Abstract
Background and objectives
Recent findings from randomized clinical trials indicate an improved patient adherence and blood pressure (BP) control by using fixed-dose combinations (FDCs) in the treatment of hypertension. The aim of the present study was to verify those data in a large real-world sample of hypertensive patients and to cross-check adherence evaluation performed by physicians and patients self-assessment.
Methods
A European multi-center, prospective, 24-week, non-interventional study was conducted including 14,979 patients with essential hypertension and new treatment with olmesartan, amlodipine and hydrochlorothiazide as an FDC. Patients’ adherence was measured using the Morisky Medication Adherence Scale (MMAS-8) and a non-standardized questionnaire was used by physicians and patients for self-assessment.
Results
The mean age of the patients was 63.9 ± 11.78 years and 46.5 % were women. One or more cardiovascular risk factors were present in 71.9 % of patients and 94.7 % had been treated for hypertension before study entry. Mean adherence to medication by MMAS-8 improved from 6.0 to 6.9 at study end. Corresponding improvements of adherence were seen on physicians’ and patients’ self-assessments throughout the study. Mean decrease of systolic/diastolic BP was 26.4/12.8 mmHg without a relevant difference between the MMAS-8 adherence levels. BP target achievement improved from 55.3 to 67.7 % in patients with low versus high adherence. The overall rate of patients with adverse drug reactions was very low (1.76 %) but more frequent in patients with low adherence.
Conclusions
Our data confirm previous clinical trial data on the improvement of medication adherence by switching antihypertensive combination therapy to an FDC and a subsequent improvement in BP target achievement. An observed trend toward a reduction in adverse drug reactions needs to be further investigated in clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The fixed-dose combination of olmesartan, amlodipine and hydrochlorothiazide increases the therapeutic adherence of unselected patients with essential hypertension. |
Blood pressure target achievement and response rates improve with increasing adherence. |
The Morisky Medication Adherence Scale is an easy-to-use tool for the assessment of patients’ adherence, which physicians should use more frequently in their daily practice. |
1 Background
Despite a variety of effective blood pressure (BP)-lowering drugs being available, BP control in most countries worldwide is not satisfactory [1, 2]. The majority of patients require dual or even triple-combination therapies to get BP at least near to a normal range [3]. Such a complex antihypertensive regimen may, therefore, require multiple pills taken at several time points during the day. This is known to decrease patient adherence to their treatment and to result in subsequent cardiovascular events [4].
To overcome this barrier, fixed-dose combinations (FDCs) of long-acting antihypertensive drugs have been developed that usually combine a renin-angiotensin-blocking agent, a calcium antagonist and a diuretic. These components are chosen based on their high efficacy to control BP and their high tolerability. FDCs result in an increased adherence with treatment and improvements of BP control (if those non-compliant are considered for computing the average) while preserving the tolerability of the free combination [5–9].
A drug with a number of FDCs available is olmesartan, which is also available as a triple FDC with amlodipine and hydrochlorothiazide. It has been demonstrated to be efficacious and safe in a number of clinical trials [10–13]. As patient populations in clinical trials may substantially differ from those in real-world clinical practice and medication adherence is artificially high in clinical trials, we aimed to verify the impact of the FDC olmesartan/amlodipine/hydrochlorothiazide on patient adherence, BP control and tolerability in a large, unselected, patient population in primary care.
2 Methods
2.1 Study Design
This open-label, non-interventional, observational study was conducted in primary care practice (general practitioners, specialists in internal medicine and cardiology) in five European countries: Austria, Belgium, Germany, the Netherlands and Switzerland. Ethical approval was obtained prior to commencement of the study by the appropriate ethics committees in the respective countries. Written informed consent was obtained from all patients prior to enrolment explaining the objectives of the study.
2.2 Patient Population and Schedule
Patients of at least 18 years of age with essential hypertension consecutively were considered for inclusion into the study if they started a treatment with SEVIKAR HCT® (olmesartan/amlodipine/hydrochlorothiazide 20/5/12.5, 40/5/12.5, 40/5/25, 40/10/12.5 or 40/10/25 mg each as a single-pill FDC), manufactured by Daiichi Sankyo Europe GmbH (Munich, Germany). There were no differences in costs or logistic of refilling between the new FDC and the pre-baseline medication. Exclusion criteria were contraindications as to the summary of product characteristics (hypersensitivity against one of the components or against sulphonamide derivates, impaired renal function, resistant hypokalaemia, hypercalcaemia, hyponatraemia and symptomatic hyperuricamia, moderate to severe liver impairment, pregnancy in the second or third trimester), as well as severe hypotension or cardiogenic shock or haemodynamically unstable heart failure and planned or current pregnancy. The planned follow-up period was 24 ± 2 weeks with optional interim visits after 8 ± 2 and 16 ± 2 weeks.
2.3 Objectives
The primary objective of the study was to gain further insights into the safety, tolerability and efficacy of the FDC of olmesartan/amlodipine/hydrochlorothiazide in an unselected patient cohort in daily practice. Furthermore, we aimed to assess the impact of patients’ adherence on the efficacy and safety of the FDC.
2.4 Blood Pressure (BP) Measurement
Office sitting BP was recorded at each visit using a calibrated standard sphygmomanometer and appropriate size cuff. All subsequent readings should have been done after the patient had been at rest for at least 5 min in a sitting position with the arm supported at the level of the heart. It was recommended to take a mean of three measurements.
2.5 Safety Analysis
For the assessment of safety, adverse drug reactions (ADRs) were recorded by severity (grades mild, moderate or severe at physicians’ estimation), their duration (start and end dates or if continuing at final examination) and seriousness (non-serious or serious ADRs).
2.6 Morisky Medication Adherence Scale
For the assessment of patients’ adherence, the modified Morisky Medication Adherence Scale (MMAS-8) [14, 15] was completed by patients on an optional basis at baseline and at study end. Country-specific local language scales were used that have been validated for hypertension according to content and construct validity. The eight questions are:
-
1.
Do you sometimes forget to take your high BP pills?
-
2.
Over the past 2 weeks, were there any days when you did not take your high BP medicine?
-
3.
Have you ever cut back or stopped taking your medication without telling your doctor because you felt worse when you took it?
-
4.
When you travel or leave home, do you sometimes forget to bring along your medications?
-
5.
Did you take your high BP medicine yesterday?
-
6.
When you feel like your BP is under control, do you sometimes stop taking your medicine?
-
7.
Do you ever feel hassled about sticking to your BP treatment plan?
-
8.
How often do you have difficulty remembering to take all your BP medication?
The coding of the answers given to questions 1–7 was “No” = 1, “Yes” = 0; for question 8 the coding was as follows: “Never/seldom” = 1, “Occasionally” = 0.75, “Sometimes” = 0.5, “Regularly” = 0.25, “Always” = 0. A score result <6 is interpreted as low adherence. A score result between 6 and <8 is interpreted as medium and a result of 8 as high adherence.
2.7 Statistical Analyses
Data were obtained on a paper case report form and entered in an electronic study database. Analyses were conducted under the responsibility of the department for Biostatistics and Data Operations at Daiichi Sankyo Europe (Munich, Germany) using Statistical Analysis System Version 9.2. The safety set included all patients for whom it is reasonable to assume that they have taken at least one dose of olmesartan/amlodipine/hydrochlorothiazide. It was used for the analysis of the demographics and safety. The full analysis set represented the safety set with valid non-missing data of the systolic and diastolic BP at baseline and at least one available post-baseline visit. The full analysis set was used for efficacy analysis.
Qualitative parameters were summarised by means of absolute and percentage numbers within the various categories. Quantitative parameters were summarised by means of standard statistics (i.e. number of non-missing and missing data, mean, standard deviation). p Values and two-sided 95 % confidence intervals (CI) were reported for the changes in systolic and diastolic BP.
3 Results
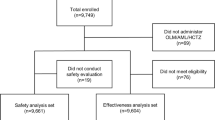
A total of 14,979 patients were included, 10,397 in Germany, 875 in Austria, 2,564 in Belgium, 282 in the Netherlands and 861 in Switzerland. The safety set comprised 14,976 patients; the full analysis set included 14,526 patients.
3.1 Patient Characteristics
Patients had a median age of 64.0 (19.0–64.0) years. Nearly half of the patients were aged between 40 and 65 years (49.2 %, 7,360 patients); 19.4 % were aged ≥75 years, 94.7 % were on treatment for hypertension before study entry. At least one cardiovascular risk factor was present in 71.9 % of the patients, for more baseline data see Table 1. The percentage of patients within the different dosage groups of the three antihypertensive substances at baseline and study end is presented in Fig. 1.
3.2 Patients’ Adherence
Information on the MMAS-8 was available for 10,798 (73.7 %) patients at both baseline and final examination. The mean score result increased from 6.0 ± 2.05 at baseline up to 6.9 ± 1.49 at study end (0.93, 95 % CI 0.90–0.97, p < 0.0001). Table 2 presents the frequency of the three adherence groups at baseline and study end and Table 3 presents the underlying items that built the MMS result at baseline and study end.
Additionally, physicians and patients were asked to evaluate medication adherence by ticking how often they have taken their medication correctly the way it was prescribed with “less than half of the time” (<50 %), “most of the time” (75 %), “almost always” (90 %) and “always” (100 %). Physicians and patients characterised adherence rather concordant at baseline with slightly higher values by the patients. The scoring result of both physicians and patients increased by a similar amount at study end, as shown in Fig. 2a and b.
We analysed all patients whose physicians’ and patients’ adherence assessments at baseline and study end were available regarding a congruent evaluation of the adherence over the course of the study (n = 10,537). A high concordance between self- and physicians’ evaluation result was found (see Table 4). Of the 3,952 patients with improved adherence in the physicians’ assessment at study end, 2370 patients (60.0 % of) voted themselves also with an improved adherence at study end and only 136 patients with a worse adherence.
3.3 BP-Lowering Efficacy According to Medication Adherence
Mean baseline BP was 160.9 ± 17.40/93.0 ± 10.95 mmHg at baseline, for BP according to MMAS-8 see Table 5 and for BP according to hypertension class see Fig. 3. Systolic/diastolic BP reduction between baseline and last available visit was 26.4 ± 18.26/12.8 ± 11.25 mmHg for both (p < 0.0001). Target BP (<140/90 mmHg) was achieved by 62.4 % of patients, BP response (systolic BP <140 mmHg and diastolic BP < 0 mmHg or change systolic BP ≥20 mmHg or diastolic BP ≥10 mmHg) by 81.5 % of all patients. BP at last available visit was 134.5 ± 11.66/80.4 ± 7.52 mmHg with no relevant differences between the adherence groups (Table 5). The mean decrease in BP was comparable among the MMSA-8 score adherence cohorts (low–medium–high) (Fig. 4).
With increasing medication adherence, BP target achievement improved considerably (Fig. 5). A weaker correlation was seen for the BP response rate (Fig. 5).
A positive relation was seen between BP target achievement and physicians’ assessment of compliance as well as between BP target achievement and patients’ self-assessment of compliance. Target achievement at study end had a range of 20.0–34.4 % in patients who scored or were scored by their physicians as having a low adherence (medication taken correctly <50% of the time) and improved to 67.4 and 66.9 % in patients with an optimal adherence (medication taken correctly 100 % of the time). A similar positive relation was observed for the BP response rate.
3.4 Safety and Tolerability
Overall, 382 ADRs were reported in 264 patients (1.76 % of all patients). Twenty-four patients (0.16 %) presented with at least one serious ADR, 207 patients discontinued from the study because of an ADR (1.38 %). No patient died during the study period.
The most frequently recorded ADRs according to preferred term were peripheral oedema (n = 89; 0.59 %), dizziness (n = 23; 0.15 %), hypotension (n = 17; 0.11 %), nausea and oedema (each n = 16; 0.11 %), and drug ineffectiveness (n = 12; 0.08 %). All other ADRs were recorded in <0.07 % (n < 10) of the patients. The percentage of patients with at least one ADR was highest for patients with low adherence while it was smallest for patients with high adherence (Table 6).
4 Discussion
The present study confirms prior, randomized, controlled trial evidence on the efficacy, safety and tolerability of the FDCs containing olmesartan/amlodipine/hydrochlorothiazide at different dosages in an unselected, hypertensive, patient population. Patients’ overall medication adherence was at the lower limit of the “medium” scale categorisation (MMAS-8 between 6 and 8) before study start, measured with the standardized MMAS. Treatment with the FDC substantially improved medication adherence measured with the MMAS-8 as well as with the non-standardized physicians’ and patient’s self-assessment questionnaire. We found a high consistency between patients’ self-assessment and physicians’ adherence assessment results.
4.1 Adherence
The impact of adherence as a key factor for achieving BP control in antihypertensive therapy becomes more and more evident. Despite numerous available drug classes, BP target achievement rates still stay below expectations [16, 17]. Poor adherence is known to be an (often hidden) major reason for not achieving BP control [18, 19] and probably an important source of preventable cardiovascular morbidity and mortality [20]. There is growing evidence showing that a high adherence is associated with a lower risk of events [21]. One recent study compared commercial health plan enrolees with index claims for an FDC of amlodipine plus olmesartan with an FDC of amlodipine plus benazepril and a free-dose combination of amlodipine plus angiotensin II receptor blockers (ARBs) and measured the proportion of days covered (PDC). Medication adherence was higher with amlodpinine/olmesartan FDC (PDC = 0.63) compared with amlodipine/benazepril (PDC = 0.55; p < 0.001) and amlodipine/ARB free-dose combination (PDC = 0.34; p < 0.001) [22].
Another large, Canadian, population-based study reconstructed outcome data of 82,320 patients treated for hypertension. High adherence level (95 %) to antihypertensive therapy compared with lower adherence level (60 %) was associated with a significant reduction in congestive heart failure events by 11 % (relative risk reduction (RR): 0.89; 95 % confidence interval (CI) 0.80–0.99) [23], coronary artery disease by 10 % (RR 0.90; 95 % CI 0.84–0.95) [24] and cerebrovascular disease by 22 % (RR 0.78, 95 % CI 0.7–0.87) [25].
Our study was not powered to measure cardio-/cerebrovascular outcomes. We measured adherence using two indirect tools, first, the validated MMAS, and second, a non-standardised patients’ self- and physicians’ adherence assessment. Our results using those tools are in line with a study assessing the validity of four indirect methods for measuring adherence to antihypertensive medication (1. knowledge regarding medication, 2. BP level, 3. attitude regarding the medication intake by Morisky-Green test and 4. self-reported adherence) [26]. None of the methods had a good positive predictive value for adherence. The best predictor was patients’ age and whether patients managed to control high BP. The highest sensitivity was shown for self-reported adherence, which is concordant with the results of the present study.
4.2 BP-Lowering Efficacy and Adherence
Our study demonstrated an increased adherence of the patients while receiving the FDC of olmesartan/amlodipine/hydrochlorothiazide throughout the study and an improving target achievement rate with increasing adherence. Two key influencing factors have to be considered as contributing to that result: 1. ARBs might have a competitive BP-lowering efficacy compared with ACE inhibitors and other antihypertensive drug classes [27, 28]. ARBs seem to display the highest rates of adherence in comparison studies with other antihypertensive drug classes [29–32]. These studies had observation periods of 1–4 years with 12-month values of between 42 and 64 % persistence with ARBs. A study by Veronesi et al. reported a persistence with ARBs of 68.5 %, while ACE inhibitors (odds ratio (OR) 0.94; 95 % CI 0.79–0.99), calcium channel blockers (OR 0.76; 95 % CI 0.54–0.85), beta blockers (OR 0.67; 95 % CI 0.57–0.79) and thiazide diuretics (0.56; 95 % CI 0.38–0.84) had a lower persistence [33]. One study identified higher age (≥65 years) and once-daily dosing to increase persistence vs. young age or multiple dosing [34].
4.3 Safety
ARBs in general and olmesartan in particular have proven to present a nearly negligible rate of side effects [35]. Therefore, the total number of ADRs to be evaluated for a correlation to adherence is very low and has to be interpreted with caution. We found a trend towards fewer ADRs and drug discontinuations with higher treatment adherence. Randomized clinical trials on this topic should further investigate this finding before any conclusions can be made.
4.4 Limitations
The present observational study was planned to reflect real life, which typically means a consecutive inclusion of suitable patients. As highly motivated patients more often agree to study participation, a bias due to higher refusal rates of low adherence patients cannot be excluded. No adjustment of the different adherence groups to age, sex or co-morbidities for creation of equal patient cohorts was performed as it was a real-life study. The scoring system of the MMAS-8 is based on eight questions that are summarised into three categories while the patients’ and physicians’ assessment was based on a four-item scale. This does not allow direct comparisons of both methods. Literature offers further different adherence-measuring tools and scoring systems, but no standardized “Morisky-styled” physicians’ questionnaire.
5 Conclusion
The present study shows that the FDC of olmesartan, amlodipine and hydrochlorothiazide increases the therapeutic adherence of unselected patients with essential hypertension. A higher adherence to medication seems to be associated with fewer ADRs and drug discontinuations. BP target achievement and response rates improve with increasing adherence. BP-lowering efficacy is nevertheless comparable and sufficient in both patient cohorts with higher and lower treatment adherence. The MMAS-8 is an easy-to-use tool for the assessment of patients’ adherence, which physicians should use more frequently in their daily practice.
References
Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Worldwide prevalence of hypertension: a systematic review. J Hypertens. 2004;22:11–9.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Task Force M. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357
Cowart JB, Taylor AA. Should two-drug initial therapy for hypertension be recommended for all patients? Curr Hypertens Rep. 2012;14:324–32.
Fifth Joint Task Force of the European Society of C, European Association of E, European Association of Percutaneous Cardiovascular I, European Heart Rhythm A, Heart Failure A, European Association for Cardiovascular P, Rehabilitation, European Atherosclerosis S, International Society of Behavioural M, European Stroke O, European Society of H, European Association for the Study of D, European Society of General Practice/Family M, International Diabetes Federation E, European Heart N. European guidelines on cardiovascular disease prevention in clinical practice (version 2012): the Fifth Joint Task Force of the European Society of Cardiology and other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur J Prev Cardiol. 2012;19:585–667.
Pimenta E, Oparil S. Fixed combinations in the management of hypertension: patient perspectives and rationale for development and utility of the olmesartan-amlodipine combination. Vasc Health Risk Manag. 2008;4:653–64.
Kjeldsen SE, Jamerson KA, Bakris GL, Pitt B, Dahlof B, Velazquez EJ, Gupte J, Staikos L, Hua TA, Shi V, Hester A, Tuomilehto J, Ostergren J, Ibsen H, Weber M. Avoiding cardiovascular events through CtiPLwSHI: predictors of blood pressure response to intensified and fixed combination treatment of hypertension: the Accomplish Study. Blood Press. 2008;17:7–17.
Geiger H, Barranco E, Gorostidi M, Taylor A, Zhang X, Xiang Z, Zhang J. Combination therapy with various combinations of aliskiren, valsartan, and hydrochlorothiazide in hypertensive patients not adequately responsive to hydrochlorothiazide alone. J Clin Hypertens. 2009;11:324–32.
Taylor AA, Shoheiber O. Adherence to antihypertensive therapy with fixed-dose amlodipine besylate/benazepril hcl versus comparable component-based therapy. Congest Heart Fail. 2003;9:324–32.
Chrysant SG, Oparil S, Melino M, Karki S, Lee J, Heyrman R. Efficacy and safety of long-term treatment with the combination of amlodipine besylate and olmesartan medoxomil in patients with hypertension. J Clin Hypertens. 2009;11:475–82.
Izzo JL Jr, Chrysant SG, Kereiakes DJ, Littlejohn Iii T, Oparil S, Melino M, Lee J, Fernandez V, Heyrman R. 24-hour efficacy and safety of triple-combination therapy with olmesartan, amlodipine, and hydrochlorothiazide: the Trinity Ambulatory Blood Pressure Substudy. J Clin Hypertens. 2011;13:873–80.
Kereiakes DJ, Chrysant SG, Izzo JL Jr, Littlejohn T 3rd, Oparil S, Melino M, Lee J, Fernandez V, Heyrman R. Long-term efficacy and safety of triple-combination therapy with olmesartan medoxomil and amlodipine besylate and hydrochlorothiazide for hypertension. J Clin Hypertens. 2012;14:149–57.
Oparil S, Chrysant SG, Melino M, Lee J, Karki S, Heyrman R. Long-term efficacy of a combination of amlodipine and olmesartan medoxomil ± hydrochlorothiazide in patients with hypertension stratified by age, race and diabetes status: a substudy of the coach trial. J Hum Hypertens. 2010;24:831–8.
Oparil S, Melino M, Lee J, Fernandez V, Heyrman R. Triple therapy with olmesartan medoxomil, amlodipine besylate, and hydrochlorothiazide in adult patients with hypertension: the trinity multicenter, randomized, double-blind, 12-week, parallel-group study. Clin Ther. 2010;32:1252–69.
Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10:348–54.
Krousel-Wood M, Islam T, Webber LS, Re RN, Morisky DE, Muntner P. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am J Manage Care. 2009;15:59–66.
Bramley TJ, Gerbino PP, Nightengale BS, Frech-Tamas F. Relationship of blood pressure control to adherence with antihypertensive monotherapy in 13 managed care organizations. J Manage Care Pharm. 2006;12:239–45.
Lee JK, Grace KA, Taylor AJ. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial. JAMA. 2006;296:2563–71.
Hill MN, Miller NH, DeGeest S, American Society of Hypertension Writing Group. Ash position paper: adherence and persistence with taking medication to control high blood pressure. J Clin Hypertens. 2010;12:757–64.
Krousel-Wood M, Joyce C, Holt E, Muntner P, Webber LS, Morisky DE, Frohlich ED, Re RN. Predictors of decline in medication adherence: results from the cohort study of medication adherence among older adults. Hypertension. 2011;58:804–10.
Corrao G, Parodi A, Nicotra F, Zambon A, Merlino L, Cesana G, Mancia G. Better compliance to antihypertensive medications reduces cardiovascular risk. J Hypertens. 2011;29:610–8.
Mazzaglia G, Ambrosioni E, Alacqua M, Filippi A, Sessa E, Immordino V, Borghi C, Brignoli O, Caputi AP, Cricelli C, Mantovani LG. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120:1598–605.
Ferrario CM, Panjabi S, Buzinec P, Swindle JP. Clinical and economic outcomes associated with amlodipine/renin-angiotensin system blocker combinations. Ther Adv Cardiovasc Dis. 2013;7:27–39.
Perreault S, Dragomir A, White M, Lalonde L, Blais L, Berard A. Better adherence to antihypertensive agents and risk reduction of chronic heart failure. J Intern Med. 2009;266:207–18.
Perreault S, Dragomir A, Roy L, White M, Blais L, Lalonde L, Berard A. Adherence level of antihypertensive agents in coronary artery disease. Br J Clin Pharmacol. 2010;69:74–84.
Kettani FZ, Dragomir A, Cote R, Roy L, Berard A, Blais L, Lalonde L, Moreau P, Perreault S. Impact of a better adherence to antihypertensive agents on cerebrovascular disease for primary prevention. Stroke J Cereb Circ. 2009;40:213–20.
Prado JC Jr, Kupek E, Mion D Jr. Validity of four indirect methods to measure adherence in primary care hypertensives. J Hum Hypertens. 2007;21:579–84.
Bramlage P, Hasford J. Blood pressure reduction, persistence and costs in the evaluation of antihypertensive drug treatment: a review. Cardiovasc Diabetol. 2009;8:18.
Law M, Wald N, Morris J. Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy. Health Technol Assess. 2003;7:1–94.
Esposti LD, Di Martino M, Saragoni S, Sgreccia A, Capone A, Buda S, Esposti ED. Pharmacoeconomics of antihypertensive drug treatment: an analysis of how long patients remain on various antihypertensive therapies. J Clin Hypertens. 2004;6:76–84.
Hasford J, Schroder-Bernhardi D, Rottenkolber M, Kostev K, Dietlein G. Persistence with antihypertensive treatments: results of a 3-year follow-up cohort study. Eur J Clin Pharmacol. 2007;63:1055–61.
Patel BV, Remigio-Baker RA, Mehta D, Thiebaud P, Frech-Tamas F, Preblick R. Effects of initial antihypertensive drug class on patient persistence and compliance in a usual-care setting in the United States. J Clin Hypertens. 2007;9:692–700.
Erkens JA, Panneman MM, Klungel OH, van den Boom G, Prescott MF, Herings RM. Differences in antihypertensive drug persistence associated with drug class and gender: a pharmo study. Pharmacoepidemiol Drug Saf. 2005;14:795–803.
Veronesi M, Cicero AF, Prandin MG, Dormi A, Cosentino E, Strocchi E, Borghi C. A prospective evaluation of persistence on antihypertensive treatment with different antihypertensive drugs in clinical practice. Vasc Health Risk Manag. 2007;3:999–1005.
Bloom BS. Continuation of initial antihypertensive medication after 1 year of therapy. Clin Ther. 1998;20:671–81.
Tocci G, Paneni F, Passerini J, Volpe M. Triple combination therapy to improve blood pressure control: experience with olmesartan-amlodipine-hydrochlorothiazide therapy. Expert Opin Pharmacother. 2012;13:2687–97.
Acknowledgments
The authors acknowledge the cooperation and commitment of all investigators and their staff, who contributed to the trial conduct.
Conflict of interest
PB, RK and RES received consultancy fees, attended advisory boards and have held lectures for a number of pharmaceutical companies including Daiichi Sankyo Germany. WPW and RS are employees of Daiichi Sankyo Germany. EMF is an employee of Daiichi Sankyo Europe, Department for Statistics. CZ has no conflict of interest to declare.
Authors’ contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data. PB and CZ have drafted the manuscript. The other authors revised the manuscript for important intellectual content and all authors granted final approval of the manuscript to be published.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bramlage, P., Ketelhut, R., Fronk, EM. et al. Clinical Impact of Patient Adherence to a Fixed-Dose Combination of Olmesartan, Amlodipine and Hydrochlorothiazide. Clin Drug Investig 34, 403–411 (2014). https://doi.org/10.1007/s40261-014-0188-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-014-0188-z