Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) is associated with a high morbidity in patients with impaired fasting glucose (IFG). Bicyclol is a synthetic compound known to protect the liver against oxidation and lipid injuries.
Objective
The objective of this study was to evaluate the efficacy and safety of metformin and bicyclol in the treatment of NAFLD patients with IFG.
Methods
After lifestyle changes and metformin treatment (500 mg orally three times daily), the 248 patients enrolled with NAFLD and IFG were equally randomized to two 24-week treatment groups: bicyclol 25 mg three times daily or vitamin E (α-tocopherol) 100 mg three times daily (control). Anthropometric measurements, serum biochemistry, liver/spleen computed tomography ratio, and changes in liver histological parameters were compared before and after treatments.
Results
A total of 223 patients completed the treatment, and there were significant improvements in body mass index, waist-to-hip ratio, and biochemical parameters in both groups (P < 0.01). Compared with the control group, the improvement in serum alanine aminotransferase levels in the bicyclol group was statistically significant (P < 0.01). Liver histological assessments revealed that steatosis, inflammation, hepatocellular ballooning, and NAFLD activity scores (NAS) were all decreased in both groups after treatment (P < 0.01). However, decreases in inflammation and NAS in the bicyclol group were statistically significant compared with the vitamin E group (P < 0.01). Adverse events in the bicyclol and control groups occurred in 1.79 and 1.80 %, respectively.
Conclusion
Metformin combined with bicyclol is effective and safe in the treatment of patients with NAFLD and IFG. However, further studies with a larger sample size are needed to confirm the efficacy and safety of the combination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Non-alcoholic fatty liver disease (NAFLD) encompasses a spectrum ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), and causes an increased risk of cardiovascular diseases, type 2 diabetes mellitus (T2DM) and liver-related complications [1]. NAFLD is now considered to be a hepatic manifestation of metabolic syndrome (MetS), and the management of patients with NAFLD consists of treating the steatohepatitis and the associated metabolic co-morbidities to prevent hepatic and metabolic complications [1, 2]. In addition to lifestyle changes, pharmacological agents have no obvious effects on hepatic and metabolic aspects in NASH patients, except for thiazolidinediones; however, the long-term safety and efficacy of thiazolidinediones have not been established [2]. On one hand, vitamin E (α-tocopherol) administered at 800 IU/day improves liver histology in non-diabetic adults with NASH, but without improvements in insulin resistance (IR) and T2DM [3]. On the other hand, metformin has no significant effect on liver histology, though it is associated with definite improvements of IR and reduction of T2DM incidence [4, 5]. Therefore, a combined pharmacological approach is necessary in NASH patients with metabolic co-morbidities.
Bicyclol (4,4′-dimethoxy-5,6,5′,6′-dimethylene-dioxy-2,2′-dicarboxylate biphenyl) is a liver protectant for patients with various liver diseases used in many countries. Further studies have shown that bicyclol plays a role in eliminating free radicals, in preventing lipid peroxidation, and in protecting hepatic cell membrane and mitochondria in a number of models of hepatic injury [6]. Bicyclol can induce glutathione and glutathione-S-transferase expression, and inhibit the accumulation of hepatic lipids [7]. Some preliminary clinical trials showed that bicyclol was effective in improving hepatic functional indexes and histological scores in NAFLD patients [8], and in improving hepatic inflammatory necrosis in chronic hepatitis B patients without influencing the therapeutic effects of antiviral agents [9]. Vitamin E can be used for the treatment of NAFLD in non-diabetic patients [3], considering its effects in diabetic patients are controversial [10, 11]. In addition, according to the Chinese Pharmacopoeia, for safety, vitamin E cannot be used in high doses. We chose a daily dose of 300 mg, the maximum acceptable dose in China. Thus, a randomized, multicenter, vitamin E-controlled trial of bicyclol for the treatment of NAFLD patients with impaired fasting glucose (IFG) was designed to evaluate the efficacy and safety of bicyclol in the context of lifestyle changes and metformin treatment.
2 Methods
2.1 Ethics and Study Design
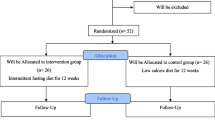
The present study was a randomized, multicenter, parallel-designed controlled trial carried out in five tertiary hospitals in China: Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (Shanghai); the Sixth People’s Hospital (Hangzhou); Xi Jing Hospital of Digestive Diseases (Xi’an); China-Japan Friendship Hospital (Beijing); and the First Affiliated Hospital of Zhengzhou University (Zhengzhou). This study was approved by the ethics committees of each of these hospitals. Written informed consent was obtained from all subjects following a detailed description of the potential benefits and risks of the study.
The randomization table was created using SAS® version 9.2 (SAS Institute, Cary, NC, USA). A total of 248 NAFLD patients with IFG were enrolled and equally (1:1) randomized to the bicyclol or vitamin E (control) group. The investigations involved in the study were in accordance with the ethical recommendations of the Declaration of Helsinki (World Medical Association) and Good Clinical Practice (GCP).
2.2 Subjects
Inclusion criteria were (1) NAFLD diagnosis, as defined by the 2010 Chinese guidelines [24] for the diagnosis and management of NAFLD; (2) body mass index (BMI) between 23 and 30 kg/m2; (3) serum alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) 1.5 to 5 times the upper limit of normal value and maintained for more than 24 weeks; and (4) fasting plasma glucose (FPG) of 6.1–7.0 mmol/L, but without a previous history of T2DM or newly diagnosed T2DM according to an oral glucose tolerance test or glycosylated hemoglobin.
Exclusion criteria were (1) other liver diseases; (2) cirrhosis and/or liver function decompensation; (3) malignant tumor and other severe systemic diseases or infectious diseases; (4) pregnant or lactating women; (5) drug addiction; (6) history of a severe allergic reaction; (7) obvious dyslipidemia needing treatment with lipid-lowering drugs; (8) use of any drugs that could influence the efficacy of the study; or (9) any other conditions making them unsuitable for the present study.
2.3 Treatment Protocols
After lifestyle intervention and a total daily dose of metformin (Glucophage™ 500 mg/tablet, Bristol-Squibb Pharmaceuticals Ltd., Shanghai, China) of 1,500 mg/day (500 mg three times daily), patients in the bicyclol group received oral bicyclol tablets (Bicyclol 25 mg/tablet, Beijing Union Pharmaceutical Factory, Beijing, China) at a total daily dose of 75 mg (25 mg three times daily). Patients in the control group received vitamin E capsules (Vitamin E 100 mg/capsule, Beijing Double-Crane Pharmaceutical Business Co., Ltd., Beijing, China) at a total daily dose of 300 mg (100 mg three times daily). Both groups received study treatment for 24 weeks. Visits were scheduled at weeks 12 and 24.
2.4 Observation Indexes
2.4.1 Subject Demographics
Demographic characteristics of the patients, such as age, sex, medical history, previous treatment history, associated co-morbidities, and concomitant medications were collected before treatment. Adverse events (AEs) were recorded following GCP requirements.
2.4.2 Anthropometric Measurements
At baseline and at 24 weeks, patients underwent anthropometric assessments (body weight, height, and waist and hip circumferences). BMI was calculated by dividing body weight (in kg) by the square of the height (m2). Waist-to-hip (W/H) ratio was also calculated. Serum biochemistry parameters such as ALT, AST, γ-glutamyltransferase, triglycerides, high-density lipoprotein cholesterol, FPG, and fasting insulin (FINS) were measured. The Homeostasis Model of Assessment – Insulin Resistance (HOMA-IR) index was calculated as: FPG (mmol/L) × FINS (µU/mL)/22.5.
2.4.3 Computed Tomography Examinations
A double-slice spiral computed tomography (CT) scan of the abdomen was performed from the upper to the lower part of liver with a slice thickness and inter-slice interval of 7.5 mm. The central plane of the liver and spleen was selected, and the CT values of the liver and spleen on the same plane were measured. The examinations were repeated three times, and the mean value was used for analysis. The ratio of liver/spleen CT values was calculated.
2.4.4 Liver Biopsy
Liver biopsies were performed at baseline and at 24 weeks. The optimal position for needle puncture was determined using ultrasound guidance. Biopsy was performed using a 16 G needle, and required hepatic tissue ≥10 mm, including more than six portal areas. The specimen was cut into serial sections, and stained by hematoxylin-eosin, reticular fiber, and Masson’s trichrome. Histological activity was assessed using the NAFLD activity score (NAS) according to the guidelines of the NASH Clinical Research Network [12].
2.5 Statistical Analysis
Results are presented as mean ± standard deviation (SD). All analyses were performed using SAS® version 9.2. Demographic characteristics of patients were compared using Student’s t test or Chi-square test according to data type. Serum biochemical values were analyzed using multilevel models to analysis the central effect, and then the repeated measures data were compared using ANOVA. The comparison of means in each group at each timepoint was performed with paired t tests for multiple comparisons. Liver histology was evaluated using non-parametric estimation. All statistical tests were two-sided. A P value <0.05 was considered statistically significant.
3 Results
3.1 Study Subjects
A total of 248 subjects were enrolled in the trial, and 25 subjects withdrew from the study during treatment. Of the remaining 223 subjects, 112 belonged to the bicyclol group and 111 to the control group. There were no significant differences in age, sex, and disease characteristics between the two groups (all P > 0.05).
3.2 Changes in Body Mass Index and Waist-to-Hip Ratio
At baseline and at 24 weeks, BMI in the bicyclol group was (mean ± SD) 26.74 ± 2.05 and 24.84 ± 1.91 kg/m2, respectively, and 26.94 ± 2.14 and 25.14 ± 1.86 kg/m2 in the control group, respectively. The W/H ratio at baseline and at 24 weeks was (mean ± SD) 0.94 ± 0.08 and 0.92 ± 0.08, respectively, in the bicyclol group and 0.94 ± 0.10 and 0.92 ± 0.09, respectively, in the control group. There were significant improvements in BMI and W/H ratio in the two groups when compared with baseline levels (both P < 0.01), but there were no differences between the two groups (P > 0.05).
3.3 Changes in Biochemical Parameters
There were significant change trends in serum biochemical parameters with time in each group (P < 0.01). In addition, significant differences in serum biochemical levels were also observed after 24 weeks of treatment when compared with baseline levels (P < 0.01). Serum ALT levels were significantly different between the two groups after 24 weeks of treatment (P < 0.01), and there was an interaction between the grouping factors and time (P < 0.01), suggesting that ALT levels showed different trends of variation between the two groups and a greater improvement in the bicyclol group after 24 weeks (Table 1).
3.4 Changes in Liver/Spleen Computed Tomography Ratio
At baseline and at 24 weeks, the liver/spleen CT ratios (mean ± SD) in the bicyclol group (0.74 ± 0.10 and 0.95 ± 0.18, respectively) and in the control group (0.73 ± 0.11 and 0.92 ± 0.17, respectively) demonstrated significant improvements compared with baseline (P < 0.01), but there were no significant differences between the two groups (P > 0.05).
3.5 Changes in Liver Histology
Liver biopsy was performed in 66 patients before the study. Nine patients were diagnosed with simple fatty liver (not NASH), 19 had possible NASH, and 38 had NASH, with five of them showing advanced fibrosis. A total of 31 patients in both groups underwent a second liver biopsy at the end of the study. Liver histological assessments revealed that steatosis, inflammation, hepatocellular ballooning, and NAS were all decreased in both groups after treatment (P < 0.01). However, decreases in inflammation and NAS in the bicyclol group were statistically significant compared with the control group (P < 0.01) (Table 2; Fig. 1).
Changes in liver histology in the bicyclol group before and after treatment (×200). a Hematoxylin and eosin staining before treatment. Hepatocytes presented with severe steatosis (covering up to 70 %), and exhibited ballooning degeneration. Parenchymal inflammation was mild and comprised a mixed population including neutrophils, lymphocytes, and Kupffer cells. b Hematoxylin and eosin staining after treatment. Fatty change (steatosis) was focal and there was no lobular inflammation and ballooning degeneration. c Masson staining before treatment: liver tissue was injured, and evident perisinusoidal fibrosis was observed. d Masson staining after treatment: hepatic tissue was normal without hyperplasia of fibrous tissues or perisinusoidal fibrosis
3.6 Comparison of Adverse Events
Two participants (1.79 %) in the bicyclol group reported abdominal distension and mild diarrhea during the study. The two AEs reported in the control group were mild abdominal distension and dizziness (incidence of 1.80 %). No abnormal laboratory results related to the study drugs were recorded.
4 Discussion
With the increasing incidence of obesity and MetS, NAFLD is becoming a common condition that is often complicated by impaired glucose regulation and T2DM. NAFLD is a well-recognized cause of liver-related morbidity and death, and it has been associated with the development of T2DM, atherosclerosis, and even malignant tumors [1, 13–16]. NAFLD has recently been recognized as a component of MetS or a hepatic manifestation of MetS [17, 18]. Prospective studies showed that NAFLD could predict the risk of MetS, T2DM, and cardiovascular diseases [19–21]. In T2DM patients with NAFLD, there is an increased risk of cardiovascular disease and death, as well as a risk of progression of end-stage liver diseases and liver-related death [2, 22]. NASH is a rate-limiting step in the process from simple fatty liver to liver cirrhosis. A retrospective study showed a lower incidence rate of cirrhosis of 0.6–3 % in NAFLD patients with simple fatty liver during a 10- to 20-year follow-up, while this rate was 15–25 % over 10 years in NASH patients [23]. With the aging society and control of other liver diseases such as viral hepatitis in China, obesity- and MetS-associated NAFLD are becoming an increasingly serious health problem, and it is of great clinical importance to prevent the development of such disease.
The results of the present study showed that there were significant improvements in BMI, W/H ratio, and biochemical parameters after 24 weeks of lifestyle intervention and metformin, in combination with bicyclol or vitamin E. However, bicyclol was better to improve serum ALT levels than vitamin E. Liver histological assessments revealed that hepatic steatosis, inflammation, hepatocellular ballooning, and NAS were all decreased in both groups after 24 weeks of treatment. However, decreases in hepatic inflammation and NAS were prominent in the bicyclol group and had statistical significance. AEs were comparable in the two groups.
Lifestyle changes are important in the treatment of T2DM. However, it has been reported that lifestyle interventions with weight loss of 3–5 % could reduce hepatic steatosis, but only an up to 10 % weight loss could improve necroinflammation [2]. In the present study, patients underwent lifestyle changes because it is part of the standard T2DM treatment. Since 10 % reduction in body weight is very difficult to achieve, we believe that hepatoprotectants are necessary in the treatment of NAFLD.
According to the Chinese guidelines for the diagnosis and management of NAFLD, the primary objective of NAFLD treatment is to improve IR, and to prevent and control MetS components and related end-stage organ lesions, thereby improving patients’ quality of life and prolonging survival. The secondary aim is to reduce fat accumulation in the liver, and to prevent or reverse steatohepatitis (lipotoxic hepatocellular injury), and to reduce or prevent the occurrence of cirrhosis and liver cancer, as well as their complications [24]. The results of the present study showed that while metformin improved glucose metabolism, combined treatment with bicyclol or vitamin E improved hepatic inflammation and liver injury in NAFLD. Therefore, we suggest that combined pharmacological therapies might play an important role in the treatment of NAFLD patients with IFG.
In addition to its anti-lipid peroxidation properties, treatment with vitamin E can inhibit the expression of inflammatory cytokines produced by monocytes and Kupffer cells, reduce the activity of nuclear factor-κB, and decrease collagen α1 gene expression, thereby lessening oxidative stress and liver injury. Vitamin E could also lead to biochemical improvements and reductions in NAS in patients with NASH, but without effects on liver fibrosis [2]. Furthermore, vitamin E supplementation might increase all-cause mortality [10, 11], and should be carefully prescribed. The present study revealed that a medium-dosage vitamin E could be used for the treatment of NASH, but that its therapeutic efficacy was less than the effects of bicyclol.
Bicyclol is a widely used liver protectant in China and other countries, and its mechanism of action may be closely related to free radical-scavenging activities, protection against lipid peroxidation, protection of cell membranes and mitochondrial function [25], and inhibition of inflammatory cytokines [26]. It was demonstrated in experimental and clinical studies that bicyclol could not only decrease serum transaminase levels, but could also lead to the improvements in pathological changes [7, 9]. Zhao et al. [27] revealed that the alleviating effects of bicyclol on hepatic inflammation was mainly due to its ability to attenuate oxidative stress, down-regulate expression of tumor necrosis factor (TNF)-α and interleukin-6, and then inhibit the secretion of cytokines such as TNF-α. In accordance with these previous studies, the results of the present study suggest that bicyclol improved NAFLD/NASH by alleviating inflammation, fibrosis, and pathological changes. Bicyclol also improved indexes of liver function. Taken together, the effects of bicyclol on the liver were greater than the effects from vitamin E.
5 Conclusion
In summary, our results showed that metformin plus bicyclol combination therapy is a safe and effective treatment regimen for patients with NAFLD complicated with IFG. Due to the limited number of patients and shorter duration of treatment, more clinical trials are still needed to verify the efficacy and safety of metformin combined with bicyclol in the treatment of patients with NAFLD complicated with IFG.
References
Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–85.
Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–23.
Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675–85.
Uygun A, Kadayifci A, Isik AT, Ozgurtas T, Deveci S, Tuzun A, et al. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2004;19(5):537–44.
Nair S, Diehl AM, Wiseman M, Farr GH Jr, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20(1):23–8.
Liu GT. Bicyclol: a novel drug for treating chronic viral hepatitis B and C. Med Chem. 2009;5(1):29–43.
Pan SY, Dong H, Yu ZL, Zhao XY, Xiang CJ, Wang H, et al. Bicyclol, a synthetic dibenzocyclooctadiene derivative, decreases hepatic lipids but increases serum triglyceride level in normal and hypercholesterolaemic mice. J Pharm Pharmacol. 2007;59(12):1657–62.
Su HL, Zhu YX, Gao ZJ, Dong XY, Zhu JY, Lei WR, et al. Efficacy comparison between bicyclol and polyene phosphatidylcholine treatments for the patients with nonalcoholic fatty liver disease. Chin J Hepatol. 2011;19(7):552–3.
Xie W, Shi G, Zhang H, Zhao G, Yu Z, Lang Z, et al. A randomized, multi-central, controlled study of patients with hepatitis B e antigen-positive chronic hepatitis B treated by adefovir dipivoxil or adefovir dipivoxil plus bicyclol. Hepatol Int. 2012;6(2):441–8.
Miller ER 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142(1):37–46.
Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297(8):842–57.
Farrell GC, Chitturi S, Lau GK, Sollano JD. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol. 2007;22(6):775–7.
Cao HX, Fan JG. Editorial: fatty liver disease: a growing public health problem worldwide. J Dig Dis. 2011;12(1):1–2.
Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52(2):774–88.
Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363(14):1341–50.
Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51(6):1972–8.
Neuschwander-Tetri BA. Nonalcoholic steatohepatitis and the metabolic syndrome. Am J Med Sci. 2005;330(6):326–35.
Rector RS, Thyfault JP, Wei Y, Ibdah JA. Non-alcoholic fatty liver disease and the metabolic syndrome: an update. World J Gastroenterol. 2008;14(2):185–92.
Nakanishi N, Suzuki K, Tatara K. Serum gamma-glutamyltransferase and risk of metabolic syndrome and type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2004;27(6):1427–32.
Kotronen A, Yki-Jarvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28(1):27–38.
Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865–73.
Fracanzani AL, Burdick L, Raselli S, Pedotti P, Grigore L, Santorelli G, et al. Carotid artery intima-media thickness in nonalcoholic fatty liver disease. Am J Med. 2008;121(1):72–8.
Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(2 Suppl 1):S99–112.
Fan JG, Jia JD, Li YM, Wang BY, Lu LG, Shi JP, et al. Guidelines for the diagnosis and management of nonalcoholic fatty liver disease: update 2010: (published in Chinese on Chinese Journal of Hepatology 2010; 18:163–166). J Dig Dis. 2011;12(1):38–44.
Liu GT, Li Y, Wei HL, Zhang H, Xu JY, Yu LH. Mechanism of protective action of bicyclol against CCl-induced liver injury in mice. Liver Int. 2005;25(4):872–9.
Wang H, Li Y. Protective effect of bicyclol on acute hepatic failure induced by lipopolysaccharide and d-galactosamine in mice. Eur J Pharmacol. 2006;534(1–3):194–201.
Zhao J, Chen H, Li Y. Protective effect of bicyclol on acute alcohol-induced liver injury in mice. Eur J Pharmacol. 2008;586(1–3):322–31.
Acknowledgments
No sources of funding were used to assist in the preparation of this study. The authors have no potential conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, Y., Shi, JP., Ma, AL. et al. Randomized, Vitamin E-Controlled Trial of Bicyclol Plus Metformin in Non-Alcoholic Fatty Liver Disease Patients with Impaired Fasting Glucose. Clin Drug Investig 34, 1–7 (2014). https://doi.org/10.1007/s40261-013-0136-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-013-0136-3