Abstract
Background and Objectives
Two new oral anticoagulants, rivaroxaban and dabigatran, with no need for anticoagulation monitoring, are available for patients with atrial fibrillation (AF). We aimed to compare their anticoagulant effects and safety when used during the AF ablation periprocedural period.
Methods
Patients undergoing AF ablation were randomly assigned to receive rivaroxaban 15 mg once daily (N = 30) or dabigatran 110 mg twice daily (N = 30). Rivaroxaban was withheld on the morning of the day before the ablation, and dabigatran was discontinued from the evening of the day before the procedure. Both anticoagulants were then resumed after haemostasis of the access site. D-dimer levels were measured just before the ablation, at the end of the ablation, and at 24 h and 48 h after the procedure.
Results
The baseline D-dimer levels were identical in both groups. However, D-dimer levels increased more markedly following the ablation procedure in patients receiving rivaroxaban than in those receiving dabigatran (mean ± standard deviation from 0.62 ± 0.16 to 1.09 ± 0.38 μg/mL vs from 0.59 ± 0.08 to 0.75 ± 0.17 μg/mL; p < 0.0001). The rate of rebleeding from the access site was similar in patients receiving rivaroxaban and those receiving dabigatran (33 vs 27 %; p = 0.78).
Conclusion
As compared with dabigatran, rivaroxaban may increase the risk of hypercoagulability when used during the periprocedural period of AF ablation, suggesting a potential rebound effect of rivaroxaban or a mismatch between its half-life and dose regimen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Following the results of the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) trial [1] and the Randomized Evaluation of Long-Term Anticoagulation Therapy (RELY) trial [2], two novel oral anticoagulants with no need for anticoagulation monitoring, rivaroxaban and dabigatran, have become used more and more in patients with atrial fibrillation (AF) all over the world. Therefore, considering that one of the important issues in the field of invasive cardiac electrophysiology is to anticoagulate patients safely during the periprocedural period of catheter ablation of AF [3], there is an urgent need to provide clinical evidence showing which new oral anticoagulant is more suitable for patients scheduled to undergo AF ablation. Given the rareness of thromboembolic and major haemorrhagic complications occurring during the periprocedural period, many thousands of subjects must be recruited to assess the safety of periablation anticoagulation with oral anticoagulants [3]. However, that is difficult and impractical. In a recent study, therefore, we reported the clinical utility of dabigatran versus warfarin by means of assessment of a coagulation marker [4]. This time, using the same methodology, we aimed to compare the anticoagulant effects of rivaroxaban and dabigatran when they were used as periprocedural anticoagulants for AF ablation.
2 Methods
2.1 Patients
This study was designed as a randomized, open-label trial, and was conducted by the Cardiovascular Center of Nagoya Daini Red Cross Hospital from May 2012 to April 2013. The study protocol was approved by the research committee of the institution. Patients with symptomatic AF were eligible for inclusion if they were scheduled to undergo radiofrequency catheter ablation for the first time and had not been previously prescribed rivaroxaban or dabigatran. Patients were excluded from the present study if they were aged >75 years; were using aspirin or other antiplatelet agents; had a prosthetic heart valve, haemodynamically significant valvular disease, advanced liver disease or an estimated glomerular filtration rate of <30 mL/min/1.73 m2; or were prescribed any azole antifungal agents. Eligible patients were enrolled after giving written informed consent.
2.2 Randomization and Study Protocol
Patients were randomly allocated at a 1:1 ratio to be treated with rivaroxaban or dabigatran as pre- and post-procedural anticoagulant therapy. The randomization was done using a block-randomization method.
If any adverse events resulting from the anticoagulants prior to the ablation at the outpatient clinic occurred, the patients were allowed to be switched to the other drug if they were unable to tolerate the adverse events.
2.3 Anticoagulation
When patients had already been treated with warfarin at the time of the randomization, rivaroxaban or dabigatran was started after confirming that warfarin had been withdrawn and that the international normalized ratio of the prothrombin time had decreased to 2.0 or less. On the basis of the results from studies including only Japanese populations [5, 6], the patients were prescribed rivaroxaban 15 mg once daily or dabigatran 110 mg twice daily, and the oral anticoagulants were started at least 3 weeks before the ablation. Considering the recommended daily number of doses of each oral anticoagulant, we instructed the patients to withhold rivaroxaban on the morning of the day before the ablation, or to discontinue dabigatran from the evening of the day before the procedure. Intravenous heparin was administered as a 100 unit/kg bolus dose after a transseptal puncture, and then additional heparin was administered when needed to maintain an activated clotting time of 300–400 s. A 20 mg dose of protamine was infused to reverse the heparin at the end of the procedure. After haemostasis at the puncture site was confirmed 4 h following the ablation, both oral anticoagulants were restarted. Bridging therapy with heparin was not used in either patient group.
2.4 Measurement of a Coagulation Marker
D-dimer levels were measured at the following four different timepoints: just before obtaining the first access to the femoral vein; at the end of the procedure before sheath removal; 24 h after the procedure; and 48 h after the procedure (Fig. 1). For quantitative D-dimer measurement, a latex-enhanced photometric immunoassay was used with a LIAS AUTO D-Dimer NEO (Sysmex Corporation, Kobe, Japan).
2.5 Catheter Ablation
All antiarrhythmic drugs were discontinued for five half-lives before the ablation. Transoesophageal echocardiography was performed in each patient prior to the procedure, to rule out the formation of any left atrial thrombi.
The details of the double-Lasso, catheter-guided, extensive encircling pulmonary vein isolation performed in the present study have been described elsewhere [7]. In brief, a 6-French decapolar catheter (Bard Electrophysiology, Lowell, MA, USA) was positioned in the coronary sinus via a 7-French short sheath (Terumo Corporation, Tokyo, Japan) inserted into the right femoral vein. One 8.5-French and two 8-French long sheaths (St. Jude Medical, St. Paul, MN, USA) were also inserted from the right femoral vein and advanced into the left atrium, using the transseptal puncture technique. Two 7-French decapolar circumferential catheters (Lasso; Biosense Webster, Diamond Bar, CA, USA) were placed within the ipsilateral superior and inferior pulmonary veins, guided by selective pulmonary vein venography. After construction of three-dimensional electroanatomical maps, using a non-fluoroscopic navigation system (CARTOSOUND, Biosense Webster), circumferential ablation lines were created around the left- and right-sided ipsilateral pulmonary veins, using a 3.5-mm irrigated-tip catheter (ThermoCool, Biosense Webster). Radiofrequency energy was delivered with a maximum power of 35 W for 20 s at each site. The temperature was limited to 43 °C. The endpoint of the pulmonary vein isolation was either elimination or dissociation of the pulmonary vein potentials recorded from the circular catheters placed within the pulmonary veins and exit block from the pulmonary veins. Finally, the cavotricuspid isthmus was ablated with an endpoint of bidirectional conduction block. The blood pressure was monitored noninvasively throughout the procedure.
2.6 Follow-Up
The patients were discharged from the hospital 2 days after the ablation and were seen at the outpatient clinic 2 weeks after the ablation, mainly to check for any postprocedural complications emerging after the hospital discharge. We then arranged to follow them up 3, 6, 9 and 12 months after the procedure to screen for any AF recurrences defined in the current guidelines [3].
2.7 Endpoints
The primary endpoint of the present study was the change over time in D-dimer levels, and the secondary endpoint was minor bleeding, defined as bleeding from any source requiring neither a transfusion nor surgery, but only medical attention.
2.8 Statistical Analysis
The sample-size estimation was based on the primary endpoint of the D-dimer levels, and our previous study [4]. In order to have 85 % power with a two-tailed alpha value of 0.05, we calculated that at least 27 patients would need to be recruited in each group to detect a difference of 0.25 μg/mL in D-dimer levels measured at any timepoint, assuming a standard deviation (SD) of 0.3 μg/mL. To allow for dropouts, we thus aimed to enrol a total of 60 patients. The analyses of the endpoints were performed on an intention-to-treat basis.
Continuous variables are presented as means ± SDs and categorical variables are presented as proportions. Differences between the patients receiving rivaroxaban and those receiving dabigatran were examined with the use of Fisher’s exact tests for categorical variables or Mann–Whitney U tests for continuous variables. Comparisons of the time-course curves of D-dimer levels were analyzed by a two-way analysis of variance for repeated measures on one factor followed by the Bonferroni correction for multiple paired comparisons. The statistical analyses were performed using IBM SPSS Statistics version 19 software (IBM SPSS, Inc., Chicago, IL, USA). For all analyses, a p value of <0.05 was considered statistically significant.
3 Results
3.1 Patients
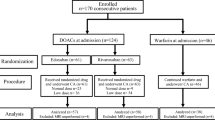
A total of 60 patients underwent randomization (Fig. 1). The mean age of the patients was 59 ± 11 years, and 46 (77 %) were male. Twenty-one patients (70 %) assigned to rivaroxaban and 19 patients (63 %) assigned to dabigatran were switched from warfarin to each new oral anticoagulant after the randomization (p = 0.78). Patients assigned to rivaroxaban had no treatment-emergent adverse events. Two patients (6.7 %) assigned to dabigatran reported gastralgia; however, they were able to tolerate it. Consequently, there were no patients who needed a treatment cross-over between the assigned treatments, or who dropped out. The clinical characteristics and findings during the ablation were similar in the patients receiving rivaroxaban and those receiving dabigatran (Tables 1, 2).
3.2 Time-Course Changes in D-Dimer Levels
D-dimer levels measured just before the ablation procedure were identical in patients receiving rivaroxaban and those receiving dabigatran (p = 0.34). However, the levels increased more markedly following the ablation procedure in patients receiving rivaroxaban than in those receiving dabigatran (mean ± SD from 0.62 ± 0.16 to 1.09 ± 0.38 μg/mL vs from 0.59 ± 0.08 to 0.75 ± 0.17 μg/mL; p < 0.0001). In patients receiving rivaroxaban, D-dimer levels continued to increase over a period of 48 h after the ablation. On the other hand, in patients receiving dabigatran, the levels peaked 24 h after the ablation (Fig. 2).
Time course of D-dimer levels in patients treated with rivaroxaban or dabigatran. The difference in the time-course curves between the groups was statistically significant (p < 0.0001). T1–4 indicate the timepoints at which D-dimer levels were measured: T1, just before the ablation procedure; T2, immediately after the ablation; T3, 24 h after the ablation; T4, 48 h after the ablation. The means and standard deviations are presented
3.3 Complications
Rebleeding from the access site occurred in some patients after they left the electrophysiology lab, and it required only prolonged bed rest but no transfusions or surgical intervention. The rate of this minor bleeding complication was similar in the patients receiving rivaroxaban and in those receiving dabigatran (33 vs 27 %; p = 0.78, Fig. 3). No other major complications, including death, stroke, cardiac tamponade, or massive bleeding requiring transfusion or surgical repair, were identified in any of the patients within the first 2 weeks after the procedure.
4 Discussion
4.1 Major Finding
To the best of our knowledge, this was the first study to compare the anticoagulation effect of rivaroxaban and dabigatran during the periprocedural period of AF ablation. We found that the increases in D-dimer levels observed following the ablation procedure were significantly greater in patients receiving rivaroxaban than in those receiving dabigatran. Studies have demonstrated that increased D-dimer levels reflect a hypercoagulable state in patients with AF [8–11], and D-dimer levels are known to be increased by radiofrequency applications to the myocardium [12, 13]. Thus, this finding may suggest that, after ablation of the left atrium, patients receiving rivaroxaban were at a higher risk of hypercoagulability than those receiving dabigatran. We discuss the details of how this result came about in the following sections.
4.2 Rebound Phenomenon
The term ‘rebound phenomenon’ is used when a transient and sudden increase in thromboembolic events is seen after cessation of anticoagulants [14]. Specifically, this phenomenon has long been known to occur when warfarin or heparin is abruptly withdrawn [14, 15]. Hermans and Claeys [16] warned in their 2006 review that new oral anticoagulants such as direct coagulation factor Xa inhibitors may also elicit a rebound effect following abrupt cessation. The authors assumed that strong monotargeted inhibition could lead to greater ‘retro-activation’ of the whole coagulation cascade when the inhibition is stopped. In the ROCKET AF trial [1], after the end of the study treatment, most patients assigned to either rivaroxaban or warfarin were transitioned to open-label warfarin. Notably, then, significantly more patients transitioning from rivaroxaban than from warfarin developed thromboembolic events during the first month after termination of the randomized treatment. This startling result perhaps indicates a rebound phenomenon. Interestingly, an appended figure showing the rates of thromboembolic events after completion of the assigned treatments in the ROCKET AF trial [1] appears to provide even more striking results than the figures depicted in the review by Hermans and Claeys [16] showing a hypothetical comparison between the drugs with and without a rebound effect. In addition, in the post hoc analysis of the data from the ROCKET AF trial [17], an extremely high rate of thrombotic events (25.60 per 100 patient-years) was reported in the patients who permanently discontinued rivaroxaban. A similar phenomenon to that seen in the ROCKET AF trial has recently been reported from sub-analyses of the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial [18], which compared apixaban, a factor Xa inhibitor, with warfarin. On the basis of the theory propounded by Hermans and Claeys [16], not only factor Xa inhibitors but also thrombin inhibitors seem to have a rebound effect. This phenomenon, however, was never reported in the RE-LY trial [2] comparing dabigatran with warfarin, although differences in the study design between the trials should be taken into account. Although there is no firm evidence, as a reason for this, we assume that the more upstream the site is where the coagulation cascade is blocked, the more likely the rebound phenomenon is to occur. We briefly withdrew both oral anticoagulants prior to the ablation procedure. That was reasonable; however, eventually it may have revealed the potential rebound effect of rivaroxaban.
4.3 Mismatch Between the Half-Life and Dose Regimen
On the basis of the manufacturer-recommended daily numbers of doses of the oral anticoagulants, we determined when to withdraw the drugs prior to the ablation. Subjects were encouraged to take dabigatran twice daily, on the basis of its short half-life. On the other hand, although the half-life of rivaroxaban is slightly shorter than that of dabigatran [19], the manufacturers recommend taking it once daily. Indeed, this recommendation on the dose regimen of rivaroxaban is surely based on the data from clinical trials [20]. However, the US Food and Drug Administration (FDA) noted that ROCKET AF’s once-daily dosing of rivaroxaban was not really supported by the available pharmacokinetic and pharmacodynamic data [21]. In this regard, we have a concern as well: what if patients happen to skip only a single dose of an oral anticoagulant? This is not uncommon in clinical practice, and it actually occurred in the present study. We provided a schema showing a virtual model for this situation (Fig. 4). It is noteworthy that in this situation, patients receiving rivaroxaban are supposed to be exposed to a less anticoagulated state for more than 24 h longer than patients receiving dabigatran. This difference in the duration of time when there was a critical decrease in the blood anticoagulant concentration might be considered unimportant; however, it should be remembered that a transient hypercoagulable condition, such as a morning surge in the blood pressure [22], can arise within a short window of time. In the present study, we performed the ablation procedure, which is known to result in a prothrombotic state, after a brief withdrawal of the oral anticoagulants. Importantly, thus, the present study may have provided a model to show what can happen when a dose of rivaroxaban is missed and at the same time some clinical event resulting in a transient hypercoagulable state occurs. Furthermore, our finding of greater increases in D-dimer levels following the ablation in patients treated with rivaroxaban may have shed light on a potential mismatch between its half-life and the number of doses.
Schema showing a hypothetical situation in which a patient skips a single dose of (a) rivaroxaban or (b) dabigatran. Each anticoagulant is skipped at 48 hours. Each dashed line represents half the maximum blood anticoagulant concentration, and each double-headed arrow shows the duration of time when the blood anticoagulant concentration is less than half the maximum concentration after the last intake of the anticoagulant
4.4 Clinical Implications
We showed, by means of assessment of a coagulation marker, that patients receiving rivaroxaban were more likely than those receiving dabigatran to be exposed to a hypercoagulable state during the early postprocedural period of AF ablation. We thus may have provided a chance for interventional electrophysiologists to reconsider how to use rivaroxaban, or even an opportunity to determine whether or not to use it for periablation anticoagulation in AF ablation.
4.5 Limitations
There is no consensus that D-dimer has a sufficient ability to stratify the risk of thromboembolic events; moreover, D-dimer levels may also reflect a factor other than myocardial damage from the radiofrequency energy that is delivered, such as the local condition of the vascular access sites [23]. Secondly, the way in which the oral anticoagulants were used in our study may not necessarily correspond to their usage in clinical practice. Finally, the number of the patients recruited in this study was limited and, further, we only enrolled patients with a low risk of thromboembolism, and probably therefore we did not encounter any thromboembolic or serious haemorrhagic complications.
5 Conclusion
As compared with dabigatran, rivaroxaban may increase the risk of hypercoagulability when it is used during the periprocedural period of AF ablation. This suggests a potential rebound effect of rivaroxaban or a mismatch between its half-life and dose regimen, and careful attention therefore needs to be paid to the periprocedural use of rivaroxaban.
References
Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91.
Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51.
Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9(632–696):e21.
Nin T, Sairaku A, Yoshida Y, et al. A randomized controlled trial of dabigatran versus warfarin for periablation anticoagulation in patients undergoing ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2013;36:172–9.
Hori M, Matsumoto M, Tanahashi N, et al. Safety and efficacy of adjusted dose of rivaroxaban in Japanese patients with non-valvular atrial fibrillation-subanalysis of J-ROCKET AF for patients with moderate renal impairment. Circ J. 2013;77:632–8.
Hori M, Connolly SJ, Ezekowitz MD, et al. Efficacy and safety of dabigatran vs. warfarin in patients with atrial fibrillation-sub-analysis in Japanese population in RE-LY trial. Circ J. 2011;75:800–5.
Ouyang F, Bänsch D, Ernst S, et al. Complete isolation of left atrium surrounding the pulmonary veins: new insights from the double-Lasso technique in paroxysmal atrial fibrillation. Circulation. 2004;110:2090–6.
Habara S, Dote K, Kato M, et al. Prediction of left atrial appendage thrombi in non-valvular atrial fibrillation. Eur Heart J. 2007;28:2217–22.
Sadanaga T, Sadanaga M, Ogawa S. Evidence that D-dimer levels predict subsequent thromboembolic and cardiovascular events in patients with atrial fibrillation during oral anticoagulant therapy. J Am Coll Cardiol. 2010;55:2225–31.
Vene N, Mavri A, Kosmelj K, et al. High D-dimer levels predict cardiovascular events in patients with chronic atrial fibrillation during oral anticoagulant therapy. Thromb Haemost. 2003;90:1163–72.
Hijazi Z, Oldgren J, Siegbahn A, et al. Biomarkers in atrial fibrillation: a clinical review. Eur Heart J. 2013;34:1475–80.
Lee DS, Dorian P, Downar E, et al. Thrombogenicity of radiofrequency ablation procedures: what factors influence thrombin generation? Europace. 2001;3:195–200.
Bulava A, Slavík L, Fiala M, et al. Endothelial damage and activation of the hemostatic system during radiofrequency catheter isolation of pulmonary veins. J Interv Card Electrophysiol. 2004;10:271–9.
Granger CB, Miller JM, Bovill EG, et al. Rebound increase in thrombin generation and activity after cessation of intravenous heparin in patients with acute coronary syndromes. Circulation. 1995;91:1929–35.
Genewein U, Haeberli A, Straub PW, et al. Rebound after cessation of oral anticoagulant therapy: the biochemical evidence. Br J Haematol. 1996;92:479–85.
Hermans C, Claeys D. Review of the rebound phenomenon in new anticoagulant treatments. Curr Med Res Opin. 2006;22:471–81.
Patel MR, Hellkamp AS, Lokhnygina Y, et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation). J Am Coll Cardiol. 2013;61:651–8.
Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92.
Bauer KA. Recent progress in anticoagulant therapy: oral direct inhibitors of thrombin and factor Xa. J Thromb Haemost. 2011;9:12–9.
Agnelli G, Gallus A, Goldhaber SZ, et al. Treatment of proximal deep-vein thrombosis with the oral direct factor Xa inhibitor rivaroxaban (BAY 59-7939): the ODIXa-DVT (Oral Direct Factor Xa Inhibitor BAY 59-7939 in Patients With Acute Symptomatic Deep-Vein Thrombosis) study. Circulation. 2007;116:180–7.
Fleming TR, Emerson SS. Evaluating rivaroxaban for nonvalvular atrial fibrillation—regulatory considerations. N Engl J Med. 2011;365:1557–9.
Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–6.
Hoke M, Koppensteiner R, Schillinger M, et al. D-dimer testing in the diagnosis of transfemoral pseudoaneurysm after percutaneous transluminal procedures. J Vasc Surg. 2010;52:383–7.
Acknowledgments
The authors thank John Martin for his grammatical assistance.
Conflict of Interest Disclosure Statement
The authors have no conflicts of interest that are directly relevant to the content of this article.
Funding Statement
This work was not supported by any external funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sairaku, A., Yoshida, Y., Ando, M. et al. A Head-to-Head Comparison of Periprocedural Coagulability Under Anticoagulation with Rivaroxaban Versus Dabigatran in Patients Undergoing Ablation of Atrial Fibrillation. Clin Drug Investig 33, 847–853 (2013). https://doi.org/10.1007/s40261-013-0134-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-013-0134-5