Abstract
Teserpaturev/G47Δ (Delytact®) is a third-generation (triple-mutated) recombinant oncolytic herpes simplex virus type 1 being developed by Daiichi Sankyo Co., Ltd. for the treatment of certain solid cancers. Teserpaturev/G47Δ has been approved for the treatment of malignant glioma in Japan and is currently in clinical development for the treatment of prostate cancer (phase II), malignant pleural mesothelioma (phase I) and recurrent olfactory neuroblastoma (phase I). This article summarizes the milestones in the development of teserpaturev/G47Δ leading to this first approval for the treatment of malignant glioma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare/15506352. |
A triple-mutated recombinant oncolytic herpes simplex virus type 1 being developed by Daiichi Sankyo Co. Ltd. for the treatment of certain solid cancers. |
Received its first approval on 11 June 2021 in Japan. |
Approved for use in malignant glioma. |
1 Introduction
Oncolytic virus therapy (OVT) is a promising anticancer treatment strategy which utilizes naturally occurring or genetically engineered viruses that are designed to selectively replicate in (and hence kill) tumor cells without harming normal cells, and initiate host anti-tumor immunity [1,2,3,4,5]. Thus, in contrast to gene therapy, which uses viruses merely as transgene delivery systems, OVT uses the virus itself as an active drug reagent [1]. Commercially available oncolytic virotherapies include Rigvir (Riga virus), an unmodified enteric cytopathogenic human orphan type 7 (ECHO-7) picornavirus, oncorine (H101), an E1B-deleted recombinant adenovirus, and talimogene laherparepvec (hereafter referred to as T-VEC), a double-mutated (second-generation) recombinant oncolytic herpes simplex virus, type 1 (HSV1) [2, 6, 7].
HSV1 is a double-stranded DNA virus with a number of features that favour its use as an oncolytic virotherapy [2, 8,9,10]. These include: a large genome suitable for insertion of foreign genes; tropism for neural cells (although it is capable of infecting a wide variety of cell types); a safety mechanism in the form of the availability of anti-HSV1 agents (e.g. acyclovir and ganciclovir); high titre generation due to its high proliferating ability; and lack of host genome integration, making it non-oncogenic [2, 8].
Teserpaturev/G47Δ (Delytact®) is a triple-mutated (third-generation) recombinant oncolytic HSV1 being developed by Daiichi Sankyo Co., Ltd. for the treatment of certain solid tumors. Based on the results of a single-arm, phase II study [11, 12], teserpaturev/G47Δ received its first approval on 11 June 2021 in Japan for the treatment of malignant glioma [13, 14]. Teserpaturev/G47Δ is therefore the first oncoviral therapy to be approved in any region of the world for the treatment of malignant glioma or any type of primary brain cancer. Because the clinical trial data are limited, the Japanese approval is conditional and time-limited [13, 15]; its continuation may be contingent upon verification and description of clinical benefit and safety in a post-marketing study [13]. Teserpaturev/G47Δ is given intratumorally to patients with malignant glioma; the recommended dosing regimen comprises administering up to six doses of the drug [1 × 109 plaque-forming units (pfu)/dose], with intervals of 5–14 days between the first and second injections and 4 weeks between all subsequent injections [14]. Adverse events occurring during teserpaturev/G47Δ therapy include cytopenia (e.g. lymphocyte count reduction, white blood cell count decrease); blood tests should be performed as appropriate [14].

Clinical development of teserpaturev/G47Δ is ongoing for the treatment of prostate cancer (phase II), olfactory neuroblastoma (phase I) and malignant pleural mesothelioma (phase I).
1.1 Company Agreements
Daiichi Sankyo Co., Ltd, the marketing authorization holder of teserpaturev/G47Δ in Japan, has developed this oncoviral therapy in collaboration with its creators, Professor Tomoki Todo and colleagues, at the Institute of Medical Science, the University of Tokyo (IMSUT) hospital [13, 16, 17]. As of Feb 2017, Daiichi Sankyo Co., Ltd, has also partnered with ActiVec Inc. for the development of teserpaturev/G47Δ in the treatment of malignant glioma [18].
2 Scientific Summary
2.1 Pharmacological Properties
Teserpaturev/G47Δ, a triple-mutated (third-generation) recombinant oncolytic HSV1, was created by adding a third mutation (α47 deletion) to its predecessor virus, G207, a double-mutated (second-generation) recombinant oncolytic HSV1 that has deletion of both copies of the γ34.5 gene as well as an ICP6 gene that has been inactivated by inserting the Escherichia coli LacZ gene [9, 19]. The incorporation of the ICP6 gene inactivation distinguishes teserpaturev/G47Δ from the commercially available second-generation recombinant oncolytic HSV1, T-VEC, which also has α47 and γ34.5 deletions [2].
Regarding the mechanism of action of teserpaturev/G47Δ, the γ34.5 deletion is mainly responsible for its cancer-selective replication and virulence attenuation. Deletion of the γ34.5 gene, which functions to negate the shut-off of protein synthesis upon viral infection by the host cell, results in a virus whose replication in normal cells is significantly attenuated, although it can still replicate in cancer cells, as these have a defect in the shut-off response. In addition, deletion of the α47 gene, which functions to inhibit the host cell transporter associated with antigen presentation, results in the persistent expression of MHC class I; this should enhance the host anti-tumor immune response. The α47 deletion also places the late US11 gene under control of the immediate-early α47 promoter, resulting in enhanced viral replication in cancer cells. The ICP6 gene encodes the large subunit of ribonucleotide reductase (RR) required for viral DNA synthesis; viruses with ICP6 gene inactivation largely replicate in dividing cells (e.g. tumor cells), that express sufficient levels of mammalian RR to complement the viral mutation [2].
In preclinical studies, intratumoral administration of teserpaturev/G47Δ either alone, ‘armed’ with (i.e. expressing) immunomodulatory transgenes and/or in association with other treatment modalities, exhibited cytotoxic activity and demonstrated antitumor efficacy in almost all cancer types and experimental models tested [2, 20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. For example, teserpaturev/G47Δ alone was effective in human glioblastoma (GBM) [23, 38] and higher-grade meningioma [28] models, and the efficacy of teserpaturev/G47Δ was enhanced when it was armed with angiostatin (human GBM models) [20], interleukin (IL)-12 (murine and human GBM models) [20, 24, 39, 43] or fms-like tyrosine kinase 3 ligand (murine glioma model) [21]. In addition, teserpaturev/G47Δ showed synergistic or combinatorial effects when combined with temozolomide chemotherapy [27], transforming growth factor beta [30] and the vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor axitinib [29] in murine and/or human GBM models. Teserpaturev/G47Δ also enhanced the efficacy of low-dose etoposide chemotherapy in a human etoposide-insensitive GBM model [25], as well as that of the poly(ADP-ribose) polymerase (PARP) inhibitor (PARPi) olaparib in human PARPi-sensitive and -resistant GBM models [26]. Of note, G47Δ replication in (and hence killing of) human GBM cells was increased following ‘transient fasting’ (i.e. growth in glucose and fetal calf serum restricted culture medium) [44].
Similarly, teserpaturev/G47Δ armed with IL-12 showed greater activity when combined with two checkpoint inhibitor (CPI) antibodies (anti-PD-1 and anti-CTLA-4), as opposed to only one CPI antibody, in a murine GBM model [39]. Notably, in murine GBM models, temozolmide showed synergistic activity with teserpaturev/G47Δ armed with IL-12 in vitro, although it did not extend survival and, moreover, abrogated the beneficial effect of the virotherapy on this outcome, when administered concurrently in vivo. This indicates that the relative timing of these two treatment modalities should be taken into account when designing clinical trials to evaluate combination therapy [45].
Teserpaturev/G47Δ showed superior efficacy to the conditionally replicative adenovirus Ad5/35.GΔ·Ki in a human GBM model [22]. In addition, triple therapy consisting of teserpaturev/G47Δ combined with intratumoral expression of measles virus fusogenic membrane glycoproteins (FMGs) and temozolomide showed superior efficacy to the corresponding Ad5/35.GΔ·Ki-based triple therapy in human GBM models [22].
Teserpaturev/G47Δ shedding into blood, saliva and urine was evaluated over time using the quantitative polymerase chain reaction method (lower limit of detection: 10 copies/μL) in 19 patients with recurrent or residual GBM who participated in the pivotal phase II UMIN000015995 study [11, 12, 14]. G47Δ DNA was detected from the blood of one patient on one occasion only (day 0, i.e. after the first dose of the virotherapy) [11, 14]. Teserpaturev/G47Δ distributed only to the central nervous system, trigeminal ganglion, and eyeball (including the optic nerve), centering on the administration site, following a single intracerebral dose of the virus in A/J mice [14]. It was not detected in the testes or ovaries [14].
2.2 Therapeutic Trials
The efficacy of intratumorally-administered teserpaturev/G47Δ in the treatment of malignant gliomas was demonstrated in a pivotal, single-arm, open-label, historically-controlled, investigator-initiated, phase II study in patients with residual or recurrent GBM (UMIN000015995) [11, 12]. Previously, the feasibility of stereotactically injecting teserpaturev/G47Δ into the tumors of patients with recurrent or progressive GBM had been established in the first-in-human (FIH), single-arm, open-label, uncontrolled, phase I/II UMIN000002661 study [46].
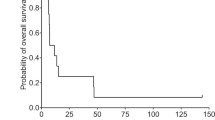
In the pivotal phase II study, the 1-year survival rate after teserpaturev/G47Δ initiation (primary endpoint) was 92.3% (95% CI 64.0–99.8%), based on an interim analysis of 13 patients [11] (data cut-off date 14 June 2018 [14]). This was higher than the 1-year survival rate in historical controls (15%); following statistical confirmation that the primary endpoint of the study had been met, the trial was terminated early in accordance with the protocol [11]. In the full analysis set, which comprised 19 patients; the 1-year survival rate after teserpaturev/G47Δ initiation was 84.2% (95% CI 60.4–96.6%; 16 of 19 patients) [11].
Regarding secondary endpoints, median progression-free survival (PFS) and overall survival (OS) after teserpaturev/G47Δ initiation were 4.7 (95% CI 3.3–6.1) and 20.2 (95% CI 16.8–23.6) months, respectively, as assessed at the subsequent data cut-off date of 1 March 2022 [11]. Median OS from the time of the initial surgery/diagnosis (exploratory endpoint) was 28.8 (95% CI 20.1–37.5) months. According to a post hoc analysis, neither median OS after teserpaturev/G47Δ initiation [wild type, 20.9 (95% CI 13.6–28.2) months; mutant type, 19.4 (95% CI 17.4–21.4) months] nor from the time of the initial surgery/diagnosis [wild type, 28.8 (95% CI 17.6–40.0) months; mutant type, 23.6 (95% CI 16.1–31.1) months] were affected by isocitrate dehydrogenase 1 mutation status. Similarly, neither median OS after teserpaturev/G47Δ initiation [MGMT−, 20.2 (95% CI 8.4–32.0) months; MGMT+, 16.2 (95% CI 7.3–25.1) months] nor from the time of initial surgery/diagnosis [MGMT−, 23.6 (95% CI 0.4–46.8) months; MGMT+, 28.8 (95% CI 25.3–32.3) months] were affected by MGMT expression. At the 1 March 2022 cut-off date, which is also the most recent observation date, three of the 19 patients were still alive and stable more than 3 years after their last teserpaturev/G47Δ injection [11].
The best overall response (BOR) during a 2-year observation period after the last teserpaturev/G47Δ administration was a partial response (PR) in one patient and stable disease (SD) in 18 patients, for an overall response rate of 5.3% (95% CI 0.1–26.0%) [11, 14].
This phase II trial enrolled 19 Japanese males and females aged ≥ 18 years with (i) pathologically-confirmed residual or recurrent GBM [as represented by magnetic resonance imaging (MRI)-enhanced lesions ≥ 10 mm] after initial therapy of surgery, radiation and temozolomide and (ii) a Karnofsky Performance Status (KPS) of ≥ 60% [11, 12]. Participants received up to six doses of teserpaturev/G47Δ (1 × 109 pfu/dose) at intervals of 5–14 days for the first and second doses and 4 (± 2) weeks for the third and subsequent doses [11, 12]. Teserpaturev/G47Δ was administered using MRI-guided stereotactic injections; it was delivered to a different coordinate for each injection and at two sites within the tumor for each dose [11, 12]. Most patients were given teserpaturev/G47Δ in addition to maintenance temozolomide chemotherapy [47].
Assessing the efficacy of teserpaturev/G47Δ in terms of PFS, OS and tumor shrinkage was a secondary objective in the FIH study [46]. Median PFS and OS after the last teserpaturev/G47Δ administration were 8 (95% CI 7–34) days and 7.3 (95% CI 6.2–15.2) months, respectively, and the 1- and 2-year survival rates after the last teserpaturev/G47Δ administration were 38.5% (95% CI 13.9–68.4%) and 23.1% (95% CI 5.0–53.8%), respectively. Median OS from the time of the initial surgery/diagnosis was 30.5 (95% CI 19.2–52.7) months. At the end of a 2-year observation period following the last teserpaturev/G47Δ administration, 3 of the 13 trial participants were still alive. This included two of the 10 patients who received two injections of the drug at the approved dose of 1 × 109 pfu; one of these two individuals continued to survive (for > 11 years after teserpaturev/G47Δ therapy) at the most recent observation date (1 March 2022). The BOR during the 2-year observation period (exploratory analysis) was complete response in one patient, PR in one patient, SD in six patients and progressive disease in five patients [46].
This phase I/II trial enrolled 13 Japanese males and females aged ≥ 18 years who had undergone prior surgery at the time of initial or recurrent disease with (i) histologically-confirmed GBM (MRI-enhanced lesions ≥ 10 mm) despite having received radiation therapy, with or with temozolomide chemotherapy, and (ii) a KPS of > 60%. Participants received two doses of teserpaturev/G47Δ [3 × 108 pfu/dose (three patients in the first cohort of the phase I part) or 1 × 109 pfu/dose (three patients in the second cohort of the phase I part plus seven patients in the phase II part)], into the same coordinates, with an interval of 5–14 days between the first and second dose. Each dose was administered using MRI-guided stereotactic injections to two sites within the tumor [46].
Features and properties of teserpaturev/G47Δ | |
|---|---|
Alternative names | Delytact, DS1647, G47 delta oncolytic virus therapy—Daiichi Sankyo/University of Tokyo |
Class | Antineoplastics; gene therapies; immunotherapies; oncolytic viruses |
Mechanism of action | Triple-mutated (third-generation), recombinant oncolytic herpes simplex virus type 1; targets, and selectively replicates in (hence killing) tumor cells, while leaving normal cells unharmed |
Route of administration | Intratumoral |
Pharmacodynamics | Either alone, ‘armed’ with (i.e. expressing) immunomodulatory transgenes and/or in association with other treatment modalities, exhibited cytotoxic activity and demonstrated antitumor efficacy in almost all preclinical studies, including models of murine and human glioblastoma |
Pharmacokinetics | Viral shedding (beyond site of intracerebral administration) only detected in blood following first dose on first day of therapy |
Most frequent adverse events | Most frequent: fever, vomiting, nausea, lymphocyte count decrease and white blood cell count decrease |
ATC codes | |
WHO ATC code | L (Antineoplastic and Immunomodulating Agents) |
EphMRA ATC code | L (Antineoplastic and Immunomodulating Agents) |
CAS registry number | 1802360-34-4 |
2.3 Adverse Events
Repeated stereotactic injections of teserpaturev/G47Δ were generally well tolerated in the pivotal phase II UMIN000015995 study in patients with recurrent or residual GBM [11].
All 19 trial participants experienced teserpaturev/G47Δ-related adverse events (AEs), most commonly fever (89.5% of patients), vomiting (57.9%), nausea (52.6%), lymphocyte count decrease (47.4%) and white blood cell count decrease (31.6%). Seven (36.8%) trial participants reported a total of nine teserpaturev/G47Δ-related AEs of grade 3 or 4 severity. The most common of these was lymphocyte count decrease, which occurred in five patients (at grade 3 severity in three and grade 4 severity in two); however, all recovered without treatment. In addition, grade 3 fever, vomiting, white blood cell count decrease and neutrophil count decrease each occurred in a single patient [11].
Among the 16 trial participants who had died by the time of the most recent observation date (1 March 2022), only one had not done so as a result of tumor progression. This death, which occurred 15 months after the patient had received their last teserpaturev/G47Δ injection, was not considered to be related to the drug [11].
Twelve of the 13 participants in the FIH phase I/II UMIN000002661 study in patients with recurrent or progressive GBM experienced a total of 69 AEs in the 90-day period following the last teserpaturev/G47Δ administration. Headache (in 61.5% of patients), fever (61.5%) and vomiting (46.2%) were the most frequently occurring AEs; they were also the most common teserpaturev/G47Δ–related AEs [46]. Five grade 3 (headache, vomiting, white blood cell count decrease, IX–XI cranial nerve disorder and neurological disorder) and one grade 4 (lymphocyte count decrease) AE were reported [46].
Key clinical trials of teserpaturev/G47Δ in Japan | |||||
|---|---|---|---|---|---|
Drug(s) | Indication | Phase | Status | Sponsor(s) | Identifier |
Teserpaturev/G47Δ | Residual or recurrent glioblastoma | II | Completed | IMSUT hospital, University of Tokyo | UMIN000015995 |
Teserpaturev/G47Δ | Recurrent glioblastoma | I-IIa | Completed | IMSUT hospital, University of Tokyo; University of Tokyo Hospital | UMIN000002661 |
Teserpaturev/G47Δ | Prostate cancer | II | Ongoing | Kyorin University and IMSUT hospital, University of Tokyo | jRCTs033210603 |
Teserpaturev/G47Δ | Castration-resistant prostate cancer | I | Completed | University of Tokyo Hospital | UMIN000010463 |
Teserpaturev/G47Δ | Malignant pleural mesothelioma | I | Ongoing | IMSUT hospital, University of Tokyo | UMIN000034063 |
Teserpaturev/G47Δ | Recurrent olfactory neuroblastoma | I | Ongoing | IMSUT hospital, University of Tokyo | UMIN000011636 |
2.4 Ongoing Clinical Trials
Currently, three single-arm, open-label, phase I or II studies of teserpaturev/G47Δ are underway.
jRCTs033210603 is a historically controlled phase II trial assessing the efficacy and safety of administering up to six doses of teserpaturev/G47Δ (1 × 109 pfu/dose) at 4-week intervals into the tumors of male patients aged ≥ 20 years with prostate cancer. The primary outcome is the 1-year failure-free survival rate; secondary outcomes include failure-free survival, overall survival, adverse event rate and serious adverse event rate. This study has a target enrolment of 30 patients.
UMIN000034063 is an uncontrolled phase I trial assessing the safety and efficacy of administering up to six fixed doses of teserpaturev/G47Δ at 4-week intervals into the pleural cavity of patients aged ≥ 20 years with malignant pleural mesothelioma that is inoperable, recurrent or progressive. Primary outcomes are types and frequencies of AEs; key secondary outcomes are change in tumor size on computed tomography scan, PFS, and OS. This study has completed the enrolment of six patients as planned.
UMIN000011636 is an uncontrolled phase I trial evaluating the safety and efficacy of administering teserpaturev/G47Δ at the same dose every 4 weeks into the tumors of patients aged ≥ 18 years with recurrent olfactory neuroblastoma that is progressive despite previous or ongoing radiation therapy. Primary outcomes are types and frequencies of AEs; key secondary outcomes are change in tumor size on MRI, PFS and OS. This study has a target enrolment of 10 patients.
3 Current Status
Teserpaturev/G47Δ received its first approval on 11 Jun 2021 for the treatment of malignant glioma in Japan.
References
Fukuhara H, Ino Y, Todo T. Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 2016;107:1373–9.
Taguchi S, Fukuhara H, Todo T. Oncolytic virus therapy in Japan: progress in clinical trials and future perspectives. Jpn J Clin Oncol. 2019;49(3):201–9.
Bommareddy PK, Shettigar M. Kaufman HL Integrating oncolytic viruses in combination cancer immunotherapy. Nat Rev Immunol. 2018;18:498–513.
Fukuhara H, Takeshima Y, Todo T. Triple-mutated oncolytic herpes virus for treating both fast- and slow-growing tumors. Cancer Sci. 2021;112(8):3293–301.
Zeng J, Li X, Sander M, et al. Oncolytic viro-immunotherapy: an emerging option in the treatment of gliomas. Front Immunol. 2021;12: 721830.
Kaufman HL, Bommareddy PK. Two roads for oncolytic immunotherapy development. J Immunother Cancer. 2019;7:26.
Mondal M, Guo J, He P, et al. Recent advances of oncolytic virus in cancer therapy. Hum Vaccines Immunother. 2020;16(10):2389–402.
Ghouse JN, Martuza SM, Rabkin SD. In situ cancer vaccination and immunovirotherapy using oncolytic HSV. Viruses. 2021;13:1740.
Mineta T, Rabkin SD, Yazaki T, et al. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1(9):938–43.
Otani Y, Yoo JY, Shimizu T, et al. Implications of immune cells in oncolytic herpes simplex virotherapy for glioma. Brain Tumor Pathol. 2022;39(2):57–64.
Todo T, Ito H, Ino Y, et al. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: a phase 2 trial. Nat Med. 2022;28:1630-9.
UMIN Clinical Trials Registry. Unique UMIN ID UMIN000015995. http://upload.umin.ac.jp. Accessed 29 July 2022.
Daiichi Sankyo Co. Ltd. DELYTACT® oncolytic virus G47∆ approved in Japan for treatment of patients with malignant glioma [media release]. 11 June 2021. https://www.daiichisankyo.com.
Daiichi-Sankyo Co. Ltd. DELYTACT: Japanese package insert. 2021.
Daiichi Sankyo Co. Ltd. Daiichi Sankyo launches DELYTACT(R) oncolytic virus G47 delta in Japan [media release]. 1 Nov 2021. https://www.daiichisankyo.com.
Daiichi Sankyo Co. Ltd. Oncolytic virus G47delta (DS-1647) designated as orphan drug under Orphan Drug/Medical Device designation system [media release]. http://www.daiichisankyo.com. Accessed 19 July 2021.
Daiichi Sankyo Co. Ltd. Daiichi Sankyo submits application for oncolytic virus teserpaturev (G47Δ) for treatment of patients with malignant glioma in Japan [media release]. http://www.daiichisankyo.com. Accessed 19 July 2021.
Daiichi Sankyo Co. Ltd. Reference data (consolidated financial results for Q3 FY2020). http://www.daiichisankyo.com. Accessed 29 July 2022.
Todo T, Martuza RL, Rabkin SD, et al. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci USA. 2001;98:6396–401.
Zhang W, Fulci G, Wakimoto H, et al. Combination of oncolytic herpes simplex viruses armed with angiostatin and IL-12 enhances antitumor efficacy in human glioblastoma models. Neoplasia. 2013;15(6):591–9.
Barnard Z, Wakimoto H, Zaupa C, et al. Expression of FMS-like tyrosine kinase 3 ligand by oncolytic herpes simplex virus type I prolongs survival in mice bearing established syngeneic intracranial malignant glioma. Neurosurgery. 2012;71(3):741–8.
Hoffmann D, Wildner O. Comparison of herpes simplex virus- and conditionally replicative adenovirus-based vectors for glioblastoma treatment. Cancer Gene Ther. 2007;14(7):627–39.
Wakimoto H, Kesari S, Farrell CJ, et al. Human glioblastoma-derived cancer stem cells: establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors. Cancer Res. 2009;69(8):3472–81.
Cheema TA, Wakimoto H, Fecci PE, et al. Multifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell model. Proc Natl Acad Sci USA. 2013;110(29):12006–11.
Cheema TA, Kanai R, Kim GW, et al. Enhanced antitumor efficacy of low-dose etoposide with oncolytic herpes simplex virus in human glioblastoma stem cell xenografts. Clin Cancer Res. 2011;17(23):7383–93.
Ning J, Wakimoto H, Peters C, et al. Rad51 degradation: role in oncolytic virus-poly(ADP-ribose) polymerase inhibitor combination therapy in glioblastoma. J Natl Cancer Inst. 2017;109(3):1–13.
Kanai R, Rabkin SD, Yip S, et al. Oncolytic virus-mediated manipulation of DNA damage responses: synergy with chemotherapy in killing glioblastoma stem cells. J Natl Cancer Inst. 2012;104(1):42–55.
Nigim F, Esaki S, Hood M, et al. A new patient-derived orthotopic malignant meningioma model treated with oncolytic herpes simplex virus. Neuro Oncol. 2016;18(9):1278–87.
Saha D, Wakimoto H, Peters CW, et al. Combinatorial effects of VEGFR kinase inhibitor axitinib and oncolytic virotherapy in mouse and human glioblastoma stem-like cell models. Clin Cancer Res. 2018;24(14):3409–22.
Esaki S, Nigim F, Moon E, et al. Blockade of transforming growth factor-β signaling enhances oncolytic herpes simplex virus efficacy in patient-derived recurrent glioblastoma models. Int J Cancer. 2017;141(11):2348–58.
Farrell CJ, Zaupa C, Barnard Z, et al. Combination immunotherapy for tumors via sequential intratumoral injections of oncolytic herpes simplex virus 1 and immature dendritic cells. Clin Cancer Res. 2008;14(23):7711–6.
Sugawara K, Iwai M, Ito H, et al. Oncolytic herpes virus G47Δ works synergistically with CTLA-4 inhibition through dynamic intratumoral immune modulation. Mol Ther Oncol. 2021;22:129–42.
Fan J, Jiang H, Cheng L, et al. Oncolytic herpes simplex virus and temozolomide synergistically inhibit breast cancer cell tumorigenesis in vitro and in vivo. Oncol Lett. 2021;21(2):99.
Sugawara K, Iwai M, Yajima S, et al. Efficacy of a third-generation oncolytic herpes virus G47Δ in advanced stage models of human gastric cancer. Mol Ther Oncol. 2020;17:205–15.
Fan J, Jiang H, Cheng L, et al. The oncolytic herpes simplex virus vector, G47Δ, effectively targets tamoxifen-resistant breast cancer cells. Oncol Rep. 2016;35(3):1741–9.
Wang L, Ning J, Wakimoto H, et al. Oncolytic herpes simplex virus and PI3K inhibitor BKM120 synergize to promote killing of prostate cancer stem-like cells. Mol Ther Oncol. 2019;13:58–66.
Yamada T, Tateishi R, Iwai M, et al. Neoadjuvant use of oncolytic herpes virus G47Δ enhances the antitumor efficacy of radiofrequency ablation. Mol Ther Oncol. 2020;18:535–45.
Ito H, Ino Y, Todo T. Therapeutic efficacy of third generation oncolytic HSV-1 (G47delta) for glioma cells with stem cell property [abstract]. In: 19th annual meeting of the American Society of Gene and Cell Therapy, 2016.
Saha D, Martuza RL, Rabkin SD. Glioblastoma eradication by combination oncolytic immunovirotherapy and immune checkpoint blockade [abstract]. Mol Ther. 2017;25(5 Suppl 1):361.
Uchihashi T, Nakahara H, Fukuhara H, et al. Oncolytic herpes virus G47Δ injected into tongue cancer swiftly traffics in lymphatics and suppresses metastasis. Mol Ther Oncol. 2021;22:388–98.
Yajima S, Sugawara K, Iwai M, et al. Efficacy and safety of a third-generation oncolytic herpes virus G47Δ in models of human esophageal carcinoma. Mol Ther Oncol. 2021;23:402–11.
Inoue K, Ito H, Iwai M, et al. Neoadjuvant use of oncolytic herpes virus G47delta prevents stage advancement of tongue cancer. Cancer Sci. 2022;113:1422.
Cheema TA, Fecci PE, Ning J, et al. Immunovirotherapy for the treatment of glioblastoma. OncoImmunology. 2014;3(1): e27218.
Esaki S, Rabkin SD, Martuza RL, et al. Transient fasting enhances replication of oncolytic herpes simplex virus in glioblastoma. Am J Cancer Res. 2016;6(2):300–11.
Saha D, Rabkin SD, Martuza RL. Temozolomide antagonizes oncolytic immunovirotherapy in glioblastoma. J Immunother Cancer. 2020;8(Suppl 1): e000345.
Todo T, Ino Y, Ohtsu H, et al. A phase I/II study of triple-mutated oncolytic herpes virus G47∆ in patients with progressive glioblastoma. Nat Commun. 2022;13(1):4119.
Todo T. Results of phase ii clinical trial of oncolytic herpes virus G47delta in patients with glioblastoma [abstract no. ATIM-14]. Neuro Oncol. 2019;21(Suppl 6):vi4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
During the peer review process the manufacturer of teserpaturev/G47Δ was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. James E. Frampton is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Frampton, J.E. Teserpaturev/G47Δ: First Approval. BioDrugs 36, 667–672 (2022). https://doi.org/10.1007/s40259-022-00553-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-022-00553-7