Abstract
Background and Objectives
Progressive multifocal leukoencephalopathy (PML) is a rare, JC-virus-mediated, demyelinating disease with a high mortality rate. As no recommended treatment exists, mirtazapine, a potential blocker of virus entry into cells, has been empirically used.
Methods
We analysed existing data on mirtazapine’s efficacy to treat PML by systematically reviewing the literature since 2005, when it was first used.
Results
Searches in PubMed, EBSCO, SCOPUS and Google Scholar between January 2005 and December 2015, identified five cohort studies and 74 case reports. No statistically significant effect of mirtazapine on PML outcome was observed in the cohort studies. From studying the case reports, mortality rate for PML was associated with the underlying circumstances, such as an older age, the use of an immunosuppressant, or PML occurring in patients with a haematological malignancy or a transplant.
Conclusions
Except for natalizumab-associated PML, we did not highlight any potential benefit of mirtazapine on disease outcomes. Further interventional studies are needed to confirm that 5-HT2AR inhibition is relevant to treat PML.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Mirtazapine may increase survival for patients with natalizumab-associated progressive multifocal leukoencephalopathy. |
Mirtazapine is neither beneficial nor detrimental for non-natalizumab-associated PML. |
Considering the high fatality of PML and the minimal adverse outcomes, we propose that mirtazapine should be started when PML is diagnosed. |
1 Introduction
Progressive multifocal leukoencephalopathy (PML) is a rare demyelinating disease caused by reactivation of JC virus (JCV) in the brain [1]. PML has been reported in the setting of various underlying conditions, such as human immunodeficiency virus (HIV) infection, haematological malignancies, autoimmune/rheumatic diseases, organ transplantation, and the use of monoclonal antibodies (Mab; i.e. natalizumab, rituximab, efalizumab) or other drugs like fingolimod and dimethyl fumarate. The prognosis of PML relies heavily on the underlying condition, the time to confirm a diagnosis and the ability to reconstitute effective immunity [1, 2]. As yet, there is no recommended treatment against JCV but early immune reconstitution is considered pivotal in the setting of HIV- or natalizumab-associated PML. This can be achieved by combined antiretroviral therapy or plasma exchange, respectively [3–5]. Nevertheless, in some other conditions, such as organ transplantation, immune suppression cannot be reversed and other options are needed. Anecdotal successful treatments using cytosine arabinoside, interferons, cidofovir, or mefloquine have been reported but none has shown a benefit in clinical trials.
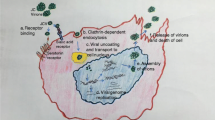
In 2004, the entry of JCV into glial cells was shown to depend on 5-hydroxytryptamine (5-HT2A) receptor [6]. As mirtazapine blocks the 5-HT2A receptor, this discovery resulted in its widespread use in PML [2]. Mirtazapine has long been used to treat depression and has a good safety profile and tolerability, with minimal adverse effects. Mirtazapine displays linear pharmacokinetics over a dose range of 15–80 mg/day and is unlikely to cause overdose toxicity [7].
Although a favourable outcome in PML patients treated with mirtazapine has been described in several case reports, no systematic investigation has analysed its efficacy and safety to treat PML.
Herein, we systematically reviewed the available data concerning the use of mirtazapine in PML patients over the past ten years, with particular focus on survival.
2 Methods
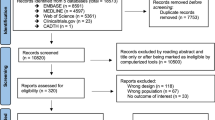
References for this systematic review were identified by searches in PubMed, EBSCO, SCOPUS and Google Scholar between January 2005 and December 2015. The search terms “progressive multifocal leukoencephalopathy”, “mirtazapine”, and “5HT2A” were used. Language was restricted to English, French, Spanish and German. References were selected only if a diagnosis of PML was definite or possible and if mirtazapine was used [8]. Articles that did not report the outcomes were excluded (Fig. 1). Two reviewers independently screened the relevant articles and extracted the data (YJ, SK). Each article was assessed for quality using a 10-point scale adapted from the CARE guidelines [9].
3 Results
3.1 Cohort Studies
We identified five cohort studies that included 167 PML patients, of which 93 received mirtazapine (Table 1). Two studies have reported a potential benefit for mirtazapine whereas three others found no difference between mirtazapine-treated and untreated patients.
In one study focusing on HIV patients, there was a non-significant trend to increased 1-year survival in patients treated with mirtazapine [2]. Two other studies analysed natalizumab-associated PML: one found no difference on survival between mirtazapine+ and mirtazapine− groups (p = 0.71) [4]; whereas in the other one all of the studied patients received a combination of mirtazapine and mefloquine and survived [5]. The latter case series was not controlled. Finally, two other studies including patients with miscellaneous underlying conditions found no significant difference between mirtazapine+ and mirtazapine− groups [10, 11]. Overall, none of these low-effective retrospective studies has demonstrated a statistically significant effect of mirtazapine on PML outcome, regardless of the underlying disease.
3.2 Case Reports
We identified 74 case reports of PML patients treated with mirtazapine (Table 2; Fig. 1; Supplementary Table S1). The mean CARE-adapted article-quality score was 5.2 (±1.4)/10. Cases were reported with increased frequency over the years (6 cases from 2005 to 2007 vs. 35 cases from 2012 to 2015). In parallel, while the incidence of HIV/AIDS-associated PML has declined over the years, immune-modulating monoclonal antibody-associated PML has been increasingly reported [12–14].
Overall, 25 % of patients were treated with corticosteroids and 11 % with at least one immunosuppressant. PML diagnosis was definite in 96 % of cases (59 % virologic, 41 % histologic). Sixty-two (84 %) patients received mirtazapine combined with another treatment (either antiviral or for immune reconstitution). The mean dosage of mirtazapine was 30 (15–60) mg/day. Overall, the mortality rate was 31 %, with 87 % of deaths directly attributable to PML. The mean time from PML onset until death was 5.6 (0.5–15) months.
Twelve patients received mirtazapine alone for the treatment of PML (HIV, n = 2; rheumatic disease, n = 2; haematological malignancy, n = 2; multiple sclerosis, n = 1; transplantation, n = 1; other, n = 4). Three patients received immunosuppressants or chemotherapy. Survival rate in this subgroup was 58 % with 4/5 cases attributable to PML. The mean time from PML onset until death was 4.5 (0.6–9) months. For two PML survivors, the time from PML onset until last report was >25 months. Patients from the monotherapy group were no different from patients with combination therapy, for all the study parameters.
According to fatality rates, three groups can be established: (1) PML patients with natalizumab-treated multiple sclerosis or other autoimmune diseases who had significantly lower fatality rates (24 and 22.5 %, respectively); (2) HIV patients who had an intermediate fatality rate (33 %); and (3) transplant recipients and patients with a haematological malignancy who had significantly higher fatality rates (71 and 60 %, respectively, Table 2; Supplementary Fig. S1). Finally, seven patients with miscellaneous underlying conditions had an intermediate fatality rate (43 %).
Mirtazapine tolerance was reported for 12 cases: it was well tolerated in 83 %, led to weight gain in one case, and had to be stopped after 5 months in another case because of paranoia. No serious adverse event was reported.
3.3 Determinants of Survival in PML Patients Treated with Mirtazapine
We performed univariate analyses to identify the determinants for survival of PML patients treated with mirtazapine (Table 3). Factors that were significantly associated with increased risk of death were: age >45 years, prior use of an immunosuppressant (Supplementary Fig. S2), and increased delay until a definite diagnosis (threshold: 1.2 months). Underlying conditions, such as transplantation and haematological malignancy, were associated with an increased risk of death. Conversely, stopping immunosuppressants and the occurrence of an IRIS were statistically associated with a decreased risk of fatality.
4 Discussion
It has been 10 years since the 5-HT2A receptor was found to facilitate JCV entry into glial cells and the subsequent use of mirtazapine in clinics; however, the benefit of this drug still remains unknown. Small cohort studies have tended to consider mirtazapine as a promising treatment for PML but the results have not been significant, hence no formal conclusion can be drawn. From studying the case reports, we confirm that the mortality rate for PML, either treated by mirtazapine or not, was associated with the underlying circumstances, such as an older age, the use of an immunosuppressant, or a PML occurring in patients with a haematological malignancy or a transplant.
Depending on the observed population, reported survival at 1 year varies from <20 to 76 % [2, 15, 16]. In HIV+ patients, reported 1-year survival is 38.6–63.6 % [3, 17], but recent observations suggest an improved 1-year survival rate for 75 % of patients who were treated aggressively early after disease onset with an effective combined antiretroviral therapy [18, 19]. These results are similar to those of the present study, suggesting mirtazapine is neither beneficial nor detrimental in treating HIV-associated PML.
Recently, the 1-year survival of 336 patients with natalizumab-associated PML was reported as 76 % [20]. In our series, survival rate was slightly higher, which could indicate a potential beneficial effect of mirtazapine in this population. We observed a similar survival rate by analysing case reports of patients with autoimmune/rheumatic disease (ARD)-associated PML under mirtazapine. Regardless of mirtazapine use, the survival rate of ARD-associated PML had been reported at 45 % [21]. The difference can be explained by the heterogeneity of ARD treatments, as all patients in Molloy and Calabrese’s study received a Mab or an immunosuppressant, whereas none of the ARD patients from case reports received a Mab and only one received an immunosuppressant [21].
Data from patients with a malignancy or an organ transplant are scarce [2, 22, 23], and a comparison between PML patients with or without mirtazapine was precluded because of the confounders, which included a heterogeneous underlying malignancy or transplanted organ/tissue, the heterogeneous underlying treatments (multiples lines of chemotherapy, various immunosuppressants), ambivalent assessment of the cause of death, and small numbers of patients per study. In a small literature review, Mateen et al reported an overall survival rate of 20 % for transplant recipients with PML [23]. This high fatality rate is similar to that of patients treated with mirtazapine from case reports, and suggests no benefit from mirtazapine.
No serious adverse event was reported. There are two main hypotheses for such results: (1) the reporting of mirtazapine tolerance was very low and some adverse events may not have been reported; and (2) mirtazapine has an excellent safety and tolerability profile [24, 25]. Of note, the use of mirtazapine for the treatment of PML is an off-label use and therefore it should be prescribed according to national rules on the off-label prescribing of drugs.
The main limitation of our work is the small number of case reports with potential publication bias. Indeed, PML is a rare disease and the opportunity to study the effect of a drug on outcomes of PML in a prospective fashion is precluded by its severity and high fatality, thus hindering placebo-controlled trials.
5 Conclusions and Pending Questions
Comparisons with historical series may suggest an increased survival for patients with natalizumab-associated PML who were treated with mirtazapine. We were not able to show any benefit of mirtazapine on PML outcomes for other conditions. Because of a low level of evidence, there are no recommendations for the use of mirtazapine in PML treatment. Nevertheless, taking into account the high fatality of PML and the minimal adverse outcomes, we propose that mirtazapine should be started when PML is diagnosed, in accordance with the rules on off-label use of drugs. At the same time, everything must be done to restore immunity in affected patients.
Some questions remain: are other 5-HT2A receptor blockers, like risperidone, ziprasidone or olanzapine, more effective against PML? Should we use higher doses of mirtazapine? Can mirtazapine be efficient for PML prophylaxis?
Further studies are needed to confirm that 5-HT2A receptor inhibition is relevant in PML treatment.
References
Koralnik IJ. Progressive multifocal leukoencephalopathy revisited: has the disease outgrown its name? Ann Neurol. 2006;60(2):162–73.
Marzocchetti A, Tompkins T, Clifford DB, et al. Determinants of survival in progressive multifocal leukoencephalopathy. Neurology. 2009;73(19):1551–8.
Berenguer J, Miralles P, Arrizabalaga J, et al. Clinical course and prognostic factors of progressive multifocal leukoencephalopathy in patients treated with highly active antiretroviral therapy. Clin Infect Dis. 2003;36(8):1047–52.
Vermersch P, Kappos L, Gold R, et al. Clinical outcomes of natalizumab-associated progressive multifocal leukoencephalopathy. Neurology. 2011;76(20):1697–704.
Dahlhaus S, Hoepner R, Chan A, et al. Disease course and outcome of 15 monocentrically treated natalizumab-associated progressive multifocal leukoencephalopathy patients. J Neurol Neurosurg Psychiatry. 2013;84(10):1068–74.
Elphick GF, Querbes W, Jordan JA, et al. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science. 2004;306(5700):1380–3.
Berling I, Isbister GK. Mirtazapine overdose is unlikely to cause major toxicity. Clin Toxicol. 2014;52(1):20–4.
Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80(15):1430–8.
Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case report guideline development. J Clin Epidemiol. 2014;67(1):46–51.
Haghikia A, Perrech M, Pula B, et al. Functional energetics of CD4+-cellular immunity in monoclonal antibody-associated progressive multifocal leukoencephalopathy in autoimmune disorders. PloS One. 2011;6(4):e18506.
Gheuens S, Ngo L, Wang X, Alsop DC, Lenkinski RE, Koralnik IJ. Metabolic profile of PML lesions in patients with and without IRIS: an observational study. Neurology. 2012;79(10):1041–8.
Casado JL, Corral I, García J, et al. Continued declining incidence and improved survival of progressive multifocal leukoencephalopathy in HIV/AIDS patients in the current era. Eur J Clin Microbiol Infect. 2014;33(2):179–87.
Carruthers RL, Berger J. Progressive multifocal leukoencephalopathy and JC Virus-related disease in modern neurology practice. Mult Scler Relat Disord. 2014;3(4):419–30.
Berger JR, Fox RJ. Reassessing the risk of natalizumab-associated PML. J Neurovirol. 2016;22(4):533–5.
De Luca A, Giancola ML, Ammassari A, et al. The effect of potent antiretroviral therapy and JC virus load in cerebrospinal fluid on clinical outcome of patients with AIDS-associated progressive multifocal leukoencephalopathy. J Infect Dis. 2000;182(4):1077–83.
Hall CD, Dafni U, Simpson D, et al. Failure of cytarabine in progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. AIDS Clinical Trials Group 243 Team. N Engl J Med. 1998;338(19):1345–51.
Falcó V, Olmo M, del Saz SV, et al. Influence of HAART on the clinical course of HIV-1-infected patients with progressive multifocal leukoencephalopathy: results of an observational multicenter study. J Acquir Immune Defic Syndr. 2008;49(1):26–31.
Gasnault J, Costagliola D, Hendel-Chavez H, et al. Improved survival of HIV-1-infected patients with progressive multifocal leukoencephalopathy receiving early 5-drug combination antiretroviral therapy. PloS One. 2011;6(6):e20967.
Fanjul F, Riveiro-Barciela M, Gonzalez J, et al. Evaluation of progressive multifocal leukoencephalopathy treatments in a Spanish cohort of HIV-infected patients: do protease inhibitors improve survival regardless of central nervous system penetration-effectiveness (CPE) score? HIV Med. 2013;14(5):321–5.
Dong-Si T, Gheuens S, Gangadharan A, et al. Predictors of survival and functional outcomes in natalizumab-associated progressive multifocal leukoencephalopathy. J Neurovirol. 2015;21(6):637–44.
Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy associated with immunosuppressive therapy in rheumatic diseases: evolving role of biologic therapies. Arthritis Rheum. 2012;64(9):3043–51.
Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113(20):4834–40.
Mateen FJ, Muralidharan R, Carone M, et al. Progressive multifocal leukoencephalopathy in transplant recipients. Ann Neurol. 2011;70(2):305–22.
Nutt DJ. Tolerability and safety aspects of mirtazapine. Hum Psychopharmacol. 2002;17(Suppl 1):S37–41.
Fawcett J, Barkin RL. Review of the results from clinical studies on the efficacy, safety and tolerability of mirtazapine for the treatment of patients with major depression. J Affect Disord. 1998;51(3):267–85.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No source of funding.
Conflict of interest
Yvan Jamilloux, Sébastien Kerever, Tristan Ferry, Christiane Broussolle, Jérôme Honnorat and Pascal Sève have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jamilloux, Y., Kerever, S., Ferry, T. et al. Treatment of Progressive Multifocal Leukoencephalopathy With Mirtazapine. Clin Drug Investig 36, 783–789 (2016). https://doi.org/10.1007/s40261-016-0433-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-016-0433-8