab-paclitaxel), dose (mg/m2), C max (µmol/L), total CL (L/h/m2), and T > 0.05 µmol/L (h). In cases where a single publication had pharmacokinetic profiles at different dose levels, every dose level was included separately. In studies with repeated pharmacokinetic profiles, only the first visit was included. Since data presentation varied among included publications, both median and mean estimates were extracted and pooled for analysis. Therefore, the summary values for all parameters reported here reflect interstudy variation and provide no indication of the significant interindividual variation in paclitaxel pharmacokinetics that is widely reported.
Correlation between dose and C max was evaluated by linear and quadratic fits and r 2 was used to evaluate the best fit (STATA® 14.2, StataCorp, College Station, TX, USA).
3 Results
An overview of included publications and the corresponding data extracted from these studies are provided as Electronic Supplementary Material (Online Resource 1). Briefly, 53 papers yielded a total of 121 pharmacokinetic profiles with a median number of six patients [interquartile range (IQR) 3–10 patients] in each study. Pharmacokinetic parameters presented in this paper are medians with IQRs (25th–75th percentiles), unless otherwise specified. A study with subtherapeutic radiolabeled paclitaxel [64] was not included in the analysis due to the nature of the paclitaxel formulation. Thus, 120 profiles were included in the final analysis.
3.1 Cremophor EL® Paclitaxel
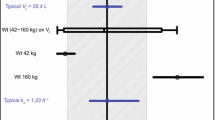
A total of 104 pharmacokinetic profiles for Cremophor EL® paclitaxel from administration of 32 different doses (15–825 mg/m2) over six different infusion times (1, 3, 6, 24, 72, and 96 h) were evaluated. The diversity of paclitaxel dosage regimens is illustrated in Fig. 2.
Distribution of dosage regimens for cremophor-diluted paclitaxel included in this analysis. The solid line represents the median dose, the box represents the interquartile range (25th–75th percentiles), and the whiskers represent 5th–95th percentiles. A single outlier with a dose of 825 mg/m2 infused over 24 h is excluded due to extension of the dose-axis and compression of the majority of the data
Ninety-one paclitaxel CL estimates were extracted from the included studies [67 for short infusion (≤6 h) and 24 for long infusion (>6 h)]. Figure 3 shows the relationship between CL and dose for short and long infusion of Cremophor EL® paclitaxel. A single CL estimate was excluded from Fig. 3b (8.1 L/h/m2 for paclitaxel 825 mg/m2) to limit the range of the dose-axis and allow for more accurate representation of the majority of the data with lower doses. Paclitaxel CL decreases up to three-fold with increasing dose, which is most evident with shorter infusion times (Fig. 3a). Furthermore, for a given dose, paclitaxel CL is approximately two-fold higher when given as a long infusion than as a short infusion.
The relationship between paclitaxel C max and dose is illustrated in Fig. 4 for short (n = 75) and long (n = 24) infusion times. A quadratic equation best described the C max–dose relationship for paclitaxel given as a short infusion; the data for the 1-h infusion time best fit this relationship (r 2 = 0.99). With a 1.7-fold increase in paclitaxel dose given as 3-h infusion the C max increases three-fold (Table 1). In contrast, a linear relationship provides the best fit for data from long infusion times. C max values for long infusions were approximately tenfold lower than for short infusions (Fig. 4). A single dose from the long infusion data (825 mg/m2) was removed from Fig. 4b to better illustrate the relationship for the majority of the values.
Maximum paclitaxel concentrations are not dose proportional with short infusion times. Maximum concentration (µmol/L) of paclitaxel during short (a) and long (b) infusion is expressed as a function of dose and infusion time. Solid lines represent a quadratic fit for short infusion times and a linear fit for long infusion times. C max maximum concentration
The non-linearity in paclitaxel pharmacokinetics is also highlighted in Table 1 with data from the most commonly used infusion time, 3 h. Median values with corresponding IQR (25th–75th percentiles) for C max and CL indicate a greater than dose-proportional increase in C max and decrease in CL with increasing dose.
In comparison to CL and C max, T > 0.05 µmol/L was less commonly reported for the paclitaxel pharmacokinetic studies included in this analysis. Only 28 values for T > 0.05 µmol/L paclitaxel were reported, 21 for short and seven for long infusion times (Fig. 5). Interestingly, increasing infusion time from 3 to 24 h does not substantially increase T > 0.05 µmol/L. All values for T > 0.05 µmol/L from a 24-h infusion are from the same study [90].
3.2 Nab-Paclitaxel
Pharmacokinetic data for nab-paclitaxel were extracted from 16 studies using nine different doses (80–375 mg/m2). The majority of these studies (n = 14) used a 0.5-h infusion; a single study used a 3-h infusion and the remaining study did not indicate an infusion time and was excluded from further analysis. The relationship between nab-paclitaxel CL and dose is illustrated in Fig. 6. With nab-paclitaxel doses above 200 mg/m2, the CL of nab-paclitaxel decreases in a similar fashion as described for the Cremophor EL® paclitaxel formulation. Non-linearity was also observed for paclitaxel C max values over a >4-fold range of nab-paclitaxel doses (Fig. 7), although the increases in C max were less striking than those observed with the cremophor-diluted formulation (Fig. 4).
3.3 Population Pharmacokinetic Modelling
Six population pharmacokinetic studies of paclitaxel monotherapy were identified [96,97,98,99,100,101] and these are summarized in Table 2. Briefly, all but one of the studies had dense sampling with study populations ranging from seven to 150 individuals. The final models included two or three compartments with unique sets of covariates. One study developed a semi-mechanistic model with paclitaxel in four different states: peripheral or central and vehicle bound or not vehicle bound [100]. In one case, elimination was considered to be saturable and represented by Michaelis–Menten pharmacokinetics [99]. The estimates of CL and volume of distribution varied significantly across the models.
4 Discussion
In this review of paclitaxel pharmacokinetics, data were extracted from 53 papers reporting paclitaxel administration as single-agent chemotherapy with either cremophor-diluted or nab-paclitaxel-bound paclitaxel. The effect of dose and infusion time on CL, C max and T > 0.05 µmol/L were the main outcomes analyzed. Based on 120 pharmacokinetic profiles for cremophor-diluted and nab-paclitaxel, paclitaxel CL is non-linear over commonly used doses and infusion times. Non-linear paclitaxel CL results in more than dose-proportional increases in C max, which is particularly apparent when paclitaxel is administered as the Cremophor EL® formulation. The most widely used dosage regimen for cremophor-diluted paclitaxel is a 3-h infusion of 175 mg/m2. This popular dosage regimen corresponds to median values for CL, C max, and T > 0.05 µmol/L of 12 L/h/m2, 5 µmol/L, and 24 h, respectively. Increasing infusion time from 3 to 24 h does not significantly increase T > 0.05 µmol/L, and with the implementation of glucocorticoid and antihistamine pretreatment to reduce hypersensitivity reactions, there is little support for longer infusion times. While non-linear CL was also observed with high doses of the nab-paclitaxel formulation, non-linearities in C max were less pronounced. The average C max of the four patients who received a dose of 375 mg/m2 in the study by Ibrahim et al. [73] was 22.6 µmol/L. Without this observation, the nab-paclitaxel C max approached a non-linear pattern similar to that of cremophor-diluted paclitaxel. Further studies are needed to fully understand the relationship between nab-paclitaxel dose and C max.
The main limitation of this analysis is the exclusion of papers where paclitaxel was given concomitantly with other chemotherapeutics. This limitation was necessary to limit variability in paclitaxel pharmacokinetic parameters and to identify robust dose-dependent changes in paclitaxel elimination. The strong correlation between C max and dose for a given short infusion time (Fig. 4a) supports the study design that was utilized. Furthermore, data regarding interindividual variability in paclitaxel pharmacokinetics were not widely available for the included studies. Both medians and means from pooled data in each study were used for the analyses described in this review. Thus, all measures for variability presented in the current study reflect interstudy variability rather than interpatient variability.
The main strength of this study is the number and diversity of paclitaxel pharmacokinetic profiles that were analyzed. Data were included for more than 30 different doses and six different infusion times, representing the most extensive analysis to date of single-agent paclitaxel pharmacokinetics. This allows for the detection of strong correlations between paclitaxel dose and C max and clearly illustrates that paclitaxel CL is dose dependent, with CL decreasing with increasing dose. The robustness of the analyses is even more striking, considering that the data was from more than 50 studies performed between 1991 and 2015 that employed a wide range of drug assays and dosage regimens. Patient heterogeneity with respect to age, ethnicity, co-morbidities, and treatment indication was also significant. The conclusions drawn from the current analysis can therefore be applied broadly across diverse patient populations and a broad range of paclitaxel dosage regimens. Due to the nature of the data mining implemented in this study, the results do not provide insight regarding interpatient variability.
The non-linearity of paclitaxel pharmacokinetics, which is easily visible for both C max and CL (Figs. 3, 4), was first recognized in the 1990s [67]. Initial reports suggested saturation of CYP-mediated metabolism of paclitaxel, but the non-linear CL is now largely attributed to the formulation of paclitaxel. Because of the high hydrophobicity of paclitaxel, it requires dilution in Cremophor EL®, a poly-oxy-ethylated oil mixed 1:1 with ethanol. Free concentrations of paclitaxel are inversely correlated with Cremophor EL® concentrations [102], which means that less paclitaxel is available for distribution at higher doses. As a result, tissue distribution and pharmacodynamics are largely assumed to be linear. In this review, we see indications of non-linear CL of nab-paclitaxel at higher doses, which could indicate saturation of metabolism at high paclitaxel concentrations. Gemfibrozil, another CYP2C8 substrate, is known for non-linear pharmacokinetics [103, 104] at higher concentrations. Although gemfibrozil non-linear pharmacokinetics have no apparent implications for its clinical use, CYP2C8 saturation could clinically affect nab-paclitaxel elimination.
Population pharmacokinetic modeling is a useful tool to describe and investigate the effect of covariates in drug variation. A number of population pharmacokinetic models have described the pharmacokinetics of paclitaxel monotherapy and have provided important insight into paclitaxel pharmacokinetics and pharmacodynamics. Hempel et al. [97] estimated the total paclitaxel plasma CL to be 6.7 L/h for 13 predominantly male patients treated with 20–50 mg/m2 as a 1-h infusion. This is in agreement with the reported values for non-compartmental analyses of higher doses of paclitaxel. While this study did not report pharmacokinetic non-linearity, this is likely due to the low paclitaxel doses that were analyzed. Zuylen et al. [96] were elegantly able to demonstrate that both non-linear distribution and elimination could be explained by micelle encapsulation of paclitaxel by Cremophor EL®. Henningsson et al. [98] concluded that the CYP2C8 genotype did not impact CL of unbound paclitaxel, a finding disputed by Bergmann et al. [32] who found a small effect of the CYP2C8*3 variant on paclitaxel CL that may depend on the ABCB1 genotype [105]. A direct relationship between liver impairment and paclitaxel elimination was linked to susceptibility to paclitaxel-induced neutropenia by Joerger et al. [99]. A model proposed by Li et al. [100] demonstrated that the similar paclitaxel concentration–time profiles of nab-paclitaxel and cremophor-diluted paclitaxel mask discordant paclitaxel tissue concentration profiles. Based on these findings, the authors conclude that the paclitaxel plasma profile is a poor marker for clinical outcome.
Paclitaxel is rarely given as monotherapy, but often administered in combination with a platinum (cisplatin, carboplatin) or doxorubicin. There are no reported pharmacokinetic interactions between paclitaxel and cisplatin [106,107,108] or carboplatin [109,110,111], although the toxicities of the drugs may be affected by the sequence of their administration [28]. When doxorubicin and paclitaxel are administered within a short time interval, the exposure to doxorubicin is significantly increased [112], which results in dose-dependent cardiotoxicity [67, 113]. Furthermore, a number of relevant pharmacokinetic interactions with paclitaxel have been reported, largely due to inhibition of the major paclitaxel efflux transporter, P-gp. Recent evidence also indicates that a metabolite of the widely used anticoagulant drug clopidogrel reduces CYP2C8-mediated paclitaxel metabolism and can lead to neurotoxicity [36, 38, 114].
Accumulation of paclitaxel in the peripheral nervous system has been associated with its toxicity. In mice, paclitaxel accumulates in the dorsal root ganglia and sciatic nerve following both single and multiple doses [115]. Paclitaxel was still detectable up to 72 h after a single dose, which was significantly after the drug could be detected in the systemic circulation. Following six doses of paclitaxel, the drug was measurable in these peripheral sites for up to 2 weeks. While such accumulation is not expected to be reflected in the plasma, these findings in mice are consistent with the observation that cumulative exposure to paclitaxel is highly correlated with the risk of paclitaxel-induced sensory neuropathy. Further exploration into the mechanisms underlying accumulation of paclitaxel in the peripheral nervous system may lead to better prediction of an individual patient’s risk for developing sensory neuropathy.
5 Conclusion
The data presented in this review demonstrates non-linearity in paclitaxel pharmacokinetics when administered as a short infusion of ≤6 h. This is largely a result of the dilution of paclitaxel in Cremophor EL® [24], and possibly to a lesser extent by saturation of CYP2C8-mediated metabolism. A strong correlation between paclitaxel C max and CL values and dose is also demonstrated. The limited data available for paclitaxel T > 0.05 µmol/L do not allow for an accurate prediction of its relationship with dose.
References
Bishop JF, Dewar J, Toner GC, Smith J, Tattersall MH, Olver IN, et al. Initial paclitaxel improves outcome compared with CMFP combination chemotherapy as front-line therapy in untreated metastatic breast cancer. J Clin Oncol. 1999;17:2355–64.
Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2003;21:3194–200.
Socinski MA. Cytotoxic chemotherapy in advanced non-small cell lung cancer: a review of standard treatment paradigms. Clin Cancer Res. 2004;10:4210s–4s.
Zasadil LM, Andersen KA, Yeum D, Rocque GB, Wilke LG, Tevaarwerk AJ, et al. Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Sci Transl Med. 2014;6:229ra43.
Mathew AE, Mejillano MR, Nath JP, Himes RH, Stella VJ. Synthesis and evaluation of some water-soluble prodrugs and derivatives of taxol with antitumor activity. J Med Chem. 1992;35:145–51.
Cresteil T, Monsarrat B, Dubois J, Sonnier M, Alvinerie P, Gueritte F. Regioselective metabolism of taxoids by human CYP3A4 and 2C8: structure-activity relationship. Drug Metab Dispos. 2002;30:438–45.
Fellner S, Bauer B, Miller DS, Schaffrik M, Fankhänel M, Spruss T, et al. Transport of paclitaxel (Taxol) across the blood–brain barrier in vitro and in vivo. J Clin Invest. 2002;110:1309–18.
Woo JS, Lee CH, Shim CK, Hwang S-J. Enhanced oral bioavailability of paclitaxel by coadministration of the P-glycoprotein inhibitor KR30031. Pharm Res. 2003;20:24–30.
Brooks TA, Minderman H, O’Loughlin KL, Pera P, Ojima I, Baer MR, et al. Taxane-based reversal agents modulate drug resistance mediated by P-glycoprotein, multidrug resistance protein, and breast cancer resistance protein. Mol Cancer Ther. 2003;2:1195–205.
Huisman MT, Chhatta AA, van Tellingen O, Beijnen JH, Schinkel AH. MRP2 (ABCC2) transports taxanes and confers paclitaxel resistance and both processes are stimulated by probenecid. Int J Cancer. 2005;116:824–9.
Lagas JS, Vlaming ML, van Tellingen O, Wagenaar E, Jansen RS, Rosing H, et al. Multidrug resistance protein 2 is an important determinant of paclitaxel pharmacokinetics. Clin Cancer Res. 2006;12:6125–32.
Smith NF, Acharya MR, Desai N, Figg WD, Sparreboom A. Identification of OATP1B3 as a high-affinity hepatocellular transporter of paclitaxel. Cancer Biol Ther. 2005;4:815–8.
Smith NF, Marsh S, Scott-Horton TJ, Hamada A, Mielke S, Mross K, et al. Variants in the SLCO1B3 gene: interethnic distribution and association with paclitaxel pharmacokinetics. Clin Pharmacol Ther. 2007;81:76–82.
Kobayashi Y, Ohshiro N, Sakai R, Ohbayashi M, Kohyama N, Yamamoto T. Transport mechanism and substrate specificity of human organic anion transporter 2 (hOat2 [SLC22A7]). J Pharm Pharmacol. 2005;57:573–8.
Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7:584–90.
Harmsen S, Meijerman I, Beijnen JH, Schellens JHM. Nuclear receptor mediated induction of cytochrome P450 3A4 by anticancer drugs: a key role for the pregnane X receptor. Cancer Chemother Pharmacol. 2009;64:35–43.
Kostrubsky VE, Lewis LD, Strom SC, Wood SG, Schuetz EG, Schuetz JD, et al. Induction of cytochrome P4503A by taxol in primary cultures of human hepatocytes. Arch Biochem Biophys. 1998;355:131–6.
Fidias P, Supko JG, Martins R, Boral A, Carey R, Grossbard M, et al. A phase II study of weekly paclitaxel in elderly patients with advanced non-small cell lung cancer. Clin Cancer Res. 2001;7:3942–9.
Chu Q, Mita A, Forouzesh B, Tolcher AW, Schwartz G, Nieto A, et al. Phase I and pharmacokinetic study of sequential paclitaxel and trabectedin every 2 weeks in patients with advanced solid tumors. Clin Cancer Res. 2010;16:2656–65.
Hotta K, Ueoka H, Kiura K, Tabata M, Kuyama S, Satoh K, et al. A phase I study and pharmacokinetics of irinotecan (CPT-11) and paclitaxel in patients with advanced non-small cell lung cancer. Lung Cancer. 2004;45:77–84.
Leyland-Jones B, Gelmon K, Ayoub J-P, Arnold A, Verma S, Dias R, et al. Pharmacokinetics, safety, and efficacy of trastuzumab administered every three weeks in combination with paclitaxel. J Clin Oncol. 2003;21:3965–71.
Eisenhauer EA, ten Bokkel Huinink WW, Swenerton KD, Gianni L, Myles J, van der Burg ME, et al. European–Canadian randomized trial of paclitaxel in relapsed ovarian cancer: high-dose versus low-dose and long versus short infusion. J Clin Oncol. 1994;12:2654–66.
Sparreboom A, van Tellingen O, Nooijen WJ, Beijnen JH. Preclinical pharmacokinetics of paclitaxel and docetaxel. Anticancer Drugs. 1998;9:1–17.
Sparreboom A, van Zuylen L, Brouwer E, Loos WJ, de Bruijn P, Gelderblom H, et al. Cremophor EL-mediated alteration of paclitaxel distribution in human blood. Cancer Res. 1999;59:1454–7.
Brouwer E, Verweij J, Bruijn PD, Loos WJ, Pillay M, Buijs D, et al. Measurement of fraction unbound paclitaxel in human plasma. Drug Metab Dispos. 2000;28:1141–5.
McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6.
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–76.
Rowinsky EK, Gilbert MR, McGuire WP, Noe DA, Grochow LB, Forastiere AA, et al. Sequences of taxol and cisplatin: a phase I and pharmacologic study. J Clin Oncol. 1991;9:1692–703.
Huizing MT, Giaccone G, van Warmerdam LJ, Rosing H, Bakker PJ, Vermorken JB, et al. Pharmacokinetics of paclitaxel and carboplatin in a dose-escalating and dose-sequencing study in patients with non-small-cell lung cancer. The European Cancer Centre. J Clin Oncol. 1997;15:317–29.
Bristol-Myers Squibb Company. Summary of product characteristics: Taxol® (paclitaxel) injection. Princeton: Bristol-Myers Squibb Company. 2011.
Hershman DL, Weimer LH, Wang A, Kranwinkel G, Brafman L, Fuentes D, et al. Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat. 2011;125:767–74.
Bergmann TK, Brasch-Andersen C, Gréen H, Mirza M, Pedersen RS, Nielsen F, et al. Impact of CYP2C8*3 on paclitaxel clearance: a population pharmacokinetic and pharmacogenomic study in 93 patients with ovarian cancer. Pharmacogenom J. 2011;11:113–20.
Baldwin RM, Ohlsson S, Pedersen RS, Mwinyi J, Ingelman-Sundberg M, Eliasson E, et al. Increased omeprazole metabolism in carriers of the CYP2C19*17 allele; a pharmacokinetic study in healthy volunteers. Br J Clin Pharmacol. 2008;65:767–74.
Apellániz-Ruiz M, Lee M-Y, Sánchez-Barroso L, Gutiérrez-Gutiérrez G, Calvo I, García-Estévez L, et al. Whole-exome sequencing reveals defective CYP3A4 variants predictive of paclitaxel dose-limiting neuropathy. Clin Cancer Res. 2015;21:322–8.
Schneider BP, Li L, Radovich M, Shen F, Miller KD, Flockhart DA, et al. Genome-wide association studies for taxane-induced peripheral neuropathy in ECOG-5103 and ECOG-1199. Clin Cancer Res. 2015;21:5082–91.
Bergmann TK, Filppula AM, Launiainen T, Nielsen F, Backman JT, Brosen K. Neurotoxicity and low paclitaxel clearance associated with concomitant clopidogrel therapy in a 60-year-old Caucasian woman with ovarian carcinoma. Br J Clin Pharmacol. 2016;81:313–5.
Shinoda Y, Kimura M, Usami E, Asano H, Yoshimura T. Potential drug interaction between paclitaxel and clopidogrel. Biomed Rep. 2016;5:141–5.
Agergaard K, Mau-Sørensen M, Stage TB, Jørgensen TL, Hassel RE, Steffensen KD, et al. Clopidogrel paclitaxel drug–drug interaction: a pharmacoepidemiologic study. Clin Pharmacol Ther. 2017;. doi:10.1002/cpt.674 (Epub 2017 Feb 22).
Mielke S, Sparreboom A, Behringer D, Mross K. Paclitaxel pharmacokinetics and response to chemotherapy in patients with advanced cancer treated with a weekly regimen. Anticancer Res. 2005;25:4423–7.
Joerger M, Huitema ADR, Richel DJ, Dittrich C, Pavlidis N, Briasoulis E, et al. Population pharmacokinetics and pharmacodynamics of paclitaxel and carboplatin in ovarian cancer patients: a study by the European organization for research and treatment of cancer-pharmacology and molecular mechanisms group and new drug development group. Clin Cancer Res. 2007;13:6410–8.
Taxol prescribing information. Princeton: Bristol-Myers Squibb; 2011. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf. Accessed 4 Feb 2017.
de Graan A-JM, Elens L, Sprowl JA, Sparreboom A, Friberg LE, van der Holt B, et al. CYP3A4*22 genotype and systemic exposure affect paclitaxel-induced neurotoxicity. Clin Cancer Res. 2013;19:3316–24.
Joerger M, von Pawel J, Kraff S, Fischer JR, Eberhardt W, Gauler TC, et al. Open-label, randomized study of individualized, pharmacokinetically (PK)-guided dosing of paclitaxel combined with carboplatin or cisplatin in patients with advanced non-small-cell lung cancer (NSCLC). Ann Oncol. 2016;27:1895–902.
Mielke S, Sparreboom A, Steinberg SM, Gelderblom H, Unger C, Behringer D, et al. Association of paclitaxel pharmacokinetics with the development of peripheral neuropathy in patients with advanced cancer. Clin Cancer Res. 2005;11:4843–50.
Sonnichsen DS, Relling MV. Clinical pharmacokinetics of paclitaxel. Clin Pharmacokinet. 1994;27:256–69.
Abu-Khalaf MM, Baumgart MA, Gettinger SN, Doddamane I, Tuck DP, Hou S, et al. Phase 1b study of the mammalian target of rapamycin inhibitor sirolimus in combination with nanoparticle albumin-bound paclitaxel in patients with advanced solid tumors. Cancer. 2015;121:1817–26.
Akerley W, Herndon JE, Egorin MJ, Lyss AP, Kindler HL, Savarese DM, et al. Weekly, high-dose paclitaxel in advanced lung carcinoma: a phase II study with pharmacokinetics by the Cancer and Leukemia Group B. Cancer. 2003;97:2480–6.
Amrein PC, Clark JR, Supko JG, Fabian RL, Wang CC, Colevas AD, et al. Phase I trial and pharmacokinetics of escalating doses of paclitaxel and concurrent hyperfractionated radiotherapy with or without amifostine in patients with advanced head and neck carcinoma. Cancer. 2005;104:1418–27.
Ando M, Yonemori K, Katsumata N, Shimizu C, Hirata T, Yamamoto H, et al. Phase I and pharmacokinetic study of nab-paclitaxel, nanoparticle albumin-bound paclitaxel, administered weekly to Japanese patients with solid tumors and metastatic breast cancer. Cancer Chemother Pharmacol. 2012;69:457–65.
Berg SL, Tolcher A, O’Shaughnessy JA, Denicoff AM, Noone M, Ognibene FP, et al. Effect of R-verapamil on the pharmacokinetics of paclitaxel in women with breast cancer. J Clin Oncol. 1995;13:2039–42.
Brown T, Havlin K, Weiss G, Cagnola J, Koeller J, Kuhn J, et al. A phase I trial of taxol given by a 6-hour intravenous infusion. J Clin Oncol. 1991;9:1261–7.
Campos SM, Matulonis UA, Penson RT, Lee H, Berkowitz RS, Duska LR, et al. Phase II study of liposomal doxorubicin and weekly paclitaxel for recurrent Müllerian tumors. Gynecol Oncol. 2003;90:610–8.
Chang SM, Kuhn JG, Robins HI, Schold SC, Spence AM, Berger MS, et al. A phase II study of paclitaxel in patients with recurrent malignant glioma using different doses depending upon the concomitant use of anticonvulsants: a North American Brain Tumor Consortium report. Cancer. 2001;91:417–22.
Chang SM, Kuhn JG, Rizzo J, Robins HI, Schold SC, Spence AM, et al. Phase I study of paclitaxel in patients with recurrent malignant glioma: a North American Brain Tumor Consortium report. J Clin Oncol. 1998;16:2188–94.
Chao Y, Chan WK, Birkhofer MJ, Hu OY, Wang SS, Huang YS, et al. Phase II and pharmacokinetic study of paclitaxel therapy for unresectable hepatocellular carcinoma patients. Br J Cancer. 1998;78:34–9.
Chen Y, Pandya KJ, Feins R, Johnstone DW, Watson T, Smudzin T, et al. Toxicity profile and pharmacokinetic study of a phase I low-dose schedule-dependent radiosensitizing paclitaxel chemoradiation regimen for inoperable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;71:407–13.
Chi KN, Chia SK, Dixon R, Newman MJ, Wacher VJ, Sikic B, et al. A phase I pharmacokinetic study of the P-glycoprotein inhibitor, ONT-093, in combination with paclitaxel in patients with advanced cancer. Invest New Drugs. 2005;23:311–5.
Chico I, Kang MH, Bergan R, Abraham J, Bakke S, Meadows B, et al. Phase I study of infusional paclitaxel in combination with the P-glycoprotein antagonist PSC 833. J Clin Oncol. 2001;19:832–42.
Diamond JR, Wu B, Agarwal N, Bowles DW, Lam ET, Werner TL, et al. Pharmacokinetic drug-drug interaction study of the angiopoietin-1/angiopoietin-2-inhibiting peptibody trebananib (AMG 386) and paclitaxel in patients with advanced solid tumors. Invest New Drugs. 2015;33:691–9.
Fidias P, Supko JG, Martins R, Boral A, Carey R, Grossbard M, et al. A phase II study of weekly paclitaxel in elderly patients with advanced non-small cell lung cancer. Clin Cancer Res. 2001;7:3942–9.
Fisherman JS, Cowan KH, Noone M, Denicoff A, Berg S, Poplack D, et al. Phase I/II study of 72-hour infusional paclitaxel and doxorubicin with granulocyte colony-stimulating factor in patients with metastatic breast cancer. J Clin Oncol. 1996;14:774–82.
Garcia AA, Keren-Rosenberg S, Parimoo D, Rogers M, Jeffers S, Koda R, et al. Phase I and pharmacologic study of estramustine phosphate and short infusions of paclitaxel in women with solid tumors. J Clin Oncol. 1998;16:2959–63.
Gardner ER, Dahut WL, Scripture CD, Jones J, Aragon-Ching JB, Desai N, et al. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin Cancer Res. 2008;14:4200–5.
Gelderblom H, Verweij J, van Zomeren DM, Buijs D, Ouwens L, Nooter K, et al. Influence of cremophor El on the bioavailability of intraperitoneal paclitaxel. Clin Cancer Res. 2002;8:1237–41.
Gelderblom H, Mross K, ten Tije AJ, Behringer D, Mielke S, van Zomeren DM, et al. Comparative pharmacokinetics of unbound paclitaxel during 1- and 3-hour infusions. J Clin Oncol. 2002;20:574–81.
Gelderblom H, Baker SD, Zhao M, Verweij J, Sparreboom A. Distribution of paclitaxel in plasma and cerebrospinal fluid. Anticancer Drugs. 2003;14:365–8.
Gianni L, Kearns CM, Giani A, Capri G, Viganó L, Lacatelli A, et al. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. J Clin Oncol. 1995;13:180–90.
Herbst RS, Madden TL, Tran HT, Blumenschein GR, Meyers CA, Seabrooke LF, et al. Safety and pharmacokinetic effects of TNP-470, an angiogenesis inhibitor, combined with paclitaxel in patients with solid tumors: evidence for activity in non-small-cell lung cancer. J Clin Oncol. 2002;20:4440–7.
Horton TM, Ames MM, Reid JM, Krailo MD, Pendergrass T, Mosher R, et al. A Phase 1 and pharmacokinetic clinical trial of paclitaxel for the treatment of refractory leukemia in children: a Children’s Oncology Group study. Pediatr Blood Cancer. 2008;50:788–92.
Huizing MT, Keung AC, Rosing H, van der Kuij V, ten Bokkel Huinink WW, Mandjes IM, et al. Pharmacokinetics of paclitaxel and metabolites in a randomized comparative study in platinum-pretreated ovarian cancer patients. J Clin Oncol. 1993;11:2127–35.
Huizing MT, Vermorken JB, Rosing H, ten Bokkel Huinink WW, Mandjes I, Pinedo HM, et al. Pharmacokinetics of paclitaxel and three major metabolites in patients with advanced breast carcinoma refractory to anthracycline therapy treated with a 3-hour paclitaxel infusion: a European Cancer Centre (ECC) trial. Ann Oncol. 1995;6:699–704.
Hurria A, Blanchard MS, Synold TW, Mortimer J, Chung CT, Luu T, et al. Age-related changes in nanoparticle albumin-bound paclitaxel pharmacokinetics and pharmacodynamics: influence of chronological versus functional age. Oncologist. 2015;20:37–44.
Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, et al. Phase I and pharmacokinetic study of ABI-007, a cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8:1038–44.
Juan O, Rocher A, Sánchez A, Sánchez JJ, Alberola V. Influence of the cyto-protective agent amifostine on the pharmacokinetics of low-dose paclitaxel. Chemotherapy. 2005;51:200–5.
Kendra KL, Plummer R, Salgia R, O’Brien MER, Paul EM, Suttle AB, et al. A multicenter phase I study of pazopanib in combination with paclitaxel in first-line treatment of patients with advanced solid tumors. Mol Cancer Ther. 2015;14:461–9.
Maier-Lenz H, Hauns B, Haering B, Koetting J, Mross K, Unger C, et al. Phase I study of paclitaxel administered as a 1-hour infusion: toxicity and pharmacokinetics. Semin Oncol. 1997;24:19-16–9.
Martin LP, Kozloff MF, Herbst RS, Samuel TA, Kim S, Rosbrook B, et al. Phase I study of axitinib combined with paclitaxel, docetaxel or capecitabine in patients with advanced solid tumours. Br J Cancer. 2012;107:1268–76.
Miller TP, Chase EM, Dorr R, Dalton WS, Lam KS, Salmon SE. A phase I/II trial of paclitaxel for non-Hodgkin’s lymphoma followed by paclitaxel plus quinine in drug-resistant disease. Anticancer Drugs. 1998;9:135–40.
Minami H, Sasaki Y, Watanabe T, Ogawa M. Pharmacodynamic modeling of the entire time course of leukopenia after a 3-hour infusion of paclitaxel. Jpn J Cancer Res. 2001;92:231–8.
Mross K, Häring B, Holländer N, Mielke S, Behringer D, Massing U, et al. Comparison of 1-hour and 3-hours paclitaxel infusion pharmacokinetics: results from a randomized trial. Onkologie. 2002;25:503–8.
Ohtsu T, Sasaki Y, Tamura T, Miyata Y, Nakanomyo H, Nishiwaki Y, et al. Clinical pharmacokinetics and pharmacodynamics of paclitaxel: a 3-hour infusion versus a 24-hour infusion. Clin Cancer Res. 1995;1:599–606.
Panday VR, ten Bokkel Huinink WW, Vermorken JB, Rosing H, Koopman FJ, Swart M, et al. Pharmacokinetics of paclitaxel administered as a 3-hour or 96-hour infusion. Pharmacol Res. 1999;40:67–74.
Panday VRN, Huizing MT, van Warmerdam LJC, Dubbelman RC, Mandjes I, Schellens JHM, et al. Pharmacologic study of 3-hour 135 mg m−2 paclitaxel in platinum pretreated patients with advanced ovarian cancer. Pharmacol Res. 1998;38:231–6.
Papadopoulos KP, Egorin MJ, Huang M, Troxel AB, Kaufman E, Balmaceda CM, et al. The pharmacokinetics and pharmacodynamics of high-dose paclitaxel monotherapy (825 mg/m2 continuous infusion over 24 h) with hematopoietic support in women with metastatic breast cancer. Cancer Chemother Pharmacol. 2001;47:45–50.
Schiller JH, Storer B, Tutsch K, Arzoomanian R, Alberti D, Feierabend C, et al. Phase I trial of 3-hour infusion of paclitaxel with or without granulocyte colony-stimulating factor in patients with advanced cancer. J Clin Oncol. 1994;12:241–8.
Sekine I, Nishiwaki Y, Watanabe K, Yoneda S, Saijo N. Phase II study of 3-hour infusion of paclitaxel in previously untreated non-small cell lung cancer. Clin Cancer Res. 1996;2:941–5.
Smorenburg CH, Sparreboom A, Bontenbal M, Stoter G, Nooter K, Verweij J. Randomized cross-over evaluation of body-surface area-based dosing versus flat-fixed dosing of paclitaxel. J Clin Oncol. 2003;21:197–202.
Sparreboom A, Scripture CD, Trieu V, Williams PJ, De T, Yang A, et al. Comparative preclinical and clinical pharmacokinetics of a cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in cremophor (Taxol). Clin Cancer Res. 2005;11:4136–43.
Takano M, Kikuchi Y, Kita T, Suzuki M, Ohwada M, Yamamoto T, et al. Phase I and pharmacological study of single paclitaxel administered weekly for heavily pre-treated patients with epithelial ovarian cancer. Anticancer Res. 2002;22:1833–8.
Tamura T, Sasaki Y, Eguchi K, Shinkai T, Ohe Y, Nishio M, et al. Phase I and pharmacokinetic study of paclitaxel by 24-hour intravenous infusion. Jpn J Cancer Res. 1994;85:1057–62.
ten Tije AJ, Synold TW, Spicer D, Verweij J, Doroshow JH, Sparreboom A. Effect of valspodar on the pharmacokinetics of unbound paclitaxel. Invest New Drugs. 2003;21:291–8.
Terwogt JMM, Malingré MM, Beijnen JH, ten Huinink Bokkel WW, Rosing H, Koopman FJ, et al. Coadministration of oral cyclosporin A enables oral therapy with paclitaxel. Clin Cancer Res. 1999;5:3379–84.
Toppmeyer DL, Gounder M, Much J, Musanti R, Vyas V, Medina M, et al. A phase I and pharmacologic study of the combination of marimastat and paclitaxel in patients with advanced malignancy. Med Sci Monit. 2003;9:PI99-104.
Wilson WH, Berg SL, Bryant G, Wittes RE, Bates S, Fojo A, et al. Paclitaxel in doxorubicin-refractory or mitoxantrone-refractory breast cancer: a phase I/II trial of 96-hour infusion. J Clin Oncol. 1994;12:1621–9.
Yamada K, Yamamoto N, Yamada Y, Mukohara T, Minami H, Tamura T. Phase I and pharmacokinetic study of ABI-007, albumin-bound paclitaxel, administered every 3 weeks in Japanese patients with solid tumors. Jpn J Clin Oncol. 2010;40:404–11.
van Zuylen L, Karlsson MO, Verweij J, Brouwer E, de Bruijn P, Nooter K, et al. Pharmacokinetic modeling of paclitaxel encapsulation in Cremophor EL micelles. Cancer Chemother Pharmacol. 2001;47:309–18.
Hempel G, Rübe C, Mosler C, Wienstroer M, Wagner-Bohn A, Schuck A, et al. Population pharmacokinetics of low-dose paclitaxel in patients with brain tumors. Anticancer Drugs. 2003;14:417–22.
Henningsson A, Marsh S, Loos WJ, Karlsson MO, Garsa A, Mross K, et al. Association of CYP2C8, CYP3A4, CYP3A5, and ABCB1 polymorphisms with the pharmacokinetics of paclitaxel. Clin Cancer Res. 2005;11:8097–104.
Joerger M, Huitema ADR, Huizing MT, Willemse PHB, de Graeff A, Rosing H, et al. Safety and pharmacology of paclitaxel in patients with impaired liver function: a population pharmacokinetic-pharmacodynamic study. Br J Clin Pharmacol. 2007;64:622–33.
Li Y, Chen N, Palmisano M, Zhou S. Pharmacologic sensitivity of paclitaxel to its delivery vehicles drives distinct clinical outcomes of paclitaxel formulations. Mol Pharm. 2015;12:1308–17.
Henningsson A, Sparreboom A, Sandström M, Freijs A, Larsson R, Bergh J, et al. Population pharmacokinetic modelling of unbound and total plasma concentrations of paclitaxel in cancer patients. Eur J Cancer. 1990;2003(39):1105–14.
van Tellingen O, Huizing MT, Panday VRN, Schellens JHM, Nooijen WJ, Beijnen JH. Cremophor EL causes (pseudo-) non-linear pharmacokinetics of paclitaxel in patients. Br J Cancer. 1999;81:330–5.
Honkalammi J, Niemi M, Neuvonen PJ, Backman JT. Dose-dependent interaction between gemfibrozil and repaglinide in humans: strong inhibition of CYP2C8 with subtherapeutic gemfibrozil doses. Drug Metab Dispos. 2011;39:1977–86.
Honkalammi J, Niemi M, Neuvonen PJ, Backman JT. Gemfibrozil is a strong inactivator of CYP2C8 in very small multiple doses. Clin Pharmacol Ther. 2012;91:846–55.
Gréen H, Söderkvist P, Rosenberg P, Mirghani RA, Rymark P, Lundqvist EA, et al. Pharmacogenetic studies of paclitaxel in the treatment of ovarian cancer. Basic Clin Pharmacol Toxicol. 2009;104:130–7.
Bhalla KN, Kumar GN, Walle UK, Ibrado AM, Javed T, Stuart RK, et al. Phase I and Pharmacologic study of a 3-hour infusion of paclitaxel followed by cisplatinum and 5-fluorouracil in patients with advanced solid tumors. Clin Cancer Res. 1999;5:1723–30.
de Jongh FE, de Wit R, Verweij J, Sparreboom A, van den Bent MJ, Stoter G, et al. Dose-dense cisplatin/paclitaxel: a well-tolerated and highly effective chemotherapeutic regimen in patients with advanced ovarian cancer. Eur J Cancer. 2002;38:2005–13.
Kurata T, Tamura T, Shinkai T, Ohe Y, Kunitoh H, Kodama T, et al. Phase I and pharmacological study of paclitaxel given over 3 h with cisplatin for advanced non-small cell lung cancer. Jpn J Clin Oncol. 2001;31:93–9.
Belani CP, Kearns CM, Zuhowski EG, Erkmen K, Hiponia D, Zacharski D, et al. Phase I trial, including pharmacokinetic and pharmacodynamic correlations, of combination paclitaxel and carboplatin in patients with metastatic non-small-cell lung cancer. J Clin Oncol. 1999;17:676–84.
Calvert AH, Boddy A, Bailey NP, Siddiqui N, Humphreys A, Hughes A, et al. Carboplatin in combination with paclitaxel in advanced ovarian cancer: dose determination and pharmacokinetic and pharmacodynamic interactions. Semin Oncol. 1995;22:91–100.
Huizing MT, van Warmerdam LJ, Rosing H, Schaefers MC, Lai A, Helmerhorst TJ, et al. Phase I and pharmacologic study of the combination paclitaxel and carboplatin as first-line chemotherapy in stage III and IV ovarian cancer. J Clin Oncol. 1997;15:1953–64.
Moreira A, Lobato R, Morais J, Silva S, Ribeiro J, Figueira A, et al. Influence of the interval between the administration of doxorubicin and paclitaxel on the pharmacokinetics of these drugs in patients with locally advanced breast cancer. Cancer Chemother Pharmacol. 2001;48:333–7.
Gianni L, Dombernowsky P, Sledge G, Martin M, Amadori D, Arbuck SG, et al. Cardiac function following combination therapy with paclitaxel and doxorubicin: an analysis of 657 women with advanced breast cancer. Ann Oncol. 2001;12:1067–73.
Itkonen MK, Tornio A, Neuvonen M, Neuvonen PJ, Niemi M, Backman JT. Clopidogrel markedly increases plasma concentrations of CYP2C8 substrate pioglitazone. Drug Metab Dispos. 2016;44:1364–71.
Wozniak KM, Vornov JJ, Wu Y, Nomoto K, Littlefield BA, DesJardins C, et al. Sustained accumulation of microtubule-binding chemotherapy drugs in the peripheral nervous system: correlations with time course and neurotoxic severity. Cancer Res. 2016;76:3332–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Financial support for this study was provided by the Danish Research Council of Independent Research | Medical Sciences (DFF-5053-00042), the A.P. Møller Foundation for the Advancement of Medical Science, and the National Institutes of Health (1R01 CA192156).
Conflicts of interest
Tore B. Stage, Troels K. Bergmann, and Deanna L. Kroetz have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stage, T.B., Bergmann, T.K. & Kroetz, D.L. Clinical Pharmacokinetics of Paclitaxel Monotherapy: An Updated Literature Review. Clin Pharmacokinet 57, 7–19 (2018). https://doi.org/10.1007/s40262-017-0563-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-017-0563-z