Abstract
Background and Objective
Breast cancer is the second leading cause of cancer death worldwide. The economic burden of breast cancer is crucial for the sustainability of healthcare systems. The objective of this study was to estimate the burden of HR+/HER2− metastatic breast cancer (MBC) in Italy, in terms of incidence, prescription patterns, healthcare resource utilisation and costs for the National Health System (NHS).
Methods
A cohort study based on healthcare administrative data (ReS database), covering > 10 million Italians, was performed. Incident cases of HR+/HER2− MBC were identified among adult women in 2013. The cohort was followed-up for 2 years to describe healthcare utilisation and integrated costs (pharmaceuticals, hospitalisations and outpatient services) for NHS. Prescription patterns were described as first-line choice and therapeutic changes. Specific therapeutic changes were used as proxies of disease progression. A survival analysis was performed to estimate the time from diagnosis to first disease progression.
Results
Of 5174,723 women, 355 cases of de novo HR+/HER2− MBC were selected (incidence: 6.9 per 100,000). During the 1st follow-up year, they generated an average cost of €7543, whereas €4834 in the 2nd year. The 85.9% received a monotherapy, while the 14.1% received a combination therapy. The most used monotherapy was nonsteroidal-aromatase-inhibitors (45.9%), while the most prescribed combination was tamoxifen + luteinizing hormone releasing hormone (LHRH) analogues (6.2%). Therapeutic changes occurred in 45.4% of patients, especially from chemotherapy to nonsteroidal-aromatase-inhibitors, after an average of 276.8 days from the first treatment. Disease progression was identified in 22.5% of patients occurring after a mean 13 ± 6 months from diagnosis.
Conclusions
This detailed picture of HR+/HER2− MBC, based on real-world data, could be helpful in health technology assessment and expenditure forecasts of future therapeutic strategies for this condition in Italy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Healthcare administrative data can be useful to investigate epidemiology and real-world practice of HR+/HER2− metastatic breast cancer. |
The incidence of HR+/HER2− metastatic breast cancer was 6.9 per 100,000, with an average cost for the National Health System of €7543 in the 1st follow-up year and €4834 in the 2nd year. |
Real-world evidence on HR+/HER2− metastatic breast cancer support health technology assessment of new therapies in order to forecast their expenditure. |
1 Introduction
According to the International Agency for Research on Cancer (IARC), breast cancer is the second most cancer overall and the fifth leading cause of cancer death worldwide [1]. It hides an extremely complex biological tapestry that can include multiple expressions of the neoplasm with varying morphology, natural history and prognosis. The most recent acquisitions on molecular biology have allowed to identify a number of oncologic determinants able to characterise different subsets of breast cancer patients. In daily clinical practice, however, the most important biological factors are oestrogen and progesterone receptors (ER and PR) and the epidermal growth factor-2 receptor (HER2). On the basis of the expression of these three biological determinants, since the early 2000s, mammary carcinomas have been classified into four distinct and reproducible molecular subtypes: (i) luminal A and (ii) luminal B, which express hormonal receptors, with Ki67 overexpressed only in luminal B; (iii) basal-like, mainly including triple negative breast carcinomas; and (iv) HER2− enriched, having the highest activation of the HER2 pathway [2,3,4,5].
Each of these four categories has a substantially different prognosis and, consequently, different therapeutic guidelines. It is therefore most interesting to analyse carefully, in a real-world setting, the outcome correlated with cost management for the National Health System (NHS). The economic burden of breast cancer is crucial for the sustainability of healthcare systems, also considering that it accounted for the highest healthcare costs in European countries [6].
To this purpose, real-world data can be a valuable tool to better understand current management of the different disease subtypes and to implement new policy programmes [7].
The aim of this study was to depict the current burden of stage IV (de novo diagnosed) hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) metastatic breast cancer (MBC) in Italy, providing real-world incidence, healthcare resource utilisation and costs for NHS. An additional aim was to describe the prescription patterns in terms of therapeutic choices, combination therapies and switches.
2 Methods
2.1 Data Source
A real-world analysis was conducted using ReS DataBase (ReS DB), coming from the collaboration between ReS (Fondazione Ricerca e Salute-Research and Health Foundation) and CINECA (Interuniversity Consortium). ReS DB is a patient-centred data warehouse, where the following healthcare administrative databases are linked with each other: socio-demographic registry, reimbursed drug prescription database, hospital discharge database, and outpatient services and visits database. Therefore, ReS DB can be analysed to describe the current disease burden by providing, for each subject, the precise healthcare pathway and related direct costs in the perspective of NHS.
From 2012 to 2015, this data source (previously named ARCO) included information on more than 10 million Italian inhabitants, resident in different Regions and Local Health Units. Quality and usefulness of data derived from administrative databases have been demonstrated by recent observational studies on other clinical populations [8, 9].
2.1.1 Cohort Selection
Patients affected by de novo HR+/HER2− MBC were identified among the female population aged ≥ 18 years at 2013 (accrual year).
MBC was identified by searching hospital discharge data where all diagnoses are codified according to the International Classification of Diseases – 9th Revision, Clinical Modification (ICD9-CM). The use of administrative hospital discharge data to detect and stage breast cancer was supported by previous studies [10,11,12]. A woman was defined by MBC if she was hospitalized in the accrual year with a diagnosis (primary or secondary) of “malignant neoplasm of female breast” (ICD-9-CM: 174.0-174.9) and, simultaneously, with a diagnosis (primary or secondary) of “secondary and malignant neoplasms” (ICD9-CM codes are listed in the supplementary Table 1S). The discharge date responding to these criteria was considered as the index date for each patient. In order to select only de novo cases, all patients who met the above criteria also in the previous year (2012) were excluded. Therefore, this strategy was able to capture MBC at the diagnosis (de novo) and it excluded MBC distant recurrence, by assuming that a patient affected by breast cancer is admitted to hospital at least one time during one year (if nothing else in a day-hospital setting).
The expression of receptors was derived using drugs as proxies, in agreement with previous researches [13, 14]. To this purpose, the reimbursed drug prescription database, where all drugs are codified according to the Anatomical Therapeutic Chemical (ATC) classification, was investigated. A patient was defined HR+ if, in the 24 months after the index date, she received at least one prescription of the following drugs: tamoxifen (ATC: L02BA01), aromatase inhibitors (L02BG), or fulvestrant (L02BA03). Finally, a patient was defined HER2− if in the 24 months after the index date, she did not receive any prescription of trastuzumab (L01XC03) or lapatinib (L01XE07).
2.2 Study Design
This was a cohort study based on historical healthcare administrative data. Each patient was selected in 2013 (accrual year, where the index date was identified). Starting from the index date, a previous period of 12 months was observed to ascertain the de novo status of the disease. A follow-up period of 24 months after the index date was also considered to define receptor expression and to describe outcome, healthcare utilisation and costs.
2.3 Analysis of Disease Burden
At the accrual year (2013), the overall annual incidence estimation (per 100,000 adult women) and its distribution across age classes were provided. Healthcare resource utilisation and direct costs, in the perspective of NHS, were calculated separately for each year of follow-up.
Hospital admissions were analysed in terms of primary diagnosis or procedure, using the DRG (Diagnosis-Related Group) classification. Outpatient services and visits were analysed using regional lists of all healthcare services reimbursed by NHS. Drug therapies were described in terms of (i) specific outpatient drugs used to treat neoplasm, (ii) chemotherapies administered in day-hospital or ambulatory settings, and (iii) other concomitant drugs.
Healthcare costs were evaluated using real price paid by NHS and tariffs for in- and outpatient services (only direct costs). Cost analyses were performed both as single expenditure item (i.e. hospital discharges, drug prescriptions and ambulatory services and visits) and as integrated costs. The total annual cost and the average annual cost per patient, in the perspective of NHS, were calculated for each follow-up year. Furthermore, the costs related to the index event (discharge with one of the diagnoses of interest) were presented separately.
2.4 Analysis of Prescription Patterns
The prescription pattern of each patient included in the study cohort was analysed along the 2-year follow-up period. Drug therapy was described in terms of first-line prescription choice, both as monotherapy and combination therapy. Combination therapy was defined as prescription of at least two drugs in a time-window of 60 days. Subsequently, changes in therapy were analysed by searching for any switch between active substances and drug additions or removals in the therapeutic plan.
Changes in drug therapy were also used as proxies of disease worsening. According to clinical opinions, a list of specific therapeutic switches identifying possible disease worsening was selected (supplementary Table 2S). This list does not include the switch from chemotherapy to endocrine therapy, due to the fact that it could be a maintenance therapy after response to chemotherapy [15, 16]. If one of the switches included in the list occurred during the follow-up period, the woman was considered in disease progression. The choice of considering as disease progression proxies only the specific drug changes occurred after 60 days was due to common clinical practice, according to which effectiveness/ineffectiveness assessment is usually performed after 2 months of therapy.
Kaplan–Meier survival analysis was performed to estimate the time from diagnosis to first disease progression.
3 Results
3.1 Incidence of De novo HR+/HER2− Metastatic Breast Cancer
From an overall population of 5,174,723 women aged ≥ 18 years, resident in 6 Italian regions at 2013 and with complete data recorded in ReS DB, 802 were found to be affected with hospitalised MBC (15.5 per 100,000). Of these, 787 were de novo cases over the year (15.2 per 100,000). Among these de novo MBC, 381 were HR+ (48.4% of de novo MBC) and, of these, 355 were HER2− (93.2% of de novo MBC with HR+). Therefore, the crude incidence of de novo HR+/HER2− MBC at 2013 was 6.9 per 100,000 adult women, without substantial differences among the Italian regions included in the analysis.
This population had a mean age of 63 ± 14 years and a median age of 65 years. Incidence distribution by age class showed an increase in estimation from 18–49 to 70–79 years, where the incidence was 13.9 per 100,000, and then a decrease in women aged 80 or more (Table 1).
3.2 Healthcare Utilisation and Costs
At the index date, all 355 women were admitted to hospital for one of the diagnoses used to select the cohort. Hospitalisations had an average annual cost per patient of €3888.
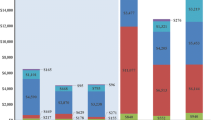
During the first year of follow-up, a woman affected by de novo HR+/HER2− MBC generated an average cost for NHS of €7543. The main cost driver was outpatient services and visits, which generated an average cost per patient of €3380, accounting for 44.8% of the overall cost. Hospitalisation costs, excluding daily admissions for chemotherapy administration, covered 22.6% of total expenditure (€1708 per patient). Chemotherapy, which is administered in day-hospital or ambulatory service, accounted for 21.2% of all expenses (€1601 per patient). Finally, drug prescriptions (both specific and concomitant drugs) generated 11.3% of the total cost (€854 per patient).
Of 355 patients followed in the first year, 326 were also followed in the second year. During the latter, a woman who met the inclusion criteria generated an average cost of €4834. The main cost driver was hospital admissions (38.5%), followed by outpatient services and visits (28.6%), drug prescriptions (17.8%), and chemotherapy administration (15.2%) (Table 2).
3.3 Prescription Patterns and Disease Progression
The prescription pattern analysis showed that, after the index date, 85.9% of the cohort was treated with monotherapy, whereas the remaining 14.1% received combination therapy. The most commonly used monotherapy schemes, as first-line choices, were nonsteroidal aromatase inhibitors (45.9%), chemotherapy (19.4%) and tamoxifen (8.7%); the most prescribed combination therapies were tamoxifen + luteinising hormone releasing hormone (LHRH) analogues (6.2%), and chemotherapy + LHRH analogues (3.1%) (supplementary Table 3S).
By analysing the 2-year prescription history for each subject, 54.4% of the cohort did not change therapy during the whole follow-up, whereas 1, 2, 3 and 4 switches were observed in 30.1%, 11.0%, 3.4% and 1.1% of patients, respectively. The first change in therapy occurred after an average of 276.8 days from the first treatment.
Overall, 237 changes were recorded during the follow-up. Of these, 83% were due to modification of the active substance, 9% to the addition of one or more drugs to a previous scheme, and 8% to removal of one drug from the previous scheme. A great number of switches occurred from chemotherapy to other therapies, most commonly to monotherapy with nonsteroidal aromatase inhibitors (supplementary Table 4S).
Disease progression was analysed by using specific therapeutic changes as proxies. According to this strategy, during the whole follow-up, 80 women (22.5% of the cohort) experienced a disease progression; in particular, 1, 2 and 3 disease progression events were recorded in 18.0%, 2.7% and 0.8% of patients, respectively. Survival analysis, which was performed on 355 women, corresponding to 7061 person-months (an average of 19.9 months for patient), found that the first disease progression occurred, on average, 13 ± 6 months after diagnosis (index date) (Fig. 1).
4 Discussion
This study provided an updated and comprehensive picture of a specific type of breast cancer, the de novo HR+/HER2− MBC. Using the well-validated Italian healthcare administrative data, our analysis showed the real-world incidence of this condition, its healthcare resource utilisation and related costs in the perspective of NHS. Moreover, real-world data were used to describe the prescription patterns and disease progression to provide more details on the current management of this type of breast cancer. This could be achieved thanks to the large ReS DB, representative of the Italian population. Indeed, since the 1970s, advances in oncology have also been made through the conduction of randomised controlled trials, but generalisability of their results to the current patient population remains an unsolved issue. Real-world evidence, with the ability to analyse large amounts of data relating to thousands of patients treated and followed up by different institutions, allows to overcome this limitation, providing reliable information on unselected patients.
The first goal of our analysis was to provide the incidence of de novo HR+/HER2− MBC, which was 6.9 per 100,000 adult women in Italy, in 2013. This result was found starting from the cohort of de novo MBC adult women whose incidence was 15.2 per 100,000, very close to the estimation by Crocetti and colleagues (13.1 per 100,000) [17]. The little discrepancy of the two incidence rates could be due to differences in the methodology: our study analysed Italian hospital discharges, whereas Crocetti et al estimated the Italian incidence by applying the US methodology by Mariotto et al. [18].
Knowledge of the epidemiology of different types of breast cancer is crucial for planning and funding effective health services. This holds particularly true for MBC, as information on its incidence and prevalence is scarce [18].
Our study included all breast cancer cases classifiable as luminal A or B, given that information on the percentage of Ki67 expression could not be obtained for each patient. In order to select patients with de novo stage IV breast cancer, hospitalisations with specific diagnosis codes were used as proxies. Therefore, despite being based on proxies, the incidence estimated from our data can be considered reliable, also given the ability of Italian administrative databases to identify specific breast cancers [10].
As for prescription patterns and disease progression regarding the 355 patients de novo HR+/HER2− MBC, these are consistent with previous literature data [19,20,21]. Specifically, the use of drug prescriptions as proxies of receptor expression reported that the rate of HR+/HER2− was 48.4% of incident cases of MBC de novo. This rate was quite lower than that reported by Gobbini et al, where HR+/HER2− represented the 65.7% of the MBC prevalent cohort [21]. This difference could be due to discrepancies between analysed cohorts (incident vs prevalent) and between data sources (administrative databases vs ad hoc data collection). Otherwise, it could be explained by the underestimation of the receptor expression, identified through drug prescriptions as proxy. However, it is not in doubt that algorithms applied to big healthcare data are built to address the multiple biological and clinical variables that characterise mammary neoplasia, allowing to extract homogeneous groups of patients that are used to perform clinical and pharmacological analyses of great interest.
The reliability of our approach was also confirmed by the results on disease progression retrieved using specific drug switches as proxies (i.e. after 24 months the 22.5% of patients experienced a disease progression at an average of 13 ± 6 months after diagnosis). As a matter of fact, these findings were comparable with the main results from randomised controlled trials on HR+/HER2− MBC: in the MONALEESA-2 trial [22], after 18 months disease progression was observed in 12% of patients treated with letrozole plus placebo, with a median progression-free survival of 14.7 months; in the PALOMA-3 trial [23], after 15 months disease progression occurred in 33% of patients in the fulvestrant plus placebo group, with a median progression-free survival of 9.5 months.
Furthermore, our study provides a comprehensive picture of healthcare resource utilisation in de novo HR+/HER2− MBC patients, in terms of drug prescriptions, hospitalisations and outpatient services, with the related costs, in the perspective of NHS. Our analysis showed that a woman affected by de novo HR+/HER2− MBC costs on average €3800 at diagnosis (index date), €7500 in the first year of follow-up and €4800 in the second year of follow-up. These costs are remarkably similar to those reported for “M1 (distant metastasis) breast cancer” in an Italian study, where administrative and cancer registry data were linked to obtain staging information [24]. Moreover, the costs incurred in the first year (diagnosis + first year of follow-up) were comparable to those retrieved in a US elderly population affected by HR+/HER2− MBC [25]. Hence, administrative data, if adequately queried, could be helpful to complete and detail the economic burden of breast cancer.
In contrast to these strengths, several study limitations should be acknowledged, some of which are pertaining to observational studies based on healthcare administrative database [26, 27]. First, proxies were used for the identification of a specific diagnosis subgroup and the receptor expressions (using the presence/absence of particular drug prescriptions). This strategy inevitably resulted in an underestimation of HR+/HER2− subjects, since a small portion of women could not be treated with drugs for different reasons (e.g. comorbidities or intolerance), or they could be included in clinical trials where the specific drug is not recorded in the pharmaceutical administrative database. However, the used methodology is highly reliable in the care setting analysed, particularly in Italy. The Italian NHS covers the healthcare costs of the entire resident population by reimbursing drug prescriptions only if they meet specific rules established by the National Medicines Agency (AIFA–Agenzia Italiana del Farmaco). In the case of drugs for breast cancer treatment, these rules are based on the specific expression of receptors. Therefore, the presence/absence of a drug prescription adequately reflects every type of receptor profile. Second, our strategy could have missed those patients affected by HR+/HER2− MBC who were neither hospitalized nor received endocrine therapy prescription, resulting in a slightly underestimated incidence.
Therefore, our results should be validated by reviewing medical records or linking with other data sources that report cancer staging information and receptor expression, to ascertain the precise diagnosis [11]. Third, administrative data were used to estimate the costs that reflect the actual expenditure paid by NHS. In some cases, these could not necessarily convey accurate information about the economic costs of procedures and services. Moreover, our analysis did not include indirect costs (e.g. lost productivity), which represent a huge part of the costs related to breast cancer, paid especially by patients and their caregivers [28]. Finally, prescription changes were used as proxies of disease progression. This methodology was adopted because no information on actual treatment failures is retrievable from administrative databases, therefore it represents the unique optimal strategy when these data sources are used.
5 Conclusion
In conclusion, using a sophisticated but easily applicable methodology, our results showed that real-world data could be helpful in health technology assessment and expenditure forecasts of future therapeutic strategies for HR+/HER2− MBC, particularly in the field of “precision medicine” or “personalised medicine” in Italy.
References
International Agency for Research on Cancer (IARC). Cancer fact sheets: Breast, Source: Globocan 2018. https://gco.iarc.fr/today/data/factsheets/cancers/20-Breast-fact-sheet.pdf. Accessed 24 July 2019.
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52.
van ‘t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415(6871):530–6.
van de Vijver MJ, He YD, van ‘t Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347(25):1999–2009.
Rivenbark AG, O’Connor SM, Coleman WB. Molecular and cellular heterogeneity in breast cancer: challenges for personalized medicine. Am J Pathol. 2013;183(4):1113–24.
Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 2013;14(12):1165–74.
Parisi M, Pelletier C, Cherepanov D, Broder MS. Outcomes research examining treatments, quality of life and costs in HER2− negative and triple-negative metastatic breast cancer: a systematic literature review. J Comp Eff Res. 2018;7(1):67–83.
Piccinni C, Ronconi G, Calabria S, Dondi L, Forcesi E, Rossi E, et al. Healthcare resources utilisation in primary progressive multiple sclerosis. Neurol Sci. 2018;39(7):1169–74.
Calabria S, Forcesi E, Dondi L, Pedrini A, Maggioni AP, Martini N. Target population of non-deferrable surgery and uncontrolled severe bleeding related to dabigatran. Cardiovasc Drugs Ther. 2018;32(3):281–6.
Schifano P, Papini P, Agabiti N, Scarinci M, Borgia P, Perucci CA. Indicators of breast cancer severity and appropriateness of surgery based on hospital administrative data in the Lazio Region, Italy. BMC Public Health. 2006;6:25.
Nordstrom BL, Whyte JL, Stolar M, Mercaldi C, Kallich JD. Identification of metastatic cancer in claims data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 2):21–8.
Kahn LH, Blustein J, Arons RR, Yee R, Shea S. The validity of hospital administrative data in monitoring variations in breast cancer surgery. Am J Public Health. 1996;86(2):243–5.
Hao Y, Li N, Fang AP, Koo V, Peeples M, Kageleiry A, et al. Real-world analysis of medical costs and healthcare resource utilization in elderly women with HR+/HER2− metastatic breast cancer receiving everolimus-based therapy or chemotherapy. Adv Ther. 2016;33(6):983–97.
Vaz-Luis I, Lin NU, Keating NL, Barry WT, Lii H, Winer EP, et al. Racial differences in outcomes for patients with metastatic breast cancer by disease subtype. Breast Cancer Res Treat. 2015;151(3):697–707.
Jacquet E, Lardy-Cleaud A, Pistilli B, Franck S, Cottu P, Delaloge S, et al. Endocrine therapy or chemotherapy as first-line therapy in hormone receptor-positive HER2− negative metastatic breast cancer patients. Eur J Cancer (Oxford, England: 1990). 2018;95:93–101.
Ciruelos E, Perez-Garcia JM, Gavila J, Rodriguez A, de la Haba-Rodriguez J. Maintenance therapy in HER2− negative metastatic breast cancer: a new approach for an old concept. Clin Drug Investig. 2019;39(7):595–606.
Crocetti E, Gori S, Falcini F. Metastatic breast cancers: Estimates for Italy. Tumori. 2018;104(2):116–20.
Mariotto AB, Etzioni R, Hurlbert M, Penberthy L, Mayer M. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol Biomark Prev. 2017;26(6):809–15.
Bertaut A, Mounier M, Desmoulins I, Guiu S, Beltjens F, Darut-Jouve A, et al. Stage IV breast cancer: a population-based study about prognostic factors according to HER2 and HR status. Eur J Cancer Care. 2015;24(6):920–8.
Tao L, Chu L, Wang LI, Moy L, Brammer M, Song C, et al. Occurrence and outcome of de novo metastatic breast cancer by subtype in a large, diverse population. Cancer Causes Control. 2016;27(9):1127–38.
Gobbini E, Ezzalfani M, Dieras V, Bachelot T, Brain E, Debled M, et al. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur J Cancer (Oxford, England: 1990). 2018;96:17–24.
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-Positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738–48.
Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2− negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–39.
Capri S, Russo A. Cost of breast cancer based on real-world data: a cancer registry study in Italy. BMC Health Serv Res. 2017;17(1):84.
Pawaskar M, Karve S, Dalvi T, Davis KL, Deeter R, editors. Health care utilization and costs among HER2− negative, HR-positive, elderly women with metastatic breast cancer in the United States. In: 2013 ASCO annual meeting; 2013 31 May–4 June 2013; Chicago, IL, USA.
van Walraven C, Austin P. Administrative database research has unique characteristics that can risk biased results. J Clin Epidemiol. 2012;65(2):126–31.
Gini R, Schuemie MJ, Pasqua A, Carlini E, Profili F, Cricelli I, et al. Monitoring compliance with standards of care for chronic diseases using healthcare administrative databases in Italy: strengths and limitations. PLoS One. 2017;12(12):e0188377.
Broekx S, Den Hond E, Torfs R, Remacle A, Mertens R, D’Hooghe T, et al. The costs of breast cancer prior to and following diagnosis. Eur J Health Econ. 2011;12(4):311–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the study.
Conflict of interest
CP, LD, GR, SC, AP, IE, NM and MM declare that they have no conflict of interest.
Ethical approval
This is an observational, non-interventional database study, re-utilising administrative data, primary collected for governance purposes. The study was conducted under specific agreements between Fondazione ReS and Italian Regional/Local Health Facilities, participating with anonymous data to the ReS database. All these institutions have general legal and ethical frameworks that allow them for conducting analyses, aimed to address governance and research questions, by making secondary use of anonymous administrative data.
Informed consent
No informed consent is required to use anonymous patient data which is analysed retrospectively.
Additional information
Part of results of this study has been submitted as abstracts to the Italian Society of Pharmacology 39th annual national meeting (20–23 November 2019, Firenze) and to AIOM (Italian Association of Medical Oncology) 21st annual national meeting (25–27 October 2019, Roma).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Piccinni, C., Dondi, L., Ronconi, G. et al. HR+/HER2− Metastatic Breast Cancer: Epidemiology, Prescription Patterns, Healthcare Resource Utilisation and Costs from a Large Italian Real-World Database. Clin Drug Investig 39, 945–951 (2019). https://doi.org/10.1007/s40261-019-00822-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-019-00822-4