Abstract
Background and Objective
Prasugrel, a P2Y12 adenosine diphosphate (ADP) receptor antagonist, inhibits ADP-mediated platelet activation and aggregation in patients with sickle cell anemia (SCA). We developed a population pharmacokinetic (popPK) model in pediatric patients from 2 to <18 years of age with SCA, and performed exposure–response evaluations to characterize the effects of prasugrel in a subset of these patients who weighed 19 kg or more and experienced at least two episodes of vaso-occlusive crises (VOC) in the past year.
Methods
A three-compartment popPK model adapted from that used in adults with acute coronary syndrome was used to describe the relationship between plasma concentrations of prasugrel’s active metabolite (Pras-AM) and time using data from phase II and III clinical studies in children. A VOC event rate model was developed from the phase III study to explore the exposure–response relationship between Pras-AM exposure and VOC, and included evaluation of covariates.
Results
The final popPK model for children with SCA provided a reasonable fit to Pras-AM plasma concentrations over time, with estimates of apparent clearance (CL/F) (172 L/h) and apparent volume of distribution (Vd/F) (51.7 L) that were comparable to previous studies in adults. The final model included weight as a covariate on both CL/F and Vd/F, and age as a covariate on CL/F. Analyses of safety (bleeding events requiring medical intervention) and efficacy (VOC event rate) variables showed no apparent relationship to model-predicted Pras-AM exposure quartiles, and no statistically significant effects of intrinsic or extrinsic factors on the VOC event rate were identified in the VOC event rate model. The effect of post hoc exposures on the VOC event rate did not reach statistical significance.
Conclusions
A popPK model was developed that provided reasonable parameter estimates, goodness-of-fit diagnostics, and visual predictive checks when applied to Pras-AM plasma concentrations in pediatric patients with SCA. Post hoc exposures obtained from this model did not correlate with measures of VOC or bleeding events in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In pediatric patients with sickle cell anemia, the pharmacokinetics of prasugrel’s active metabolite (Pras-AM) were adequately characterized by a structurally simplified model previously established in adult patients with acute coronary syndrome. |
There were no observable relationships between the Pras-AM exposure quartile and either the primary efficacy endpoint (vaso-occlusive crises [VOC]) or the primary safety endpoint (spontaneous bleeding requiring medical intervention) in pediatric patients with sickle cell anemia. |
A VOC event rate (response) model was developed to test several intrinsic and extrinsic factors, including exposure to Pras-AM, for their effects on the VOC event rate. None of these covariates reached the prespecified level of statistical significance (p < 0.001) to be retained in the final model. |
1 Introduction
Affecting up to 330,000 infants born annually, [1] sickle cell anemia (SCA) is an inherited blood disorder in which a more hydrophobic variant of hemoglobin (HbS) is expressed rather than normal hemoglobin (HbA) [2]. A principal complication of SCA is vaso-occlusive crisis (VOC), also termed ‘painful crisis’ or ‘severe pain episode,’ which manifests clinically as pain in various locations and can result in ischemia or infarction/necrosis of the affected organ [2].
No approved medicines affect the underlying pathology of SCA in pediatric patients, many of whom receive limited benefit from palliative first-line treatments, such as analgesia and intravenous hydration. Accordingly, approved treatments for SCA in pediatric patients are essential in combating symptoms of the disorder. Hydroxyurea induces a state of ‘stress erythropoiesis’ and increases fetal hemoglobin (HbF) and nitric oxide production, increasing blood flow by means of reduced intercellular adhesion and other mechanisms [3]. National Heart Lung and Blood Institute [4] and European Medicines Agency [5] guidelines recommend hydroxyurea for adults and children with SCA, but it has not been approved for use in children by the US Food and Drug Administration (FDA) [6]. While hydroxyurea treatment can provide significant benefits regarding rates of pain, acute chest syndrome, hospitalizations, and transfusions, decreases in markers of organ damage appear to vary [7].
The complex pathophysiology of vaso-occlusion in SCA provides many possibilities for therapeutic intervention, including agents that reduce platelet activation/aggregation, implicated in vaso-occlusion. Prasugrel, a P2Y12 platelet adenosine diphosphate (ADP) receptor antagonist, inhibits platelet activation and aggregation mediated by ADP [8, 9]. Prasugrel inhibits platelet activation and aggregation in adults [10, 11] and children/adolescents with SCA [12]. Prasugrel is not detected in plasma; it is rapidly hydrolyzed by carboxylesterases in the intestine to form a thiolactone precursor (Pras-TL, R-95913), which is converted to an active metabolite (Pras-AM, R-138727) by a single, cytochrome P450 (CYP)-dependent step in the liver and/or intestine [13]. Pras-AM concentration in plasma is, therefore, the primary analyte in pharmacokinetic analyses, as well as an essential component in exposure–response analyses in adult and pediatric patients.
Prasugrel pharmacokinetics and pharmacodynamics have been well-characterized in adults across several clinical studies using non-compartmental and population methods, and population pharmacokinetic (popPK) and pharmacokinetic/pharmacodynamic (PK/PD) models have remained largely unaltered throughout the development of prasugrel for adults with acute coronary syndrome (ACS) [14, 15]. Non-compartmental analysis was also used to characterize Pras-AM exposures in both adults and children with SCA, as previously reported [11, 12], but post-dose sampling schedules truncated at 4 h in these studies precluded accurate measurement of terminal-phase parameters such as apparent clearance (CL/F) and apparent volume of distribution (Vd/F). In addition to addressing this shortcoming, popPK modeling allows for quantitative testing of potential covariates on individual pharmacokinetic parameters as well as pharmacodynamics or response measures. This is particularly important in pediatric studies due to the limited numbers of patients and samples available for analyses, which may in turn restrict the ability to determine causes of variability [16]. The observed variability in exposures observed across a range of weight-based doses in the aforementioned study of prasugrel in children with SCA [12], a component of the present popPK analysis, is a noteworthy example.
The objectives of this study were to (1) develop a popPK model characterizing the relationship between Pras-AM plasma concentrations over time in pediatric patients with SCA using data from the phase II and III clinical studies TACX and TADO, respectively; (2) descriptively analyze and report the relationships between (A) Pras-AM exposure quartile and efficacy (VOC event rate) and (B) Pras-AM exposure quartile and safety (bleeding events requiring medical intervention) in the phase III clinical study (TADO); and (3) develop a response (VOC event rate) model exploring several covariates in Study TADO.
2 Methods
2.1 Study Design
Study TACX was a phase II, open-label, multicenter, dose-ranging study in pediatric patients with SCA. It was the first study in which prasugrel was given to individuals <18 years old. In this two-part study, patients received up to three single doses of prasugrel (Part A) and/or once-daily doses of prasugrel for 20–36 days (Part B). In Part B, all subjects received a dose that targeted 30% platelet inhibition for the first 14 ± 4 days and a second dose that targeted 50% platelet inhibition for the second 14 ± 4 days [12].
Study TADO was a phase III, double-blind, randomized, parallel-group, multinational, efficacy and safety comparison of prasugrel and placebo once daily for 9–24 months in pediatric patients with SCA. The primary objective of Study TADO was to assess the efficacy of prasugrel compared to placebo in pediatric patients with SCA as measured by reduction in the rate of VOC, which is a composite endpoint of painful crisis or acute chest syndrome. Patients received a starting dose of prasugrel 0.08 mg/kg based on PK/PD relationships elucidated in Study TACX. That study showed that a single dose level was not appropriate for all patients given the wide range of exposures observed across weight-based doses and variability in PK/PD relationships [12]; therefore, a dose-titration scheme was implemented. In Study TADO, patients received an initial daily dose of 0.08 mg/kg, followed by doses ranging from 0.04 to 0.12 mg/kg (absolute doses from 1 to 10 mg) titrated to a prespecified range of pharmacodynamic response as previously described [17]. Low body-weight patients receiving the lowest dose of 1 mg and requiring a dose reduction were treated with 1 mg every other day and discontinued from the study if their pharmacodynamic response (platelet reactivity) was still not within the predefined range.
2.2 Participants
Details pertaining to the study population have been published previously [12, 17]. In short, pediatric patients from 2 to <18 years of age, with body weight ≥12 kg (Study TACX) or ≥19 kg (Study TADO), and who have SCA (homozygous hemoglobin S [HbSS] genotype or compound heterozygous hemoglobin S/beta zero [HbS/β°] thalassemia) were eligible to participate. Patients taking hydroxyurea had to have been on a stable dose for 60 days before enrollment.
2.3 Sample Collection and Bioanalysis
In Study TACX, blood samples were collected for pharmacokinetic analysis at 0.5, 1, 1.5, 2, and 4 h post-dose. In Study TADO, patients followed one of three sampling schedules, each of which likewise involved sample collection through 4 h post-dose but collected either at different times or with lesser frequency. In two of the schedules, five samples were collected from each patient, emphasizing either earlier or later timepoints, which enabled better characterization of either the absorption or the elimination portion of individual concentration–time profiles. This aided overall fit of the population model when all data were combined. A third schedule, used in younger patients, collected only three samples to minimize overall blood volume collection.
Plasma samples from studies TACX and TADO were analyzed at Q2 Solutions (Ithaca, NY, USA) for the stably derivatized Pras-AM (R-138727_MP) using a validated liquid chromatography–tandem mass spectrometric detection method. The lower limit of quantification was 0.5 ng/mL and the upper limit of quantification was 250 ng/mL. Samples above the limit of quantification were diluted to yield results within the calibrated range. The interassay accuracy (defined as relative error) for R-138727_MP during initial validation ranged from −2.96 to 4.80%. The interassay precision (defined as relative standard deviation) during validation ranged from 2.61 to 6.49%.
2.4 Datasets
A comprehensive popPK analysis dataset was generated using Statistical Analysis System (SAS®; SAS Institute, Cary, NC, USA) software programs to merge Pras-AM plasma concentrations with dosing information and demographic data (e.g., weight, age, sex). Efficacy (VOC event rate) data and safety (bleeding event) data were then merged with post hoc exposure estimates from the popPK model, dosing data, and demographic features to create the exposure–response (E–R) analysis dataset. Both popPK and VOC event rate modeling employed the non-linear mixed-effects modeling software NONMEM®, version 7.3 (ICON Development Solutions, Hanover, MD, USA).
2.5 Model Development
Data from the phase II study TACX were combined with data from the phase III study TADO for popPK analyses, which provided the following: (1) an assessment of Pras-AM pharmacokinetics in the pediatric SCA population; (2) a characterization of interpatient variability in pharmacokinetics; (3) an assessment of intrinsic and extrinsic factors that could significantly influence Pras-AM pharmacokinetic parameters; and (4) post hoc exposure estimates for individuals to allow E–R analyses in Study TADO. In general, these analyses were exploratory and did not test a priori hypotheses. Graphical visualization and population modeling, based on NONMEM® in conjunction with such ancillary tools as Perl Speaks NONMEM (PsN), TIBCO Spotfire® S+ (TIBCO Software Inc., Palo Alto, CA, USA), and R 3.2.2, were the principal analysis techniques. Analyses were conducted in accordance with the tenets contained in the most recent FDA Guidance for Industry: Population Pharmacokinetics [18].
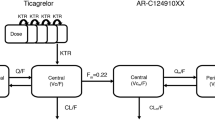
Attempts to fit the sparse Pras-AM concentration data from these pediatric patients using simple one- and two-compartment models with linear absorption were unsuccessful. Efforts subsequently shifted toward adapting the prasugrel popPK model in adults with ACS [14, 15] to pediatric patients with SCA, which was challenging due to differences in populations, the small number of blood samples collected from each child, and the small number of pediatric patients in each age cohort. Furthermore, visit durations were intentionally limited to minimize the burden of clinical procedures on pediatric patients and their caregivers, in turn limiting the post-dose pharmacokinetic sampling period and preventing reliable characterization of Pras-AM CL/F. Also, Pras-TL concentrations were not measured in these studies. In an effort to address these challenges, popPK model development commenced by simplifying the four-compartment model in adults to a three-compartment model in pediatric patients with SCA by eliminating the Pras-TL peripheral compartment and reducing the number of estimated parameters (Fig. 1). This required k 13, the Pras-TL formation rate constant, to instead be parameterized in terms of the Pras-AM first-pass formation rate constant (k 13 = k 12 × (1 − FFP)/FFP) (where k 12 is the rate constant for first-pass formation of Pras-AM and FFP is the fraction formed first-pass), an approach that was successfully employed in previous adult studies where concentrations of Pras-TL were likewise not available. Because exposure increased with lower body weight in adults with ACS [19] and was associated with an increased bleeding risk in patients <60 kg who received prasugrel 10 mg once daily, body weight was included as a covariate on both CL/F and Vd/F in the base pediatric model for the combined studies (i.e., before formal covariate testing) based on the wide range and probable influence of body weight in the pediatric population. Parameter estimation was further aided by including Bayesian post hoc estimates and variance–covariance matrix from Study TABR, an adult phase II stable cardiac patient study [15]. These were implemented as normal Wishart priors (NWPRI), added to the NONMEM® control stream using the $PRIOR coding block ($PRIOR NWPRI NTHETA=9, NETA=3, NTHP=7, NETP=3, NPEXP=1, where NTHETA denotes the total number of THETAs in the model and THETAs 8 and 9 correspond to covariate effects of weight on Vd/F and age on CL/F, which were not influenced by priors [NTHP]).
Schematic of the simplified population pharmacokinetic model for pediatric patients with sickle cell anemia. CL/F apparent clearance, D1 duration of absorption, FFP fraction formed first-pass, k 12 Pras-AM first-pass formation rate, k 13 Pras-TL (precursor) formation rate, k 32 rate of Pras-AM formation from precursor (Pras-TL), k 20 elimination rate, Vd/F apparent volume of distribution
2.5.1 Covariate Model Development
Having established an appropriate base pharmacokinetic model, the effects of potential covariates/patient factors (i.e., age, body weight, sex, race, hydroxyurea use) were assessed for their statistical significance and clinical relevance on the disposition of prasugrel after standard forward inclusion and backward elimination methodology (Fig. 2). Because body weight was already included as a covariate on both CL/F and Vd/F in the base model, it was not re-tested on these parameters during covariate testing, but was instead tested for significance on other structural parameters.
The relationships between NONMEM®-derived Bayesian estimates of individual parameters from the final base model and clinically relevant patient factors were explored graphically and also formally tested by measuring changes in objective function value (OFV). If a covariate was considered significant and was included on more than one parameter, the covariate was tested in combination on the parameters (e.g., CL/F and Vd/F in combination).
2.5.2 Final Pediatric Model
A full pharmacokinetic model was developed by combining covariates that were individually significant. Body weight and age are known to be highly correlated and physiologically related factors, especially in pediatric patients. Because an additive effect of one with the other could not be dismissed, each was tested independently and both were to be retained in the full model if statistically significant. Once the full model was established, the significance of the potential covariates was evaluated using a backward elimination (model reduction) technique as previously described [14, 15] to yield the final pharmacokinetic model (Fig. 2).
2.6 Model Evaluation
Besides OFV and goodness-of-fit plots (Electronic Supplementary Material [ESM] Fig. 1), visual predictive checks (VPCs) were performed on the final base and final pharmacokinetic models to ensure that each maintained fidelity with the data used for its development. The VPC entails simulating concentration–time data with the model, taking into account interpatient variability and residual error. The distributions of simulated concentrations, conditional on the posterior distribution of model parameters, were then compared to the corresponding observed distributions to ensure concordance. Simulated and observed distributions were compared by calculating the median, 5th, and 95th percentiles for each. Prediction correction [20] was used throughout to allow comparison of model performance across the range of absolute dose levels required to achieve predetermined weight-based dosing regimens.
In addition to VPCs, bootstrap analyses were conducted to assess the precision of the final parameter estimates of both the final base and the final pharmacokinetic models (ESM Fig. 2). The bootstraps were carried out using the PsN bootstrap routine, sampling from the analysis dataset with replacement, to produce re-sampled datasets with the same number of patients. Five hundred bootstrap datasets were created in this way for final base model evaluation (increased to 1000 for the final model, 993 of which ran successfully), and the model was fit to each one. The 95% confidence intervals for each parameter were calculated from the distribution of bootstrap parameter estimates (Table 2; ESM Fig. 2).
2.7 Exposure–Response: Descriptive Analyses and Vaso-Occlusive Crisis (VOC) Event Rate Model
Efficacy (VOC event rate) and safety (bleeding events requiring medical intervention) were initially assessed graphically by Pras-AM model-predicted area under the concentration–time curve (AUC) quartile to determine their relationship, if any. The exposure–VOC relationship was then investigated further with a quantitative VOC event rate model in NONMEM®, in which the typical value and distribution of the VOC event rate in the Study TADO population were determined and the significance of exposure and several intrinsic and extrinsic factors were tested.
Models to describe the central tendency and distribution of the VOC event rate were evaluated using log-transformed data due to their skewed distribution. The general structure of the model included one parameter to represent the typical value (central tendency) and another parameter (random effect) for interpatient variability as shown in the equation below:
where VOC i is the event rate for individual patient i, VOCpop is the population typical value for the event rate, and η i is the random effect for individual patient i, representing their difference from the typical value.
Due to the nature of the distribution of the VOC event rate, various transformations of the random effects in the model were tested, including a log-normal distribution, logit, Box–Cox, and heavy-tailed transformations [21]. Ultimately, the VOC event rate model described the typical value and distribution of the VOC event rate in the Study TADO population using a mixture model to describe the bimodal distribution. The general process used for VOC event rate model development was similar to that used for popPK model development (Fig. 2). Intrinsic factors tested for their effect on VOC included age as a continuous variable; age as categories of 2 to <6 years, 6 to <12 years, and 12 to <18 years; body weight; race; ethnicity; sex; and individual CL/F of Pras-AM. Extrinsic factors tested included hydroxyurea use and study arm. Pras-AM exposure was also tested together with the covariates. For the drug effect, a maximum-effect model was tested in addition to linear and power models. Evaluation was based on model fit to the distribution of VOC data before and after transformation, and included a comparison of individual predictions (IPRED) to observations (dependent variable [DV]) as well as a VPC (ESM Fig. 3). Because there was only one VOC event rate per patient, residual error could not be separated from interpatient variability. Residual plots were therefore not created.
3 Results
3.1 Demographics
The demographics and clinical characteristics for patients in the popPK dataset are listed in Table 1. The median (range) age of 11 (2–18) years and weight of 30 (13–81) kg were calculated from all 167 patients who received at least one dose of prasugrel and contributed samples for bioanalysis, while centering for the other parameters was calculated from the 145 patients remaining after excluding missing or unusable data. Most patients were Black/African (79%) with HbSS genotype (93%). About half of the patients were taking concomitant hydroxyurea.
3.2 Final Population Pharmacokinetic Model
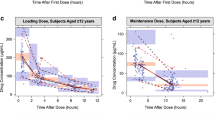
Overall, parameter estimates and associated standard errors were quite similar between the base and final pharmacokinetic models (base model results not shown), with a reduction in interpatient variability associated with CL/F (33.9 vs. 38.8% coefficient of variation) after adding age as a covariate (Table 2). Formal covariate testing on the final base pharmacokinetic model included weight at visit, age at visit, race, sex, and concomitant hydroxyurea use. Of these, only the effect of age on CL/F reached the predefined significance threshold to be retained in the final pharmacokinetic model, in addition to the weight covariates that were included in the base model. Given the paucity of patients with the HbS/β° thalassemia genotype, it was not evaluated as a covariate. Interpatient variability for the duration of absorption (92%) and apparent Vd/F of Pras-AM distribution (128%) values were both high, in accordance with the variability in pharmacokinetics across the pediatric population. Goodness-of-fit plots (ESM Fig. 1) were reasonable, especially in the region of lower concentrations, where most of the data reside. At higher concentrations, where data are sparse, they exhibit a general bias toward under-prediction in population prediction (PRED), IPRED, and conditional prediction (CPRED) relative to observations. This may reflect that stepwise conversions of the parent drug to the precursor (Pras-TL) and then to Pras-AM occur at different rates within the pediatric population, and/or that concentration data (Pras-AM collection points or the absence of Pras-TL concentrations) in the pediatric studies are inadequate to better characterize the absorption, distribution, and early elimination phases of Pras-AM (near maximum concentration [C max]), where concentrations are highest and most variable. Regardless of causality, the discrepancy is minimized after accounting for interpatient variability, as shown in the IPRED versus DV plot, and overall model fit, as shown in the prediction-corrected confidence interval VPC of the final pharmacokinetic model (Fig. 3).
Prediction-corrected, confidence interval visual predictive check of the final population pharmacokinetic model. Solid circles denote prediction-corrected observed data, dashed lines denote the median, 5th, and 95th percentiles of prediction-corrected observations, and shaded regions indicate the 90% confidence intervals for the median, 5th, and 95th percentiles of the simulated predictions
3.3 VOC Event Rate
The analysis of VOC event rate by Pras-AM popPK model-predicted AUC quartile showed no discernible relationship between the rate of VOC and Pras-AM exposure over the range of estimated exposures in Study TADO. Not all prasugrel-treated patients contributed to pharmacokinetic analysis, and therefore not all treated patients experiencing VOC events have corresponding exposure estimates. The annual rate of VOC was similar across all four quartiles, with similar median VOC event rates and considerable overlap in the interquartile ranges of VOC/year (Fig. 4). To further explore the relationship between Pras-AM exposure and VOC events, a VOC event rate model was developed to describe the typical value and distribution of the VOC event rate in the Study TADO population. Data from this model showed no statistically significant effect of the covariates tested, including drug treatment (prasugrel vs. placebo), Pras-AM exposure, age, race, and concomitant hydroxyurea use. The final VOC event rate model included a mixture component for the bimodal distribution, and the parameter estimates are shown in Table 3. Approximately 30% of patients were predicted not to experience an annual VOC event. Results from this analysis are consistent with the overall statistical analysis of Study TADO data, which did not meet the primary study objective of VOC event rate reduction.
Annual number of vaso-occlusive crises in Study TADO versus model-predicted Pras-AM AUC quartile. Boxes denote 25th and 75th percentiles, lines within boxes represent median values, whiskers extend to the 95th percentile, and datapoints above whiskers denote observed values above the 95th percentile. AUC population pharmacokinetic model-predicted area under the curve of Pras-AM concentration versus time, N number of patients, Pras-AM prasugrel active metabolite, VOC vaso-occlusive crisis
3.4 Safety Events
The relationship between safety and the popPK model-predicted Pras-AM exposure quartile in Study TADO is illustrated in Fig. 5. Not all prasugrel-treated patients contributed to pharmacokinetic analysis, and therefore not all treated patients experiencing bleeding events have corresponding exposure estimates. The number of bleeding events requiring medical intervention—the greatest safety concern in the pediatric sickle-cell population—was low overall, with 13 events among prasugrel-treated patients (9 in patients with exposure estimates and 4 in patients without exposure estimates) and 9 in placebo-treated patients. The number of patients experiencing a bleeding event requiring medical intervention was also low, with 11 prasugrel-treated patients (8 with exposure estimates and 3 without exposure estimates) and 8 placebo-treated patients. The quartile analyses of the total number of bleeding events requiring medical intervention (Fig. 5a) and the number of patients who had such an event (Fig. 5b) show no relationship to Pras-AM exposure quartile, with more bleeding events and patients associated with quartiles below the median AUC than above. Given the limited number of events, formal exposure–response modeling of safety events was not pursued.
Bleeding events requiring intervention versus model-predicted Pras-AM AUC quartile in Study TADO. Summary by number of bleeding events (a) and by number of patients with bleeding events (b). In a, percentages represent the number of bleeding events in the Pras-AM AUC quartile or the placebo group divided by the total number of bleeding events across all patients (n = 22). There were 4 (18.2%) bleeding events with intervention in patients randomized to prasugrel from whom Pras-AM data (AUC) were unavailable. In b, percentages represent the number of patients with a bleeding event in the Pras-AM AUC quartile or the placebo group divided by the number of patients in that quartile or the placebo group. There were 3 (5.1%) patients with bleeding events requiring intervention in patients randomized to prasugrel from whom Pras-AM data (AUC) were unavailable. Q1: N = 28, AUC <17.3 ng·h/mL; Q2: N = 28, AUC 17.3–21.8 ng·h/mL; Q3: N = 28, AUC 21.8–28.6 ng·h/mL; Q4: N = 28, AUC ≥28.6 ng·h/mL; patients randomized to prasugrel from whom Pras-AM data were unavailable: N = 59; Placebo: N = 170. AUC model-predicted area under the concentration versus time curve, n number of bleeding events (a) or patients with bleeding events (b) requiring intervention, N total number of patients in AUC quartile or placebo group, Pras-AM prasugrel active metabolite, Q quartile
4 Discussion
PopPK models, similar to those we describe here, may help overcome barriers to drug use in pediatric patients with SCA by providing predictions of drug pharmacokinetics that optimize dose selection and maximize the chance of improved outcomes. This is exemplified by the popPK models describing hydroxyurea dosage in pediatric patients that predict hydroxyurea exposure [22, 23] and provide a pharmacokinetic model-based individualized hydroxyurea dosing strategy with body weight as a significant predictor of CL/F [23]. Thus, popPK models in pediatric patients with SCA have aided in scaling dosage appropriately, thereby enhancing the potential for therapeutic effect while minimizing the risk of overdose.
This analysis demonstrates that the application of population-based methods to children with SCA are not limited to dose selection, but may also be used to better understand intrinsic and extrinsic factors (covariates) that influence pharmacokinetics and allow better understanding of E–R relationships. Differences between patient populations and study designs between adult ACS and pediatric SCA clinical studies of prasugrel resulted in a limited number of patients, relatively short visit durations, and fewer blood samples per patient in the pediatric SCA studies than in adults with ACS. Therefore, the four-compartment popPK model developed for adult ACS patients [14, 15] could not be directly applied to pediatric SCA patients without modification. The complexity of the model was incompatible with the quantity and range of concentration data to which it was being applied, leading to over-parameterization and subsequent estimation failures. Application of simple one- and two-compartment models with linear absorption and first-order elimination was similarly unsuccessful, likely owing to the unique metabolism of prasugrel parent drug to thiolactone precursor (Pras-TL) en route to the active metabolite (Pras-AM) and subsequent conversion to inactive metabolites [15].
To account for these aspects of the metabolism and activation of prasugrel, as well as to accommodate the paucity of data in the pediatric SCA studies, the adult popPK model was structurally simplified to a three-compartment model by removing the Pras-TL distribution compartment. This structural simplification was complemented by the use of informative priors—Bayesian post hoc estimates and variance–covariance matrix—from a phase II study in adults with stable atherosclerosis. This reduced the number of estimated parameters and simplified the estimation routine. Cumulatively, these changes resulted in a base model that provided a reasonable fit to plasma concentrations of Pras-AM in the dataset comprising pediatric SCA studies TACX and TADO.
Having identified a suitable structural popPK model, efforts shifted to covariate testing. Identifying predictive covariates to explain interpatient variability in parameter estimates is one of the major benefits of population approaches in general, and pediatric studies in particular benefit by including the effects of age and/or body size [16]. Because growth and development are simultaneous processes, age and body size are considered primary covariates for pharmacokinetic models in pediatric patients [16, 24, 25]. Age is a significant covariate describing maturation of drug metabolism [16] associated with the passage of time [25], and body weight is a significant covariate for CL/F and Vd/F [22, 23]. Accordingly, the popPK pediatric SCA model we describe included body weight as a covariate on both CL/F and Vd/F in the base model (prior to formal covariate testing) given its known significance in both adults and children, as well as the wide range of body weights in the pediatric SCA population. During formal covariate testing, age was found to provide an additional, statistically significant benefit, accounting for a notable percentage of interpatient variability in CL/F. Age was therefore retained as a covariate on CL/F in the final popPK model.
Population pharmacokinetic modeling provided useful estimates for key pharmacokinetic parameters, including more precise approximations of individual CL/F values and subsequent calculation of steady-state exposures for exposure–response evaluations. Analyses of efficacy (defined as VOC event rate) and safety (defined as bleeding events requiring medical intervention) variables relative to Pras-AM exposure quartiles showed no discernable relationship. Study TADO failed to meet its primary objective, which was a reduction in the frequency of VOC in children with SCA treated with prasugrel. This relationship was also investigated with a VOC event rate model in which the typical value and distribution of the VOC event rate in the Study TADO population was used to directly test covariates, including treatment group (drug vs. placebo) and exposure on VOC event, and likewise showed no significant treatment effect on the primary outcome measure based on predefined criteria. A graphical analysis of safety events from Study TADO, specifically bleeding events requiring medical intervention, showed no discernible correlation with exposure quartile. Based on the very few bleeding events throughout the course of the study, as well as the lack of an observable trend by exposure quartile, exposure–safety relationships were not explored further.
Limitations to model development and analyses include the small number of plasma samples from which to measure Pras-AM, the limited number and short duration of clinic visits at which pharmacokinetic data were collected (no pharmacokinetic sample collection beyond 4 h post-dose), limited number of clinical events, and the intrinsic challenge of developing and implementing population models that make assumptions about a population with variable clinical characteristics. Pursuant to this, a key assumption in the use of priors from an adult ACS study in a pediatric SCA analysis was that the two were comparable in their pharmacokinetic characteristics. This was corroborated by simulation–observation overlay (Eli Lilly and Company, data on file), and justified the use of priors from Study TABR to guide the fitting of the pharmacokinetic data from studies TACX and TADO. While exposures among adults [11] and children [12] with sickle cell anemia are quite similar, in the current analysis we have avoided a direct comparison of adults to children given differences in study designs, dosing regimens, and the inherent physiological and clinical differences between the two populations. Another limitation was the predefined dose range in Study TADO, specifically the upper limit of 0.12 mg/kg (not to exceed an absolute dose of 10 mg). While this is the daily tablet strength for most adults in the ACS population, some children in Study TADO exhibited lower than anticipated platelet inhibition at this dose level. Although this might suggest that higher doses were warranted, the primary concern when defining the dose range in Study TADO was that of patient safety, specifically the threat of spontaneous or injury-induced bleeding events in active children. This concern precluded exploration beyond the upper dose limit of 10 mg/day. Genotyping of CYP2C19 was not included in the current study since in non-cardiac subjects, and in contrast with clopidogrel, the pharmacokinetics and pharmacodynamics of prasugrel are minimally affected by polymorphisms in this gene [26]. In cardiac patients, CYP2C19 polymorphisms have been reported to affect the pharmacodynamics of prasugrel, but to a much lesser extent than their effect on clopidogrel [27, 28].
5 Conclusions
PopPK modeling and simulation in pediatric patients with SCA may help predict drug exposure, and thereby improve treatment decisions and ultimately patient outcomes. A popPK model for prasugrel in adult ACS patients was adapted to pediatric patients with SCA, and corroborated the weight effect found in previous adult ACS studies [14, 15]. Age was identified as a significant covariate on CL/F in addition to (and independent of) body weight. Subsequent E–R analyses showed no discernable trend between either VOC or safety (bleeding events requiring medical intervention) and post hoc Pras-AM exposure quartiles obtained from the final popPK model across the dose range evaluated in Study TADO. Moreover, among the covariates tested, there were no statistically significant effects of intrinsic or extrinsic factors—including treatment (drug vs. placebo)—in the VOC event rate model.
References
Piel FB, Patil AP, Howes RE, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381:142–51.
Serjeant GR. Sickle-cell disease. Lancet. 1997;350:725–30.
Green NS, Barral S. Emerging science of hydroxyurea therapy for pediatric sickle cell disease. Pediatr Res. 2014;75:196–204.
National Heart, Lung, and Blood Institute. U.S. Department of Health and Human Services. Evidence-based management of sickle cell disease. Expert panel report. 2014. https://www.nhlbi.nih.gov/sites/www.nhlbi.nih.gov/files/sickle-cell-disease-report.pdf. Accessed 3 Feb 2017.
European Medicines Agency. Public summary of positive opinion for orphan designation of hydroxyurea for the treatment of sickle cell syndrome. 2008. http://www.ema.europa.eu/docs/en_GB/document_library/Orphan_designation/2009/10/WC500006488.pdf. Accessed 3 Feb 2017.
Centers for Disease Control. U.S Department of Health and Human Services. Evidence-based management of sickle cell disease. 2016. https://www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines/. Accessed 27 Apr 2016.
Fitzhugh CD, Hsieh MM, Allen D, et al. Hydroxyurea-increased fetal hemoglobin is associated with less organ damage and longer survival in adults with sickle cell anemia. PLoS One. 2015;10:e0141706.
Niitsu Y, Jakubowski JA, Sugidachi A, et al. Pharmacology of CS-747 (prasugrel, LY640315), a novel, potent antiplatelet agent with in vivo P2Y12 receptor antagonist activity. Semin Thromb Hemost. 2005;31:184–94.
Jakubowski JA, Winters KJ, Naganuma H, et al. Prasugrel: a novel thienopyridine antiplatelet agent: a review of preclinical and clinical studies and the mechanistic basis for its distinct antiplatelet profile. Cardiovasc Drug Rev. 2007;25:357–74.
Wun T, Soulieres D, Frelinger AL, et al. A double-blind, randomized, multicenter phase 2 study of prasugrel versus placebo in adult patients with sickle cell disease. J Hematol Oncol. 2013;6:1–10.
Jakubowski JA, Zhou C, Small DS, et al. A phase 1 study of prasugrel in patients with sickle cell disease: pharmacokinetcs and effects on ex vivo platelet reactivity. Br J Clin Pharmacol 2012;75(6):1433–44.
Styles L, Heiselman D, Heath LE, et al. Prasugrel in children with sickle cell disease: pharmacokinetic and pharmacodynamic data form an open-label, adaptive design, dose-ranging study. J Pediatr Hematol Oncol. 2015;37:1–9.
Small DS, Farid NA, Payne CD, et al. Effect of intrinsic and extrinsic factors on the clinical pharmacokinetics and pharmacodynamics of prasugrel. Clin Pharmacokinet. 2010;49:777–98.
Wrishko RE, Ernest S, Small DS, et al. Population pharmacokinetic analyses to evaluate the influence of intrinsic and extrinsic factors on exposure of prasugrel active metabolite in TRITON-TIMI 38. J Clin Pharmacol. 2009;49:984–98.
Ernest CS, Small DS, Rohatagi S, et al. Population pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel in aspirin-treated patients with stable coronary artery disease. J Pharmacokinet Pharmacodyn. 2008;35:593–618.
Vinks AA. Important role of population pharmacokinetic/pharmacodynamic modeling in pediatric patients. J Pediatr. 2011;159:361–3.
Hoppe CC, Styles L, Heath L, et al. Design of the DOVE (Determining effects of platelet inhibition on vaso-occlusive events) trial: a global phase 3 double-blind, randomized, placebo-controlled multicenter study of the efficacy and safety of prasugrel in pediatric patients with sickle cell anemia utilizing a dose titration strategy. Pediatr Blood Cancer. 2016;63:299–305.
United States Food and Drug Administration. Guidance for industry: population pharmacokinetics. 1999. http://www.fda.gov/downloads/Drugs/…/Guidances/UCM072137.pdf. Accessed 12 May 2016.
Jakubowski JA, Angiolillo DJ, Zhou C, et al. The influence of body size on the pharmacodynamic and pharmacokinetic response to clopidogrel and prasugrel: a retrospective analysis of the FEATHER study. Thromb Res 2014;134:552–7.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–51.
Petersson KJ, Hanze E, Savic RM, Karlsson MO. Semiparametric distributions with estimated shape parameters. Pharm Res. 2009;26:2174–85.
Wiczling P, Liem RI, Panepinto JA, et al. Population pharmacokinetics of hydroxyurea for children and adolescents with sickle cell disease. J Clin Pharmacol. 2014;54:1016–22.
Dong M, McGann PT, Mizuno T, et al. Development of a pharmacokinetic-guided dose individualization strategy for hydroxyurea treatment in children with sickle cell anaemia. Brit J Clin Pharmacol. 2016;81:742–52.
Manolis E, Osman TE, Herold R, et al. Role of modeling and simulation in pediatric investigation plans. Pediatr Anaesth. 2011;21:214–21.
Anderson BJ, Holford NHG. Tips and traps of analyzing pediatric PK data. Pediatr Anaesth. 2011;21:222–37.
Brandt JT, Close SL, Iturria SJ, et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost. 2007;5:2429–36.
Cuisset T, Loosveld M, Morange PE, et al. CYP2C19*2 and *17 alleles have a significant impact on platelet response and bleeding risk in patients treated with prasugrel after acute coronary syndrome. JACC Cardiovasc Interv. 2012;5:1280–7.
Grosdidier C, Quilici J, Loosveld M, et al. Effect of CYP2C19*2 and *17 genetic variants on platelet response to clopidogrel and prasugrel maintenance dose and relation to bleeding complications. Am J Cardiol. 2013;111:985–90.
Acknowledgements
The authors wish to acknowledge the contributions of Chunmei Zhou, Patricia Brown, and Lori Heath of Eli Lilly and Company for aid in patient-level data acquisition and verification, as well as confirmation of trial details; C. Steven Ernest II of Eli Lilly and Company for valuable insight and suggestions regarding the adult population pharmacokinetic model and its adaptation to the pediatric studies reported herein; Timothy H. Waterhouse of Eli Lilly and Company for assistance in reviewing and providing comments on the manuscript; and Gina Moore, MS, inVentiv Health Clinical, for technical assistance in preparing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Brian A. Moser, Elizabeth S. LaBell, Emmanuel Chigutsa, Joseph A. Jakubowski, and David S. Small are employees and minor stockholders of Eli Lilly and Company.
Ethics
Clinical protocols were reviewed and approved by ethical/institutional review boards, and all study procedures were performed under ethical standards originating in the Declaration of Helsinki. Study participants were required to provide written informed consent before participating in study activities.
Funding
These studies were sponsored by Eli Lilly and Company and Daiichi-Sankyo.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moser, B.A., LaBell, E.S., Chigutsa, E. et al. Population Pharmacokinetic and Exposure–Response Analyses of Prasugrel in Pediatric Patients with Sickle Cell Anemia. Clin Pharmacokinet 57, 243–254 (2018). https://doi.org/10.1007/s40262-017-0556-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-017-0556-y