Abstract
Background and Objective
Piperaquine–dihydroartemisinin combination therapy has established efficacy for the treatment of malaria; however, a more comprehensive understanding of the pharmacokinetic properties and factors contributing to inter- and intra-individual variability is critical to optimize clinical use. This study assessed the effects of food on the pharmacokinetics of combination piperaquine–dihydroartemisinin administration in healthy volunteers.
Methods
This was an open-label, single-dose, parallel-group study. Participants were randomly allocated to receive oral piperaquine–dihydroartemisinin either after an overnight fast or immediately after a standardized, high-fat, high-calorie meal. Blood samples were collected for analysis of plasma piperaquine and dihydroartemisinin concentrations, which were utilized for calculation of pharmacokinetic parameters, using a standard model-independent approach.
Results
Consumption of a high-fat, high-calorie meal resulted in substantial increases in the extent of exposure to piperaquine (ratio between area under the plasma concentration–time curve [AUC] values from 0 to 168 h in the fed and fasted states [AUC0–168 h FED/AUC0–168 h FASTED] = 299 %, 90 % confidence interval [CI] 239–374 %). This likely reflects an increase in the oral bioavailability of the drug, directly related to the fat content of the meal. Co-administration of food was also found to result in both delayed and enhanced absorption of dihydroartemisinin (ratio between AUC values from time zero to infinity in the fed and states [AUC∞ FED/AUC∞ FASTED] = 142 %, 90 % CI 113–178 %; ratio between mean transit time [MTT] values in the fed and fasted states [MTTFED/MTTFASTED] = 135 %, 90 % CI 114–160 %).
Conclusion
Although food was found to significantly impact on the pharmacokinetics of piperaquine and dihydroartemisinin, given the low fat content of standard meals within endemic regions and the anorexic effects of malaria infection, these results are unlikely to impact on the clinical utility of these drugs. However, co-administration of food with these anti-malarials by populations consuming a typical Western diet should be avoided to reduce the risk of toxic side effects. It is therefore a general recommendation that piperaquine–dihydroartemisinin not be administered within ±3 h of food consumption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The influence of food on piperaquine pharmacokinetics has been examined with equivocal findings, whereas the food–drug interaction of dihydroartemisinin has not been investigated; a more comprehensive understanding of the factors contributing to pharmacokinetic variability is critical in order to optimize the use of these drugs in clinical practice. |
Consumption of a high-fat, high-calorie meal with administration of piperaquine–dihydroartemisinin combination therapy resulted in a substantial increase in the extent of exposure to piperaquine and delayed and enhanced the absorption of dihydroartemisinin. For piperaquine, the food effect appeared to be directly related to the fat content of the meal. |
On the basis of these findings, the general recommendation is that piperaquine–dihydroartemisinin combination therapy not be administered within ±3 h of food consumption. |
1 Introduction
The current World Health Organization (WHO) recommendation for the treatment of uncomplicated Plasmodium falciparum malaria is artemisinin-based combination therapy comprising two anti-malarial drugs with independent modes of action, specifically a rapid-acting artemisinin-derived compound administered with a longer-acting anti-malarial [1]. The complementary modes of action of these two anti-malarial drugs are thought to increase treatment effectiveness and prevent or delay the emergence of resistance.
The combination of piperaquine and dihydroartemisinin is a well-tolerated treatment for uncomplicated falciparum malaria, with established efficacy in practice [2–6]. Despite previous long-term clinical use of these drugs, the pharmacokinetics of piperaquine and dihydroartemisinin have been described only within the last decade [7–10]. The pharmacokinetics of piperaquine are characterized by slow oral absorption (with a time to reach the maximum plasma concentration [T max] of 5 h in the fasted state), a large apparent volume of distribution (~700 L/kg), extensive protein binding (>99 %) and a long elimination half-life (~20 days) [11]. On the other hand, dihydroartemisinin is rapidly absorbed (T max 1–2 h) and displays a small apparent volume of distribution (0.8 L/kg) and a rapid elimination half-life (~1 h) [11]. A more comprehensive understanding of the pharmacokinetics of these drugs and the factors contributing to inter- and intra-individual variability is critical in order to optimize their use in clinical practice.
Food–drug interactions are often associated with alterations in drug pharmacokinetics due to changes in gastric emptying, gastric pH or other physiological changes, resulting in a reduction, delay, increase and/or acceleration in drug absorption [12]. Although a number of studies have investigated the effects of food on the pharmacokinetics of various drugs, given the varied contribution of factors such as the physiochemical properties of the drug and the composition and timing of the meal, there is still no scientific basis to predict food–drug interactions.
Previous studies have examined the influence of food on the pharmacokinetic properties of piperaquine, with some studies demonstrating substantial alterations in piperaquine concentrations after a meal [13, 14], whereas others have reported no significant effects [15–18]. On the other hand, the impact of food on the pharmacokinetics of dihydroartemisinin has not been reported, and while this aspect has been examined for other artemisinin-derived compounds, the results have been equivocal [19–21]. This study was conducted to assess the effects of food on the extent and rate of absorption of piperaquine and dihydroartemisinin, administered as a fixed-dose combination, in healthy adult male volunteers.
2 Methods
2.1 Ethical Considerations
The study was reviewed and approved by the Bellberry Human Research Ethics Committee (HREC; reference no. C24/10). Participants were fully informed of the study procedures and provided written informed consent prior to study initiation. The study was conducted in accordance with the Declaration of Helsinki, the National Statement on Ethical Conduct in Human Research issued by the National Health and Medical Research Council (Australia) and the principles of Good Clinical Practice.
2.2 Study Design
This was a randomized, open-label, single-dose, parallel-group study conducted to evaluate the effects of a high-fat, high-calorie meal on the pharmacokinetics of piperaquine and dihydroartemisinin in 36 healthy, adult male volunteers (18 subjects planned per treatment group). Given the long half-life of piperaquine (~20 days), a parallel study design was considered appropriate to address the study objectives.
The clinical component of the study was conducted by CPR Pharma Services (Adelaide, SA, Australia) at clinical sites within Australia.
Prior to treatment administration, volunteers were screened and were required to meet the eligibility criteria for the study: male; Caucasian; aged 18–50 years; body weight >75 kg; body mass index 19.0–27.0 kg/m2; free from clinically significant illness or disease as determined by medical and surgical history, physical examination, vital signs, electrocardiogram and clinical laboratory determinations; provision of written informed consent.
Participants were randomly allocated to receive a single oral dose of 1280 mg of piperaquine phosphate/160 mg of dihydroartemisinin, either with 240 mL of water after an overnight fast of at least 10 h or with 200 mL of water after a standardized, high-fat, high-calorie meal (consumed within 30 min prior to dose administration).
The study treatment was administered as Eurartesim™ tablets containing 320 mg of piperaquine phosphate and 40 mg of dihydroartemisinin, manufactured by Sigma-Tau Industrie Farmaceutiche Riunite SpA (Rome, Italy; batch no. PP091166). Subjects randomized to the fed treatment were provided with a high-fat, high-calorie breakfast (50 % fat/800–1000 kcal), comprising two fried eggs, two strips of bacon, two slices of toast with two serves of butter, 4 oz (114 g) of hash brown potatoes and 8 oz (240 mL) of whole milk.
Blood samples were collected for analysis of plasma piperaquine concentrations prior to dosing (at 0 h) and 1, 2, 3, 4, 5, 6, 8, 12, 16, 24, 30, 36, 42, 48, 60, 72, 96, 120 and 168 h after treatment administration. Blood samples were collected for analysis of plasma dihydroartemisinin concentrations prior to dosing (at 0 h) and 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10 and 12 h after treatment administration.
2.3 Analytical Methods
Plasma samples were analysed for piperaquine and dihydroartemisinin concentrations by CPR Pharma Services (Adelaide, SA, Australia).
Piperaquine and dihydroartemisinin concentrations were quantified by high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS) methods validated according to US Food and Drug Administration (FDA) guidelines. Validation studies and study sample analyses were conducted according to the principles of Good Laboratory Practice (GLP).
Piperaquine and the internal standard (deuterated piperaquine) were purified from plasma by protein precipitation followed by chromatographic separation and MS/MS detection. To 50 µL of human plasma, 250 µL of 0.5 % trifluoroacetic acid in acetonitrile was added; after 1 min of mixing, 50 µL of supernatant was transferred to a polypropylene tube and a further 100 µL of ultra-pure water was added, after which samples were injected into the HPLC–MS/MS system. A Waters X-Bridge™ 3.5 µm C18 column was used for chromatographic separation under gradient conditions at a 0.3 mL/min flow rate. Mobile phase (MP) A was 10:90 acetonitrile/0.1 % trifluoroacetic acid in water (v/v), and MP B was 90:10 acetonitrile/0.1 % trifluoroacetic acid in water (v/v); the gradient program was set from 0 to 3.00 min: 0 to 40 % MP A; from 3.00 to 3.10 min: 40 to 80 % MP A; from 3.10 to 3.40 min: 80 % MP A; from 3.40 to 3.50 min: 80 to 0 % MP A; and from 3.50 to 5.00 min: 0 % MP A. Piperaquine and the internal standard were monitored by an MS/MS detector in positive multiple reaction monitoring (MRM) mode. The single charged Q1/Q3 transitions were 535.2/288.3 atomic mass units (amu) for piperaquine and 541.4/294.1 amu for the internal standard. The typical retention time was 2.70 min for both piperaquine and the internal standard. The lower limit of quantification (LLOQ) was 5 ng/mL, and the calibration curve range was 5–500 ng/mL. Independent quality-control samples had concentrations of 15.0, 75.0 and 375 ng/mL of piperaquine. Samples were analysed for piperaquine determination in a total of 11 analytical runs. The linearity and reproducibility of the calibration curves were evaluated from repeated analysis of the calibration curve samples. The inter-assay accuracy and precision were within 5.0 % and 8.0 %, respectively. The mean R 2 value was 0.9968. The accuracy and precision evaluated from repeated analysis of the quality-control samples were within 3.0 and 11.0 %, respectively.
Dihydroartemisinin and the internal standard (artemisinin) were purified from plasma by liquid/liquid extraction followed by chromatographic separation and MS/MS detection. All sample processing was carried out in ice, i.e. from thawing through addition of the extraction solvent. To 100 µL of human plasma, 3 mL of chlorobutane was added; after 10 min of mixing, samples were centrifuged at 3000 rpm for 5 min and placed in a freezer at −80 ± 15 °C for 15 min. The upper extraction solvent was then transferred to a polypropylene tube and evaporated to dryness under a gentle stream of nitrogen; samples were reconstituted with 50:50 methanol/10 mM ammonium acetate [pH 4] (v/v) before injection into the HPLC–MS/MS system. A Waters X-Bridge™ 3.5 µm C18 (50 × 2.1 mm) column equipped with a Waters X-Bridge C18 3.5 µm guard cartridge was used for chromatographic separation under isocratic conditions, the mobile phase being 63.5:36.5 methanol/10 mM ammonium acetate [pH 4] (v/v) at a 0.2 mL/min flow rate. Dihydroartemisinin and the internal standard were monitored by an MS/MS detector in positive MRM mode. The single charged Q1/Q3 transitions were 302.3/163.0 amu for dihydroartemisinin and 300.3/209.1 amu for the internal standard. The typical retention times were 2.95 and 2.84 min for dihydroartemisinin and the internal standard, respectively. The LLOQ was 10 ng/mL, and the calibration curve range was 10–1600 ng/mL. Independent quality-control samples had concentrations of 25.0, 250 and 1200 ng/mL of dihydroartemisinin. Samples were analysed for dihydroartemisinin determination in a total of seven analytical runs. The linearity and reproducibility of the calibration curves were evaluated from repeated analysis of the calibration curve samples. The inter-assay accuracy and precision were within 5.0 and 7.0 %, respectively. The mean R 2 value was 0.9986. The accuracy and precision evaluated from repeated analysis of the quality-control samples were both within 6 %.
2.4 Pharmacokinetic and Statistical Methods
Plasma analyte concentrations were utilized for calculation of pharmacokinetic parameters, using a standard model-independent approach.
For piperaquine, the area under the plasma concentration–time curve (AUC) from 0 to 168 h (AUC0–168 h) was calculated using the linear trapezoidal method. The maximum observed plasma concentration (C max) and T max were taken directly from the data without interpolation. The mean transit time (MTT) over the sampling interval (MTT0–168 h) was calculated as AUMC0–168 h/AUC0–168 h, where AUMC0–168 h is the area under the first moment of the plasma concentration–time curve (AUMC) from 0 to 168 h.
For dihydroartemisinin, the AUC from time zero to the time of the last quantifiable concentration (AUC0–last) was calculated using the linear trapezoidal method. The AUC from time zero to infinity (AUC0–∞) was calculated as AUC0–last + Clast/λ z, where C last is the last quantifiable concentration and λz is the terminal slope of the Ln-transformed concentration–time profile. C max and T max were taken directly from the data without interpolation. The MTT was calculated as AUMC0–∞/AUC0–∞. The terminal half-life (T ½) was calculated as Ln(2)/λ z.
A linear mixed-effects analysis of variance (ANOVA) model was used to analyse Ln-transformed AUC, C max, MTT and T ½ parameters. The pooled variance was used to construct the 90 % confidence intervals (CIs) for the ratios of the fed and fasted treatment geometric least squares means. In constructing these 90 % vCIs, the fasted treatment was used as the reference. A non-parametric Mann–Whitney U test was used to assess treatment differences for the T max data. A Student’s t test was used to determine differences in demographic parameters between the treatment groups.
Significance was set at an α level of 0.05. Treatment equivalence was concluded if the 90 % CIs were within the limits of 80–125 %.
Phoenix® WinNonlin®, Version 1.3 (Pharsight Corporation, Mountain View, CA, USA) was used for pharmacokinetic and parametric statistical analyses. SPSS for Windows, Version 19 (SPSS Inc., Chicago, IL, USA) was used for non-parametric statistical analysis.
3 Results
Complete concentration–time data were available for 36 participants (18 fasted, 18 fed) for assessment of piperaquine pharmacokinetics, and for 37 participants (19 fasted, 18 fed) for assessment of dihydroartemisinin pharmacokinetics. One subject (in the fasted group) withdrew from the study prior to collection of the 168 h sample. Demographic information for the study population is summarized in Table 1; there were no statistically significant differences in demographic parameters between the two treatment groups.
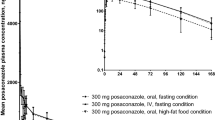
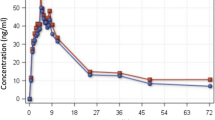
Plasma concentration–time profiles in the fed and fasted states are displayed for piperaquine in Fig. 1 and for dihydroartemisinin in Fig. 2. Pharmacokinetic parameters are summarized in Table 2, and the ratios of the fed/fasted data and associated 90 % CIs for the pharmacokinetic parameters are summarized in Fig. 3.
90 % confidence intervals of piperaquine and dihydroartemisinin pharmacokinetic parameters obtained after administration of a single oral dose of 160 mg of dihydroartemisinin/1280 mg of piperaquine in the fed and fasted states. The data are expressed as the 90 % confidence interval around the ratio (%fed/fasted) of the geometric least squares mean. AUC area under the plasma concentration–time curve, AUC 0–168 h AUC from 0 to 168 h, AUC 0–last AUC from time zero to the time of the last quantifiable concentration, AUC 0–∞ AUC from time zero to infinity, C max maximum observed plasma concentration, MTT mean transit time, MTT 0–168 h MTT from 0 to 168 h, T ½ terminal half-life
3.1 Piperaquine
The study results indicated that there was a substantial increase in plasma piperaquine concentrations after consumption of a high-fat, high-calorie meal (Fig. 1), with average AUC and C max parameters increasing approximately 3- to 4-fold (AUC0–168 h FED/AUC0–168 h FASTED = 99 %; C max FED/C max FASTED = 395 %). Equivalence assessment of the piperaquine pharmacokinetic data indicated that the meal resulted in significantly higher AUC0–168 h values (p < 0.001) and C max values (p < 0.001), with 90 % CIs well above the 80–125 % limits (Fig. 3). On the other hand, no treatment differences were detected for MTT0–168 h (MTT0–168 h FED/MTT0–168 h FASTED = 87 %; 90 % CI 80–95 %). No significant differences in T max values (p = 0.309) were found.
3.2 Dihydroartemisinin
Statistical analysis of the plasma dihydroartemisinin data indicated that consumption of a high-fat, high-calorie meal resulted in significantly higher AUC0–last values (p = 0.022), AUC0–∞ values (p = 0.013) and MTT values (p = 0.005) [AUC0–last FED/AUC0–last FASTED = 142 %; AUC0–∞ FED/AUC0–∞ FASTED = 142 %; MTTFED/MTTFASTED = 135 %). In addition, the 90 % CIs for the ratios of the fed/fasted data extended beyond the 80–125 % limits, indicating that the treatments could not be considered equivalent with respect to AUC0–last, AUC0–∞, C max and MTT (Fig. 3). The meal was also shown to result in significantly later maximum plasma concentrations (2.47 ± 1.37 h vs 1.16 ± 0.448 h; p < 0.001). No significant differences in C max values (p = 0.239) and T ½ values (p = 0.598) were found.
4 Discussion
A comprehensive understanding of the pharmacokinetics of drugs and the factors contributing to inter- and intra-individual variability is critical in order to develop evidence-based guidelines for appropriate prescription of these drugs in clinical practice. This study was conducted to examine potential food–drug interactions for piperaquine and dihydroartemisinin in healthy adult volunteers.
Given the poor water solubility and likely low oral bioavailability of piperaquine [22], it is proposed that administration with food results in enhanced bioavailability and increases in drug exposure. A number of studies have examined the influence of food on piperaquine pharmacokinetics, with varying results. In a small (n = 8), randomized, crossover study, Sim et al. [14] demonstrated significant increases in both the rate of absorption (213 % increase in C max) and the extent of absorption (107 % increase in AUC0–last) of piperaquine after administration with a high-fat, high-calorie meal (53.4 g/900–1000 kcal), in comparison with the values observed in the fasted state. Similarly, Chinh et al. [13] reported significant increases in C max and AUC parameters after consumption of a standard Vietnamese noodle meal (with 16.7 g of fat) in comparison with fasting values in 26 healthy subjects. On the other hand, Hai et al. [16] reported no significant impact of a similar Vietnamese meal (with 17 g fat) on the pharmacokinetics of piperaquine; however, it should be noted that the reported C max values and AUC from 0 to 24 h (AUC0–24) values were higher in the fed group (n = 16) than in the fasted group (n = 16) [C max 212 vs 130 µg/L; AUC0–24 2.2 vs 1.7 mg·h/L). Recently, Annerberg et al. [15] investigated the impact of consumption of a small amount of fat (chocolate milk containing 6.7 g of fat) on piperaquine pharmacokinetics in malaria patients and were unable to detect any significant differences in pharmacokinetics parameters between the fasted group (n = 14) and the fed group (n = 15).
In the present study, a significant increase in plasma piperaquine concentrations was demonstrated after consumption of a high-fat, high-calorie meal (Fig. 1), in keeping with the findings reported by Sim et al. [14]. Given the 3- to 4-fold increases in AUC and C max values, it can be concluded that the meal resulted in increases in the extent of exposure to piperaquine. Assuming that the meal had no effects on the clearance or volume of distribution of piperaquine, these findings are likely to reflect a substantial increase in the oral bioavailability of the drug. Although no difference in the MTT values for piperaquine was observed, this is not unexpected given that the mean residence time (MRT) far exceeds the mean absorption time (MAT) for piperaquine and, as such, it is unlikely that small differences in the absorption time could be detected. As a result, limited conclusions can be drawn with regard to the effect of food on the rate of absorption of piperaquine. It should be noted that because of the considerably long half-life of piperaquine, the sampling schedule employed for this study was unable to characterize the full piperaquine concentration–time profile; consequently, the terminal phase and associated pharmacokinetic parameters could not be determined. However, the sampling schedule was considered adequate to ensure complete absorption of the drug; therefore the C max and AUC0–168 h values were considered sufficient to characterize the peak and total drug exposure as per the FDA guidelines.
While the effects of food on the pharmacokinetics of orally administered dihydroartemisinin have not been previously investigated, studies have examined the impact of food on the pharmacokinetics of other artemisinin-derived compounds, with varying results. Fitoussi et al. [20] reported a significant reduction in artesunate and dihydroartemisinin (metabolite) C max and AUC0–last values and increased dihydroartemisinin Tmax values after administration of artesunate in combination with a meal, in comparison with doses administered in the fasted state. On the other hand, Tan et al. [21] identified food as a significant covariate for the absorption rate constant in a population pharmacokinetic model of artesunate, with an associated increase in the absorption half-life of artesunate from 10.8 min (fasted) to 67.5 min (fed); however, the extent of absorption was not significantly affected. Dien et al. [19] investigated the impact of food on artemisinin pharmacokinetics and concluded that consumption of a meal had no effects on AUC, C max, T max, apparent oral clearance or MTT values.
The present study was able to demonstrate that administration of dihydroartemisinin in combination with a high-fat, high-calorie meal resulted in significant increases in AUC0–last, AUC0–∞ and MTT values. As MTT comprises MRT + MAT, and given that MRT was likely to be similar in the two treatment groups (as the clearance and volume of distribution would not be expected to be affected by the meal, and T ½ values were not significantly altered), it can be assumed that the increases in MTT values were reflective of increased MAT. The observed increases in AUC0–last, AUC0–∞ and MTT values therefore demonstrate that consumption of a high-fat, high-calorie meal results in delayed and enhanced absorption of orally administered dihydroartemisinin in comparison with absorption in the fasted state.
The effects of food on the absorption of piperaquine and dihydroartemisinin are likely to reflect a number of factors, including delayed gastric emptying, increased bile salt secretion, increased solubility and dissolution of the drugs and/or changes in gastric pH [12]. While the exact mechanism is unknown, it is interesting to note that the absorption of piperaquine appears to be directly related to the fat content of the meal. In fact, combining our findings with those of other studies, it can be seen that there is a significant correlation between the fat content of the administered meal and the ratio of the fed/fasted C max and AUC data (Fig. 4).
Relationship between the fat content of the administered meal and the ratio of the piperaquine fed/fasted data in terms of the maximum observed plasma concentration (black circles; R 2 = 0.995, p < 0.001) and the area under the plasma concentration–time curve (white circles; R 2 = 0.930, p = 0.008). The data are taken from the present study, Chinh et al. [13], Sim et al. [14], Annerberg et al. [15] and Hai et al. [16]
Despite the reported significant alterations in the pharmacokinetics of piperaquine and dihydroartemisinin with food, the clinical relevance of these findings is questionable. Given that the fat content of standard meals within endemic areas is relatively low in comparison with Western countries [23], and patients with malarial infection are typically anorexic [1], these results are unlikely to impact on the clinical utility of these drugs. While it has been suggested that piperaquine–dihydroartemisinin should be co-administered with a high-fat meal to improve treatment exposure (as is recommended for artemether–lumefantrine [20, 24]), given the established efficacy of piperaquine–dihydroartemisinin [2–6], this practice would be unlikely to improve treatment effectiveness—rather, it would impact on the safety profile of these drugs. In particular, as a potential risk of corrected QT (QTc) interval prolongation has been noted for piperaquine–dihydroartemisinin, concurrent consumption of food with treatment administration would likely result in increased drug exposure and consequently an increased risk of cardiac adverse effects [11]. It should also be noted that while the standard diets of populations within malaria-endemic regions are not expected to impact significantly on the safety profiles of these drugs, co-administration of food with these anti-malarials by populations consuming typical Western diets should be avoided, especially given that food has been shown to have no impact on the efficacy of piperaquine–dihydroartemisinin [17]. It is therefore a general recommendation that piperaquine–dihydroartemisinin not be administered within ±3 h of food consumption.
5 Conclusion
This study demonstrated that administration of piperaquine–dihydroartemisinin combination therapy with a high-fat, high-calorie meal results in a substantial increase in the extent of exposure to piperaquine and both delayed and enhanced absorption of dihydroartemisinin. The mechanism of the food–drug interaction for piperaquine and dihydroartemisinin is unknown; however, for piperaquine, the data indicate that the effect is directly correlated with the fat content of the meal. Although malaria patients in endemic regions are unlikely to consume a high-fat diet, given that piperaquine–dihydroartemisinin has been associated with a potential risk of QTc interval prolongation, it is prudent to recommend that these drugs not be administered within ±3 h of food consumption.
References
World Health Organization. Guidelines for the treatment of malaria. 2015. http://apps.who.int/iris/bitstream/10665/162441/1/9789241549127_eng.pdf?ua=1. Accessed 27 July 2015.
Ashley EA, McGready R, Hutagalung R, Phaiphun L, Slight T, Proux S, Thwai KL, Barends M, Looareesuwan S, White NJ, Nosten F. A randomized, controlled study of a simple, once-daily regimen of dihydroartemisinin–piperaquine for the treatment of uncomplicated, multidrug-resistant falciparum malaria. Clin Infect Dis. 2005;41(4):425–32.
Myint HY, Ashley EA, Day NPJ, Nosten F, White NJ. Efficacy and safety of dihydroartemisinin–piperaquine. Trans R Soc Trop Med Hyg. 2007;101(9):858–66.
Karema C, Fanello CI, van Overmeir C, van Geertruyden JP, van Doren W, Ngamije D, D’Alessandro U. Safety and efficacy of dihydroartemisinin/piperaquine (Artekin) for the treatment of uncomplicated Plasmodium falciparum malaria in Rwandan children. Trans R Soc Trop Med Hyg. 2006;100(12):1105–11.
Smithuis F, Kyaw MK, Phe O, Aye KZ, Htet L, Barends M, Lindegårdh N, Singtoroj T, Ashley E, Lwin S, Stepniewska K, White NJ. Efficacy and effectiveness of dihydroartemisinin–piperaquine versus artesunate–mefloquine in falciparum malaria: an open-label randomised comparison. Lancet. 2006;367(95828):2075–85.
Tran TH, Dolecek C, Pham PM, Nguyen TD, Nguyen TT, Le HT, Dong TH, Tran TT, Stepniewska K, White NJ, Farrar J. Dihydroartemisinin–piperaquine against multidrug-resistant Plasmodium falciparum malaria in Vietnam: randomised clinical trial. Lancet. 2004;363(9402):18–22.
Binh TQ, Ilett KF, Batty KT, Davis TM, Hung NC, Powell SM, Thu LT, Thien HV, Phuöng HL, Phuöng VD. Oral bioavailability of dihydroartemisinin in Vietnamese volunteers and in patients with falciparum malaria. Br J Clin Pharmacol. 2001;51(6):541–6.
Hung TY, Davis TM, Ilett KF, Karunajeewa H, Hewitt S, Denis MB, Lim C, Socheat D. Population pharmacokinetics of piperaquine in adults and children with uncomplicated falciparum or vivax malaria. Br J Clin Pharmacol. 2004;57(3):253–62.
Na-Bangchang K, Krudsood S, Silachamroon U, Molunto P, Tasanor O, Chalermrut K, Tangpukdee N, Matangkasombut O, Kano S, Looareesuwan S. The pharmacokinetics of oral dihydroartemisinin and artesunate in healthy Thai volunteers. Southeast Asian J Trop Med Public Health. 2004;35(3):575–82.
Tarning J, Ashley EA, Lindegårdh N, Stepniewska K, Phaiphun L, Day NP, McGready R, Ashton M, Nosten F, White NJ. Population pharmacokinetics of piperaquine after two different treatment regimens with dihydroartemisinin–piperaquine in patients with Plasmodium falciparum malaria in Thailand. Antimicrob Agents Chemother. 2008;52(3):1052–61.
European Medicines Agency. Eurartesim: piperaquine tetraphosphate/dihydroartemisinin. Product information: 28/07/2014 Eurartesim -EMEA/H/C/001199 -N/0013. 2014. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001199/human_med_001450.jsp&mid=WC0b01ac058001d124. Accessed 5 Aug 2015.
Singh BN. Effects of food on clinical pharmacokinetics. Clin Pharmacokinet. 1999;37(3):213–55.
Chinh NT, Quang NN, Thanh NX, Dai B, Travers T, Edstein MD. Short report: pharmacokinetics of the antimalarial drug piperaquine in healthy Vietnamese subjects. Am J Trop Med Hyg. 2008;79(4):620–3.
Sim IK, Davis TME, Ilett KF. Effects of a high-fat meal on the relative oral bioavailability of piperaquine. Antimicrob Agents Chemother. 2005;46(6):2407–11.
Annerberg A, Lwin KM, Lindegårdh N, Khrutsawadchai S, Ashley E, Day NPJ, Singhasivanon P, Tarning J, White NJ, Nosten N. A small amount of fat does not affect piperaquine exposure in patients with malaria. Antimicrob Agents Chemother. 2011;55(9):3971–6.
Hai TN, Hietala SF, Van Huong NV, Ashton M. The influence of food on the pharmacokinetics of piperaquine in healthy Vietnamese volunteers. Acta Trop. 2008;107(2):145–9.
Lwin KM, Phyo AP, Hanpithakpong W, Ashley EA, Lee SJ, Cheah P, Singhasivanon P, White NJ, Lindegårdh N, Nosten F. Randomized, double-blind, placebo-controlled trial of monthly versus bimonthly dihydroartemisinin–piperaquine chemoprevention in adults at high risk of malaria. Antimicrob Agents Chemother. 2012;56(3):1571–7.
Tarning J, Lindegårdh N, Lwin KM, Annerberg A, Kiricharoen L, Ashley E, White NJ, Nosten F, Day NP. Population pharmacokinetic assessment of the effect of food on piperaquine bioavailability in patients with uncomplicated malaria. Antimicrob Agents Chemother. 2014;58(4):2052–8.
Dien TK, de Vries PJ, Khanh NX, Koopmans R, Binh LN, Duc DD, Kager PA, van Boxtel CJ. Effect of food intake on pharmacokinetics of oral artemisinin in healthy Vietnamese subjects. Antimicrob Agents Chemother. 1997;41(5):1069–72.
Fitoussi S, Thang C, Lesauvage E, Barré J, Charron B, Filali-Ansary A, Lameyre V. Bioavailability of a co-formulated combination of amodiaquine and artesunate under fed and fasted conditions: a randomised, open-label crossover study. Arzneimittelforschung. 2009;59(7):370–6.
Tan B, Naik H, Jang IJ, Yu KS, Kirsch LE, Shin CS, Craft JC, Fleckenstein L. Population pharmacokinetics of artesunate and dihydroartemisinin following single- and multiple-dosing of oral artesunate in healthy subjects. Malar J. 2009;8:304.
Tarning J, Lindegårdh N, Sandberg S, Day NJ, White NJ, Ashton M. Pharmacokinetics and metabolism of the antimalarial piperaquine after intravenous and oral single doses to the rat. J Pharm Sci. 2008;97(8):3400–10.
Premji ZG, Abdulla S, Ogutu B, Ndong A, Falade CO, Sagara I, Mulure N, Nwaiwu O, Kokwaro G. The content of African diets is adequate to achieve optimal efficacy with fixed-dose artemether–lumefantrine: a review of the evidence. Malar J. 2008;7:244.
Djimdé A, Lefèvre G. Understanding the pharmacokinetics of Coartem®. Malar J. 2009;8(Suppl 1):S4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was co-funded by Sigma-Tau Farmaceutiche Riunite SpA and the Medicines for Malaria Venture.
Disclosure of potential conflicts of interest
The authors SER, AME and DU declare that they have no conflict of interest. SS, YL and BF are employed by institutions that received funding (commercial sponsorship of contract research) from Sigma-Tau Industrie Farmaceutiche Riunite SpA for the submitted research. GV, AB and SP are employees of Sigma-Tau Industrie Farmaceutiche Riunite SpA.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Reuter, S.E., Evans, A.M., Shakib, S. et al. Effect of Food on the Pharmacokinetics of Piperaquine and Dihydroartemisinin. Clin Drug Investig 35, 559–567 (2015). https://doi.org/10.1007/s40261-015-0312-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-015-0312-8