Abstract
Background
Infliximab and etanercept biosimilars present significant potential cost savings to the NHS. Patients need to be involved in the decision to use these medicines but there is limited published literature on their knowledge and attitudes about these biosimilars.
Objectives
The aim of this study was to investigate ankylosing spondylitis and rheumatoid arthritis patients’ knowledge and attitudes towards infliximab and etanercept biosimilars in the UK.
Methods
A self-administered web survey was conducted among the members of the National Rheumatoid Arthritis Society and the National Ankylosing Spondylitis Society in the UK between 2 March 2017 and 2 June 2017.
Results
A total of 182 patients participated in this survey. The majority of participants (73%) were on etanercept, and 66 and 80% of patients on originator biologic and biosimilars, respectively, understood what biosimilars were. Patients who were currently on biosimilars had greater confidence in their effectiveness and the doctor’s decision to initiate than those who were originally on originator biologics that doctors proposed to switch to biosimilars. The majority (82%) of participants on biosimilars thought that biosimilars help to save money for the NHS, while just over half (54%) of participants on the originator biologics thought the cost of treatment should not be considered when prescribing biosimilars.
Conclusions
Survey participants had a good knowledge and understanding of biosimilars. Participants on biosimilars were confident and positive about biosimilars’ safety, efficacy and switching, whereas participants on the originator biologics were more reluctant to switch to biosimilars. Those patients who expressed concerns felt that more clinical trials on switching biosimilars, better communication and reassurance by healthcare professional teams and further involvement in decision making would increase their acceptance of biosimilars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ankylosing spondylitis and rheumatoid arthritis patients on biosimilars were better informed and confident about biosimilars than those on originator biologics. |
Both groups of patients surveyed wanted clarity about reasons for switching. |
Those on biosimilars accepted that switching saved money for the NHS. Those on originator biologics thought that cost should not be an issue. |
Both patient groups felt that more clinical trials and better communication with healthcare professionals would increase reassurance. |
1 Introduction
Ankylosing spondylitis (AS) and rheumatoid arthritis (RA) are chronic and disabling conditions [1]. The management of AS and RA has improved with the introduction of anti-tumour necrosis factor (TNF) (infliximab, etanercept and adalimumab) [2]. Anti-TNFs are increasingly used to treat AS and RA in clinical practice due to their ability to reduce or reverse signs, symptoms, disability and progression of joint damage, improving patient’s quality of life and functional capacity [3]. Anti-TNFs have a considerable impact on healthcare budgets. According to the Health and Social Care Information Centre report in 2015, the expenditure on infliximab, etanercept and adalimumab in England was £760 million, which represents just under 5% of the total medicines expenditure [4].
Infliximab and etanercept were the first anti-TNFs to lose patent protection in Europe in 2013 and 2015, respectively, and have had competition from biosimilars [5]. Biosimilars for rituximab and adalimumab have recently been marketed and biosimilars for certolizumab pegol, golimumab and ustekinumab are in development [6]. The lower cost of biosimilars presents significant potential cost savings in a financially constrained health system such as the UK National Health Service (NHS) [7]. Thus, in theory, a lower acquisition cost potentially removes one barrier to prescribing biologics [8].
Healthcare professionals (HCPs) have been surveyed on their knowledge, attitude and practice towards biosimilars in the UK [9]. The results of that survey showed a high level of knowledge and awareness about biosimilars and that national as well as discipline-specific guidance influenced their uptake of biosimilars. Rheumatologists were more concerned than other HCPs about switching patients to biosimilars.
Despite the need for active involvement of patients in decisions about anti-TNFs [10], there have been limited publications on the views, opinions and the attitude of patients toward infliximab and etanercept biosimilars [11]. A survey of the literature revealed only three studies on patients’ attitudes towards anti-TNF biosimilars; only one was in rheumatology, and none involved UK patients only. The Jacobs et al. [12] study showed a low awareness of biosimilars with gaps in knowledge about biosimilars among US and European patients with ulcerative colitis (UC), Crohn’s disease (CD), RA and psoriasis. Sullivan et al. [13] and Waller et al. [14] showed reservations about biosimilars amongst UC and CD patients and AS and RA patients in Germany, respectively. Both studies showed that a quarter of the participants were concerned that they did not know enough about their medications (biosimilars) [13, 14].
Given the importance of biologic medicines in the UK NHS, we aimed to address this gap in our understanding of patients’ knowledge and attitudes to anti-TNF biosimilars. This study aimed to explore AS and RA patients’ understanding and attitude towards infliximab and etanercept biosimilars in the UK.
2 Methods
This was an anonymised, self-administered, web-based survey among AS and RA patients in UK. This survey was conducted between 2 March, 2017 and 2 June, 2017. The study was approved by the Ethics Review Panel at Keele University (Ref. ERP393).
A convenience sample of AS and RA patients who were registered members of the National Rheumatoid Arthritis Society and the National Ankylosing Spondylitis Society in the UK participated in this survey. The survey was an open survey. A request was sent to both societies to post the web survey advertisement and link to the survey on their Facebook page. A reminder post was sent via the society after 4 weeks of initial posting. The survey front page included information describing the survey and asking members for voluntary participation. An electronic consent of voluntary participation was sought from the respondents by clicking an ‘agree’ button. All the respondents were able to review and change their responses by scrolling up and down the page before submission. Cookies were used by the survey tool to minimise the chance of more than one response per computer. The survey tool was designed to allow only fully completed questionnaires to be submitted for analysis.
Survey themes were developed from concerns reported in the current literature on biosimilars [13, 14], in particular those relating to patients’ understanding and awareness about the biological nature of their medication and the concept of biosimilarity (questions 1–7), patients’ attitude toward biosimilars (questions 8–11) and concerns and questions about switching (question 12).
A questionnaire comprising 12 questions (see Electronic Supplementary Material) was designed using an electronic website (Survey Monkey). All the questions were closed multiple-choice questions (MCQ) with the exception of question 12 which was an open question. Questions were developed to explore knowledge, understanding and opinions towards biosimilars. The survey was piloted on a small number of lay individuals (the primary care user group at Keele University) and revised appropriately to eliminate redundancy and difficult or ambiguous questions. The questionnaire did not ask for any personal identifying information.
The survey responses to closed MCQs (1–11) were collated and summarised as number and percentage of responding patients using Survey Monkey and Microsoft Excel 2013. The open question (12) was analysed by thematic analysis. A t test analysis was used to test the difference in the percentages between participants on originator biologics and participants on biosimilars.
3 Results
3.1 Participants
A total of 182 patients participated in this survey and responses were evenly distributed (50/50) between AS patients and RA patients. The majority of AS and RA patients (71 and 73%, respectively) were on etanercept. A significantly higher percentage of participants (41%; p < 0.05) were on an etanercept biosimilar (Benepali®) compared with 24% on infliximab biosimilars (Remsima® and Inflectra®) (Table 1).
All survey participants (patients on originator biological and biosimilars) were aware that the medicine they used (infliximab or etanercept) was a biological medicine. The percentage of participants who knew that biosimilar versions of their branded biological medication existed was significantly higher among participants on biosimilars (83%) than in participants on originator biologics (60%) (p < 0.001). The majority of participants on biosimilars (80%) thought biosimilars were similar copies of biological medicines, 15% thought they were an identical copy of a biological medicine and 5% thought they were a new brand of a biological medicine (Table 2).
3.2 Confidence in Biosimilars and Doctor’s Decision
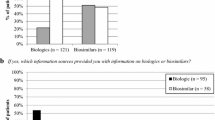
Participants on biosimilars had greater confidence in biosimilars to be as effective as the originator biologics and in their doctor’s decision to initiate and/or to switch to biosimilars than participants on originator biologics (Fig. 1).
3.3 Biosimilars’ Safety and Efficacy
Most participants on biosimilars (72%) thought biosimilars were as safe and effective as the originator. In contrast, 45% of participants on the originator biologics thought that biosimilars were less safe and 60% thought that biosimilars were less effective than originator biologics (Fig. 2).
3.4 Switching to Biosimilars
A significantly higher percentage of participants on biosimilars (74%) were comfortable and open to switching to other biosimilars compared with 28% of participants on originator biologics (p < 0.001) (Fig. 3).
3.5 Cost of Biosimilars
Fifty-eight percent of participants on biosimilars thought that using less expensive biosimilars would result in more patients being treated with biological medicines and 82% thought that biosimilars would help save money for the NHS. In contrast, 54% of participants on originator biologics thought that cost of treatment should not be considered when prescribing biological medicines (Fig. 4).
3.6 Patients’ Concerns and Queries
The open question of what would be your main question(s) if your healthcare professional wanted to switch you from branded infliximab/etanercept to biosimilar infliximab/etanercept elicited some similarities in the groups but also some clear differences. More participants on biosimilars wanted to know about side effects and safety, whereas more participants on originator biologics wanted more evidence from trials. Similar proportions in both groups wanted to ask about the reasons for switching and if they could switch back. A small percentage of those on the originator biologic had been switched to a biosimilar, had a bad experience after switching and were switched back. None of the participants currently on a biosimilar had a bad experience when switching and a small but significant proportion had switched without any concerns (Table 3).
4 Discussion
Almost three quarters of the participants were on etanercept. This is not surprising due to the preferred method of administration of etanercept (self-administration by subcutaneous injections or pen injectors at home) compared with intravenous infusion of infliximab in a hospital setting. This finding was in line with other studies that showed etanercept and adalimumab were the market-dominant anti-TNF biologics in rheumatology [9, 15]. The uptake of biosimilars was also higher among participants on etanercept (40%) in comparison with those on infliximab (24%), which may also be due to the greater experience of rheumatologists with etanercept than infliximab and is in line with our previous study on HCPs [9].
All AS and RA patients on originator biologics and biosimilars were aware of the biological nature of their medicine. Similarly, the majority of participants on biosimilars were well informed that they were on a biosimilar version of the biological medicine and understood correctly what biosimilars were (Table 2). This is perhaps a reflection of the role that both HCPs and patients’ associations have played in providing information about the disease and the treatment for patients [16]. A similar web survey did show a lower level of knowledge amongst HCPs but this did include HCPs who were not prescribers [9]. Despite the high awareness and understanding of biosimilars amongst the patients in our survey currently using them, more than one third of patients on the originator biologics were not aware of the availability of a biosimilar version of their medicine (Table 2). This may be due to the fact that patients stabilised on their originator biologic had not been offered a biosimilar. Our results also showed that UK AS and RA patients have a higher level of knowledge and awareness about biosimilars than US and European patients on biological medicine and UC and CD patients in Europe, where up to 70% had not heard of a biosimilar [12, 17].
Patients on biosimilars were more confident in the efficacy and safety of biosimilars and their doctors’ decision to initiate or switch patients to biosimilars than patients on the originator biologic (Figs. 1, 2). Participants on biosimilars were more comfortable switching to another biosimilar than participants on originator biologics (Fig. 3). This indicates healthcare professional teams have been successful in communicating with and educating patients starting biosimilars. The fact that no patients currently using biosimilars had a bad experience when switching (Table 3) may also be a reflection of this support. This result was in line with Waller et al., who assessed the acceptance of biosimilars among RA and AS patients in Germany [14].
Patients on biosimilars appeared to better understand the reason for prescribing biosimilars (i.e. cost saving to the NHS, from using less expensive biologics) (Fig. 4), while participants on originator biologics thought that cost should not be taken into account when prescribing biological medicines (Fig. 4). A European study of UC and CD patients found a similar proportion felt that cost should not come before efficacy and safety, although this study did not differentiate between patients on originator biologics and biosimilars [17]. This may be due to the public misconception, previously found with generics, that less expensive medicines would be less effective [18].
Patients on originator biologics and those on biosimilars had similar levels of questions about the side effects and effectiveness of biosimilars compared with the originator biologics, the reasons for switching, and the ability to switch back, whereas a higher proportion of participants on originator biologics wanted more evidence from clinical trials on biosimilars (Table 3). Similar concerns had been reported by UC, CD, RA and AS patients in Europe [14, 17]. In the latter study, which was in RA and AS patients similar to our cohort, 36–41% felt they did not know enough about biosimilars and a higher proportion of patients on an originator biologic had similar concerns to those identified in our study. Both groups of patients surveyed felt that more clinical trials on switching to biosimilars, pharmacovigilance studies and continuous education for HCPs would alleviate and answer patients’ concerns and queries. This has already been identified by the British Society of Rheumatology, which has requested more clinical data before they will recommend switching and close monitoring of patients switched to biosimilars for non-clinical reasons to ensure efficacy and safety [19].
The strength of this study is that it is the first study in a UK cohort of patients on anti-TNFs to compare and contrast the attitudes of patients on originator biologics with those on biosimilars. Our study has some limitations, since it was not possible to calculate the response rate as the total number of members of the National Rheumatoid Arthritis Society and National Ankylosing Spondylitis Society are confidential. The web (Facebook) community may not be a representative sample of the whole population, and results obtained with questionnaires on the web can be biased towards self-selection. Opinions surveyed were those of patients who were members of specialised associations and on biological medicines (infliximab and etanercept), which potentially could lead to bias, but we believe this unlikely as there are no advantages or disadvantages to the individual as a result of negative or positive results from the survey. Patients who have been switched to biosimilars had mentioned that in the open question, but the exact number of patients on biosimilars who were new users or have been switched from the originator was not known.
5 Conclusion
Within this survey of UK patients with AS and RA, knowledge and understanding of biosimilars was good. Patients on biosimilars were more confident and positive about biosimilar safety, efficacy and switching, whereas participants on originator biologics were more reluctant to switch to biosimilars. Evidence from clinical trials and information about the safety and efficacy and the possibility of switching back were the main questions participants had about biosimilars. More communication and reassurance of the patient by healthcare professional teams and further involvement in the decision making concerning biosimilars is required to increase biosimilars acceptance. Our results are similar to other European patient studies but provide more detail on the attitudes of patients already on a biosimilar and those on an originator biologic.
References
Salaffi F, Carotti M, Gasparini S, Intorcia M, Grassi W. The health-related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: a comparison with a selected sample of healthy people. Health Qual Life Outcomes. 2009;7(1):25.
Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370(9602):1861–74.
Van der Kooij SM, de Vries-Bouwstra JK, Goekoop-Ruiterman YP, Ewals JA, Han KH, Hazes JM, Kerstens PJ, Peeters AJ, van Zeben D, Breedveld FC, Huizinga TW, Dijkmans BA, Allaart CF. Patient-reported outcomes in a randomized trial comparing four different treatment strategies in recent-onset rheumatoid arthritis. Arthritis Care Res. 2009;61(1):4–12. doi:10.1002/art.24367.
Health and Social Care Information Centre. Prescribing Costs in Hospitals and the Community England 2014–15. 2015. http://content.digital.nhs.uk/catalogue/PUB18973/hosp-pres-eng-201415-report.pdf. Accessed 30 May 2017.
Azevedo VF, Galli N, Kleinfelder A, D’Ippolito J, Urbano PC. Etanercept biosimilars. Rheumatol Int. 2015;35(2):197–209.
European Medicine Agency. European public assessment reports. 2017. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar_search.jsp&mid=WC0b01ac058001d124&searchTab=searchByAuthType&keyword=Enter%20keywords&searchType=name&alreadyLoaded=true&status=Authorised&jsenabled=false&searchGenericType=biosimilars&orderBy=authDate&pageNo=1. Accessed 8 June 2017.
Aladul M, Fitzpatrick R, Chapman S. CP-024 factors affecting uptake of biosimilars. Eur J Hosp Pharm. 2017;24:A10–1. doi:10.1136/ejhpharm-2017-000640.23.
IMS Institute. Delivering on the potential of biosimilar medicines: the role of functioning competitive markets. 2016. http://www.imshealth.com/files/web/IMSH%20Institute/Healthcare%20Briefs/Documents/IMS_Institute_Biosimilar_Brief_March_2016.pdf. Accessed 11 July 2017.
Chapman SR, Fitzpatrick RW, Aladul MI. Knowledge, attitude and practice of healthcare professionals towards infliximab and insulin glargine biosimilars: result of a UK web-based survey. BMJ Open. 2017;7:e016730. doi:10.1136/bmjopen-2017-016730.
Nota I, Drossaert CH, Taal E, Vonkeman HE, van de Laar MA. Patient participation in decisions about disease modifying anti-rheumatic drugs: a cross-sectional survey. BMC Musculoskelet Disord. 2014;15(1):333.
NHS England. What is a biosimilar medicine? 2015. https://www.england.nhs.uk/wp-content/uploads/2015/09/biosimilar-guide.pdf. Accessed 30 May 2017.
Jacobs I, Singh E, Sewell KL, AL-Sabbagh A, Shane LG. Patient attitudes and understanding about biosimilars: an international cross-sectional survey. Patient Prefer Adherence. 2016;10:937.
Sullivan E, Piercy J, Waller J, Black CM, Kachroo S. Assessing gastroenterologist and patient acceptance of biosimilars in ulcerative colitis and Crohn’s disease across Germany. PloS One. 2017;12(4):e0175826.
Waller J, Sullivan E, Piercy J, Black CM, Kachroo S. Assessing physician and patient acceptance of infliximab biosimilars in rheumatoid arthritis, ankylosing spondyloarthritis and psoriatic arthritis across Germany. Patient Prefer Adherence. 2017;11:519.
PMLiVE. Brand leaders in RA will lose market share, predict rheumatologists. 2013. http://www.pmlive.com/pmhub/healthcare_market_research/109066_the_research_partnership/white_papers_and_resources/brand_leaders_in_ra_will_lose_market_share,_predict_rheumatologists. Accessed 4 April 2017.
Lyons BA. Selecting and evaluating sources of patient education materials. In: Muma RD, Lyons BA, editors. Patient education: a practical approach. Massachusetts, Burlington: Jones & Bartlett Publishers; 2011. p. 10–4.
Peyrin-Biroulet L, Lönnfors S, Roblin X, Danese S, Avedano L. Patient perspectives on biosimilars: a survey by the European Federation of Crohn’s and Ulcerative Colitis Associations. J Crohn’s Colitis. 2017;11(1):128–33.
Dunne SS, Dunne CP. What do people really think of generic medicines? A systematic review and critical appraisal of literature on stakeholder perceptions of generic drugs. BMC Med. 2015;13(1):173.
The British Society of Rheumatology. British Society for Rheumatology Position statement on biosimilar medicines (Revised January 2017). 2017. http://www.nras.org.uk/data/files/revised_bsr_biosimilars_position_statement_jan_2017.pdf. Accessed 1 June 2017.
Acknowledgements
The authors would like to acknowledge the National Rheumatoid Arthritis Society and the National Ankylosing Spondylitis Society, everyone who provided assistance in the dissemination of the survey, and all participants who completed the questionnaire. Mohammed Aladul was sponsored by the Higher Committee for Education Development in Iraq.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was not funded by any organisation and the researchers are independent of any funding bodies.
Conflict of interest
Mohammed I. Aladul, Raymond W. Fitzpatrick and Stephen R. Chapman declare that they have no conflict of interest.
Informed consent
Electronic informed consent was obtained from all individual participants included in the study.
Ethical approval
This study approved by the Ethics Review Panel at Keele University (Ref. ERP393).
Contributors
All authors have contributed to this study and all authors reviewed and approved the final version of the manuscript. MIA participated in the study design, data collection, and interpretation of results, prepared the manuscript draft, and performed all analytical testing and manuscript review. RWF participated in the study design, interpreted the results and reviewed the manuscript and corrected the final version of the manuscript. SRC designed the study, interpreted the results and reviewed the manuscript and corrected the final version of the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aladul, M.I., Fitzpatrick, R.W. & Chapman, S.R. Patients’ Understanding and Attitudes Towards Infliximab and Etanercept Biosimilars: Result of a UK Web-Based Survey. BioDrugs 31, 439–446 (2017). https://doi.org/10.1007/s40259-017-0238-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-017-0238-1