Abstract
Breast cancer (BC) is the most common cancer in women worldwide, and has an undeniable negative impact on public health. The advent of molecular biology and immunotherapy has made targeted therapeutic interventions possible, providing treatments tailored to the individual characteristics of the patient and the disease. The over-expression of human epidermal growth factor receptor (HER) 2 is implicated in the pathophysiology of BC and represents a clinically relevant biomarker for its treatment. Trastuzumab, a recombinant antibody targeting HER2, was the first biological drug approved for the treatment of HER2-positive BC. Although there are currently other anti-HER2 agents available (e.g. pertuzumab and lapatinib), trastuzumab remains the gold standard for treatment of this disease subtype. Nonetheless, concerns have been raised regarding potential cardiotoxicity and treatment resistance. Moreover, several other therapeutic issues remain unclear and have been addressed in an inconsistent way. The current literature lacks a comprehensive review of trastuzumab providing useful information for clinical practice, including pharmacokinetic and pharmacodynamic aspects, its clinical use, existing controversies and future advances. This detailed review of trastuzumab in the pharmacotherapy of BC attempts to fill this gap.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In line with the current evidence, trastuzumab is the standard of care for human epidermal growth factor receptor (HER) 2-positive breast cancer (BC). |
Trastuzumab should be considered initially for early and advanced stages of the disease, bearing in mind that combination with taxanes seems advantageous over its use as monotherapy. |
When available, subcutaneous administration of trastuzumab is preferable in terms of cost effectiveness, patient convenience and satisfaction. |
Trastuzumab–emtansine is now the first-line therapeutic option for cases of advanced BC that are resistant to trastuzumab. |
1 Introduction
Breast cancer (BC) is by far the most common cancer and is also the leading cause of cancer-related mortality in women worldwide, representing a significant public health concern [1–4]; it has therefore warranted special attention from the scientific community [5]. Current treatment options for BC include surgery, radiotherapy and systemic pharmacotherapy, namely chemotherapy, endocrine and biological therapy. The treatment strategy is based on several factors, including the status of specific biomarkers such as human epidermal growth factor receptor (HER) 2 [5–7]. The over-expression of HER2 has been associated with a more clinically aggressive tumour and a worse prognosis [5, 7–9]. In such cases, trastuzumab has assumed a key role in the therapeutic armamentarium for BC; it is considered to be the standard of care for patients with HER2-positive BC. In fact, its introduction in clinical practice revolutionised the treatment and natural history of this BC subtype, although not without shortcomings [5, 10]. Trastuzumab has been implicated in cardiovascular adverse effects, limited therapeutic response against brain metastases and treatment resistance [11, 12]. Other topical issues include the disease stages at which trastuzumab should be used, the most appropriate treatment scheme (e.g. monotherapy or in combination with chemotherapy drugs), the timing of the chemotherapy regimen (concomitant or sequential administration), the optimal treatment duration and the ideal administration route (intravenous or subcutaneous) [7, 9, 10, 13, 14]. Recent updates of international consensus guidelines for the treatment of BC that encompass HER2-positive BC have addressed these issues [15–17]. However, a comprehensive review of the pharmacokinetic and pharmacodynamics aspects of trastuzumab, its clinical use, existing controversies and future advances, which could provide useful information for clinical practice, is still lacking. The present article attempts to fill this gap by offering a detailed review of trastuzumab in the pharmacotherapy of BC.
2 Search Strategy
Clinical trials, case reports, review articles and other relevant study designs published from 2005 onwards on the use of trastuzumab in the treatment of HER2-positive BC were identified by searching the MEDLINE, EMBASE and Cochrane databases. Additionally, the bibliographies of eligible papers were searched. Only articles published in English and in peer-reviewed journals were included. Combinations and iterations of the search terms “Breast cancer”, “HER2-positive”, “Trastuzumab”, “Herceptin” and “Biological treatment” were used. Searches were last updated on 26 December 2015. In line with our aim to comprehensively map the literature on the topic, we included a broad range of study designs, with the intention of providing an overview of the sources of the available evidence. Additional data were also obtained from the summary of product characteristics of trastuzumab, which are available on the websites of the European Medicines Agency (EMA) and US Food and Drug Administration (FDA) [23, 43].
3 The Role of Human Epidermal Growth Factor Receptor (HER) 2 in the Pathophysiology of Breast Cancer (BC)
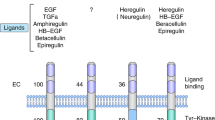
HER2 belongs to the tyrosine kinase receptor family and mediates critical signalling functions both in normal and malignant breast tissues, including cell proliferation and differentiation. This receptor lacks ligand-binding activity and is activated by the formation of homodimers and/or heterodimers with other HER family members (HER1, 3 and 4), which are ligand activated [8, 14, 18]. These mechanisms involving HER2 are shown in Fig. 1.
Putative mechanism of action of trastuzumab [5, 7, 14, 18–20]. ADCC antibody-dependent cellular cytotoxicity, Akt protein kinase B, CDK cyclin-dependent kinase, ERK extracellular signal-regulated kinase, HER human epidermal growth factor receptor, HIFα hypoxia inducible factor α, MEK mitogen-activated protein kinase/ERK kinase, mTOR mammalian target of rapamycin, p27 cyclin-dependent kinase inhibitor, p95 HER2 active terminal fragment of human epidermal growth factor receptor 2, PI3K phosphatidylinositol 3-kinase, PIP2 phosphatidylinositol (4,5)-bisphosphate, PIP3 phosphatidylinositol (3,4,5)-triphosphate, PTEN phosphatase and tensin homologue, RAC1 Ras-related C3 botulinum toxin substrate 1, RAF kinases, VEGF vascular endothelial growth factor
Typically, HER2 is expressed at a low level on the surface of epithelial cells, but high levels of HER2 have been found in approximately 15–30 % of BCs (HER2-positive BC subtype), a factor that has been linked to increased tumourigenicity [5, 7–9, 14, 18]. It has been recognised that the overabundance of HER2 stimulates a constitutive HER2 phosphorylation and the activation of downstream signalling pathways, culminating in tumour growth. Moreover, a further ligand-independent mechanism of HER2 activation in HER2-positive tumours has been proposed: a metalloprotease-mediated shedding of the HER2 extracellular domain, which produces a catalytically active HER2 terminal fragment, known as p95HER2 [14, 18].
In other words, the increased levels of HER2 on BC cells promote cell proliferation and angiogenesis, the inhibition of apoptosis and the development of metastasis. These aspects are clinically translated into a more aggressive BC phenotype, with high-grade tumours, increased growth rates, early systemic metastasis, and decreased rates of disease-free (DFS) and overall survival (OS) [5, 7–9]. Thus, targeting HER2 is an attractive therapeutic approach [11] and the assessment of its status has become crucial in BC management. According to McKeage and Lyseng-Williamson [9], HER2 over-expression is a prerequisite for the cytotoxic effect of trastuzumab and it should only be used in this subtype of tumour. In this respect, international guidelines recommend that screening for the biomarker should be performed in all new diagnoses of invasive BC through immunohistochemical (IHC) analysis or in situ hybridisation (fluorescent, chromogenic or silver in situ hybridisation); borderline IHC samples (IHC 2+) should be retested by in situ hybridisation [8, 16].
4 Trastuzumab: Pharmacodynamics and Pharmacokinetics
4.1 Mechanism of Action
Trastuzumab binds with high affinity to the extracellular domain of HER2, but the global mechanism underlying the therapeutic effect is not yet completely understood. However, there is consensus that it results from the co-influence of multiple actions [5, 7, 14, 18–20].
Recently, De et al. [14] reviewed the mechanism of action of trastuzumab, proposing that it acts in a direct manner on three main levels:
-
By inhibiting the ligand-independent HER2–HER3 heterodimerisation that occurs under conditions of HER2 over-expression;
-
By preventing the proteolytic cleavage of the HER2 extracellular domain and the formation of the active p95HER2 fragment; and
-
By inducing the antibody-dependent cellular cytotoxicity (ADCC) toward HER2-positive tumours by engaging with Fc receptors on immune effector cells [14].
As a result of these actions, downregulation of the signalling pathways, which involves phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) and mitogen-activated protein kinase (MAPK), is triggered. In turn, this results in enhanced nuclear import, stabilisation of the cyclin-dependent kinase (CDK) inhibitor p27, reduction of the secretion of angiogenic factors and an impaired DNA damage response [14].
Accordingly, several reviews have identified these mechanisms as being responsible for the clinical efficacy of trastuzumab [7, 18–21]. However, it should be noted that the immunological mechanism of ADCC has also been considered to be a key factor in the efficacy of trastuzumab-based therapies [7, 14, 18, 19]. Numerous studies have described the ability of trastuzumab to target immune cells in tumour sites over-expressing HER2 [7, 14, 18, 22]. Furthermore, some authors have hypothesised that trastuzumab also triggers the degradation of HER2, although the exact mechanism of this remains obscure [18–20].
The mechanism of action of trastuzumab is represented in Fig. 1. Globally, the final result of the direct and indirect actions discussed above is two-fold: increased cell cycle arrest and apoptosis plus suppression of cell proliferation and angiogenesis [18, 19].
4.2 Pharmacokinetic Properties
The pharmacokinetic profile of intravenous trastuzumab has been extensively studied and, more recently, the development of the subcutaneous formulation has driven the comparison between these two administration routes [10, 23, 24]. Table 1 summarises the pharmacokinetic properties of trastuzumab and Table 2 compares intravenous and subcutaneous administration of the drug.
Trastuzumab is administered by intravenous perfusion weekly or every 3 weeks, in body weight-adjusted doses (Table 2). Garnock-Jones et al. [7] reported mean steady-state concentrations of 60 mg/mL for the once a week regimen and 65.47 mg/mL for the 3-weekly regimen [7]. The mean minimum concentrations (C min) were about 20 % lower and the mean maximum concentrations (C max) were about 70 % higher for the latter, indicating a higher fluctuation index of drug concentrations in blood. Nevertheless, the average exposure at any time during trastuzumab treatment was found to be similar in the two dosing regimens [7].
The distribution of trastuzumab is limited (Table 1; low distribution volume) and only minimal amounts of the drug penetrate into the cerebrospinal fluid [7, 23, 24, 35]. This is consistent with the observed development of brain metastases in patients receiving the antibody [35].
The elimination pathways of trastuzumab are unknown. However, its clearance does not appear to occur by excretion and liver metabolism as in conventional drugs, but rather by an analogue mechanism relating to the homeostatic regulation of endogenous immunoglobulin G. While binding of the trastuzumab variable region with its target induces the ADCC, target destruction and the destruction of the immunoglobulin itself (contributing to its clearance), the constant region mediates a sophisticated homeostatic mechanism of protection against lysosomal proteolysis, which explains the long half-life of trastuzumab [23, 24, 35].
A phase III open-label multicentre trial (HannaH [enHANced treatment with NeoAdjuvant Herceptin] study) was recently performed to test the non-inferiority of the new subcutaneous formulation and dosing scheme in relation to the intravenous formulation, in terms of efficacy, safety and pharmacokinetic profile [28]. A total of 596 HER2-positive BC patients with operable, locally advanced or inflammatory tumours were randomized to either a fixed dose of trastuzumab 600 mg subcutaneously every 3 weeks or the 3-weekly intravenous schedule of trastuzumab. The data from this study showed that the subcutaneous formulation was non-inferior with respect to the primary pharmacokinetics endpoint (mean C min measured after seven cycles). The trial also demonstrated a similar therapeutic response and safety profile in both groups (Table 2). However, more patients had adverse effects in the subcutaneously treated group (21 vs. 12 %), particularly infections and infestations, which are not easily explained. It has been suggested that this could be attributable to the open-label design of the study [10, 23, 25, 28, 32].
Other studies have assessed different forms of subcutaneous administration. For example, 114 healthy male volunteers were randomly assigned to either subcutaneous trastuzumab given by a self-administration device (currently not approved) or a normal syringe; similar pharmacokinetic profiles were observed [23, 25, 26, 36].
In summary, from a pharmacokinetic point of view, the subcutaneous option represents a viable alternative to intravenous trastuzumab. This issue is addressed again in Sect. 8.
5 Therapeutic Indications for Trastuzumab
5.1 Early BC
There are two modalities of treatment for early BC: neo-adjuvant/primary treatment and adjuvant treatment. All therapeutic regimens used in the adjuvant setting, including those with trastuzumab, may also be used preoperatively. Nevertheless, if neo-adjuvant trastuzumab chemotherapy is used, maintaining the planned treatment is recommended, without dividing it into preoperative and postoperative periods, independent of the magnitude of tumour response. It should also be noted that treatment should be started within 2–6 weeks after surgery in the adjuvant setting, since administration more than 12 weeks after surgery has been associated with a clinically relevant decrease in the efficacy of systemic therapy [16].
Several large randomised, open-label, multicentre trials have assessed the efficacy of trastuzumab as concurrent or sequential treatment (in relation to chemotherapy) in patients with HER2-positive early BC, both in neo-adjuvant and adjuvant settings. These studies have been extensively reviewed [7, 8, 10, 27, 37–40] and international guidelines for the treatment of early BC have been published [15, 16, 41, 42].
The approved therapeutic indications of trastuzumab in the EU and USA for HER2-positive early and advanced BC are presented in Table 3. In contrast to the EU, the scope of utilisation of trastuzumab in the treatment of early BC in the USA does not encompass the neo-adjuvant setting and considers combination with an anthracycline (doxorubicin) legitimate.
The benefit of adding trastuzumab to the chemotherapy protocols in the treatment of patients with HER2-positive early BC, in both neo-adjuvant and adjuvant regimens, is clear. This therapeutic strategy provides better therapeutic outcomes than chemotherapy alone, namely higher pathological complete response (pCR) rates and a lower risk of disease relapse and patient death [7, 16, 27, 39–41].
Two initial studies were seminal in establishing trastuzumab as a routine therapy for HER2-positive early BC, specifically the NSABP (National Surgical Adjuvant Breast and Bowel Project NSABP) B-31 and NCCTG (North Central Cancer Treatment Group) N9831 trials [45, 46]. These studies investigated, in a joint analysis, the clinical utility of adding 1 year of trastuzumab to adjuvant chemotherapy. HER2-positive BC patients were randomised to receive doxorubicin and cyclophosphamide followed by paclitaxel plus trastuzumab during 1 year or doxorubicin and cyclophosphamide followed by paclitaxel. Overall, the combination of trastuzumab with paclitaxel resulted in therapeutic benefit at all timepoints evaluated, significantly improving DFS and OS [23, 45, 46]. The last update of the results from this joint analysis at a mean follow-up of 8.4 years reported a 37 % decrease in the risk of death and an increase in the 10-year OS rate from 75.2 to 84 % with the addition of trastuzumab to chemotherapy. Additionally, a 40 % improvement in DFS and an increase in the 10-year DFS rate from 62.2 to 73.7 % were found [46].
In turn, the NOAH (NeOAdjuvant Herceptin) trial also found better therapeutic outcomes with the use of neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab than with neoadjuvant chemotherapy alone. Trastuzumab significantly improved event-free survival (3-year event-free survival 71 % with trastuzumab vs. 56 % without) and pCR rates (pCR in breast tissue 43 % with trastuzumab vs. 22 % without; total pCR 38 % with trastuzumab vs. 19 % without) in patients with HER2-positive locally advanced BC [47].
The BCIRG006 [12] and BO16348 [23] trials and a recent meta-analysis of randomised controlled trials published by the Cochrane Collaboration [37] further support the role of trastuzumab in the treatment of early BC.
Moreover, pharmacoeconomic data support the cost effectiveness of adjuvant trastuzumab in this group of patients. When compared with chemotherapy alone, trastuzumab administered subsequent to or concurrently with chemotherapy was associated with favourable incremental costs per quality-adjusted life-year or life-year gained in several countries (Switzerland, Norway, Belgium, France, Poland, the UK, Australia, Japan, Taiwan and Brazil) [9].
As previously noted, trastuzumab can be used concomitantly or sequentially with chemotherapeutic drugs. Whether there are relevant therapeutic differences between these combinations and, if so, which is the best approach, is still under debate. Considering the data available presently, there is a strong trend favouring concomitant administration in terms of therapeutic efficacy, including in early BC [10, 16, 38–41].
However, recent European Society for Medical Oncology guidelines regarding the diagnosis, treatment and follow-up of primary BC subscribed to by the EMA warn that while trastuzumab may routinely be co-administered with non-anthracycline-based chemotherapy and with endocrine therapy, concomitant use with anthracyclines is not recommended due to the risk of cardiotoxicity [16, 17, 23]. In support of this recommendation, Du and co-workers [40] concluded in a meta-analysis of clinical trials that there is an increased risk of cardiac toxicity with concurrent administration of anthracyclines and trastuzumab in both neo-adjuvant and metastatic settings, even in a relatively shorter period [40]. For instance, the BCIRG006 clinical trial observed fewer cardiac events in the group of patients who received the docetaxel–carboplatin–trastuzumab combination than in those who received the anthracycline schedule [12, 41]. On the other hand, concurrent administration of anthracyclines and trastuzumab in the ACOSOG (American College of Surgeons Oncology Group) Z1041 trial did not result in a significant increase in cardiac events, although it also did not show additional benefits in relation to sequential administration [48, 49].
Although the combination of trastuzumab with anthracycline therapy is approved by the FDA, the combination with taxanes is safer and has been demonstrated to be more effective than the sequential treatment [16, 23, 43]. A meta-analysis of sequential and concomitant arms of six trastuzumab adjuvant trials in BC demonstrated that concomitant adjuvant trastuzumab therapy offers a greater benefit than sequential administration in both DFS and OS [39]. For most patients, the use of an anthracycline-based regimen followed by a taxane–trastuzumab-based regimen is the preferred choice [16, 17]. This recommendation is also applicable to the treatment of advanced BC.
In contrast, recommendations for the duration of treatment with trastuzumab are distinct in the two main stages of BC [15, 16]. Patients with early BC should be treated with trastuzumab during 1 year or, in Europe, until disease recurrence (whichever occurs first); extending treatment beyond 1 year is not recommended (Table 3) [23, 43]. No further benefits were demonstrated with the administration of trastuzumab for 2 years and the risk of adverse effects (mainly cardiotoxicity) can increase [16, 17]. The reduction of the duration of treatment of HER2-positive early BC with trastuzumab has been evaluated: similar improvements were obtained with only 9 weeks of treatment, but the studies were unable to show non-inferiority at 6 months. Twelve months of treatment remains the standard of care [16, 17, 50, 51]. In contrast, the optimal duration of trastuzumab treatment for advanced BC is currently unknown.
Lastly, it is important to discuss efficacy data for adjuvant trastuzumab treatment in specific groups, such as patients with small BC and patients who are over 70 years of age. Randomised and non-randomised trials support the use of adjuvant therapy in tumours of 2 cm or less [52–54], but trastuzumab use in clinical practice under these circumstances has not yet been approved [23, 43]. Nevertheless, due to a relatively high failure risk, international guidelines have recommended that trastuzumab should also be considered for this patient group, in particular in estrogen receptor (ER)-negative disease [16, 17]. Likewise, trastuzumab regimens should be considered for adjuvant treatment in elderly women, particularly in those at higher risk of relapse, with a lack of extra risk factors for trastuzumab-associated cardiotoxicity and with a prolonged estimated life expectancy [16, 17, 52].
5.2 Advanced BC
Worldwide recommendations have advised that anti-HER2 treatment should be offered early to all patients with HER2-positive metastatic BC [15, 42, 44]. The therapeutic indications of trastuzumab for advanced BC are more restrictive and objective in the EU than in the USA (Table 3), in line with the conclusions reached through extensive research for this stage of the disease [23, 43, 44]. Beyond the approved indications (Table 3), patients with ER-/HER2-positive metastatic BC, for whom endocrine therapy was chosen over chemotherapy, are also eligible to initiate an anti-HER2 agent such as trastuzumab. This is based on the significant benefit that has been observed on progression-free survival (PFS) with the combination of anti-HER2 therapy (either trastuzumab or lapatinib) and endocrine therapy [15, 42]. The combination of chemotherapy plus trastuzumab has shown superiority versus chemotherapy plus lapatinib in terms of PFS and OS as the first-line treatment for patients with HER2-positive metastatic BC who have been previously treated (in the adjuvant setting) or untreated with trastuzumab. Moreover, it is now agreed that the combination of chemotherapy plus trastuzumab and pertuzumab as first-line therapy is superior to chemotherapy plus trastuzumab in terms of OS, primarily for previously untreated HER2-positive metastatic BC [15]. Since this treatment option is preferable, it is likely that the therapeutic indications for trastuzumab will be updated to encompass dual HER2 blockade.
The place of other anti-HER2 options, such as trastuzumab–emtansine, has not been considered in the treatment of advanced BC [15, 41].
Regarding the duration of trastuzumab treatment for metastatic BC, the optimal interval is currently unknown and patients are treated until disease progression [23, 42, 43].
Recent international consensus guidelines for advanced BC recommend maintenance of HER2 pathway blockade, even with progression of the disease, under a treatment scheme using an anti-HER2 agent combined with a cytotoxic or endocrine agent [15].
While trastuzumab-based treatment is generally the first-line treatment for advanced BC, a pertinent issue is the best pharmacotherapy for patients whose BC has progressed after a trastuzumab-based treatment. Until recently, there were two viable options: continue trastuzumab in combination with another cytotoxic agent or change to lapatinib in combination with capecitabine; the preferred option in terms of the benefit/risk relationship was unknown [16]. In 2013, trastuzumab–emtansine was approved by the FDA specifically for the treatment of patients with HER2-positive metastatic BC who had previously received trastuzumab and a taxane [55, 56]. The regimen consists of an antibody–drug conjugate that associates the anti-tumour properties of trastuzumab with the cytotoxic effects of a microtubule-disrupting chemotherapy drug (mertansine, also known as DM1) [55, 56]. After first-line trastuzumab-based therapy, trastuzumab–emtansine provides superior efficacy as a second-line therapy over other HER2-based therapies [15]. In the phase III clinical trial EMILIA (Emtansine vs. Capecitabine plus Lapatinib in Patients with HER2-Positive Locally Advanced or Metastatic Breast Cancer), women with advanced HER2-positive BC that is resistant to the combination of trastuzumab and a taxane showed a significantly prolonged PFS and OS with less toxicity when treated second-line with trastuzumab–emtansine than with lapatinib plus capecitabine [55, 56]. Thus, trastuzumab–emtansine should be the preferred therapeutic option in patients who have progressed through at least one line of trastuzumab-based therapy [15]. Other clinical trials are currently being carried out to advance the understanding of the role of trastuzumab–emtansine in the treatment of early and advanced BC [56]. Data on the treatment of patients with HER2-positive advanced BC who relapse on or shortly after adjuvant trastuzumab are scarce; further studies, that take this population with a poor prognosis into account, need to be undertaken.
6 Safety Profile of Trastuzumab
Trastuzumab is a relatively safe and usually well-tolerated drug [16]. As an antibody, trastuzumab displays an atypical pharmacokinetics, not modulated by the classic routes of drug elimination, and a targeted mode of action. Thus, problems such as drug–drug interactions and interference with unwanted targets (frequently one of the main causes of drugs’ ‘adverse effects’) are unlikely. However, the HER2 receptor mediates multiple signalling pathways, not only in tumour cells. HER2 also has a homeostatic function in normal cells and the worrying safety issues seem to be related to the lack of specificity of trastuzumab for the tumour cells.
In more detail, the most common adverse reactions with trastuzumab are infusion-related symptoms, such as fever and chills. It is estimated that about 40 % of patients who are treated with trastuzumab experience some form of infusion-related reaction, although most are mild to moderate and frequently occur with the first infusions [6, 23].
Other frequent adverse reactions are gastrointestinal effects (nausea, vomiting, diarrhoea, constipation), haematotoxicity (in particular neutropenia), infections, rash, erythema, headaches, asthenia, arthralgia and myalgia [6, 7, 23, 43, 57, 58]. Ishizuna et al. [59] warned that although the development of hepatotoxicity is rare, periodic monitoring of liver function is necessary during trastuzumab therapy [59].
Cardiotoxicity and pulmonary toxicity are considered the most clinically important adverse effects involving trastuzumab. Severe, occasionally fatal, pulmonary toxicity events have been documented. An important risk marker for the development of pulmonary events is the presence of dyspnoea at rest; patients with this clinical status should not receive trastuzumab [23]. Cardiotoxicity, ranging from left ventricular systolic dysfunction to congestive heart failure, is the most extensively studied adverse effect. Congestive heart failure is a common adverse reaction (frequency of ≥1/100 to <1/10) and has been related to fatal outcomes [23]. Interestingly, trastuzumab-associated cardiac dysfunction is not dose related and is often reversible, unlike that observed for anthracyclines [8]. As already discussed, the combination of anthracyclines and trastuzumab is not recommended, since anthracyclines can induce cardiotoxicity and the concurrent administration offers no additional benefit [6, 48]. Nevertheless, cardiotoxic events have been observed equally in patients receiving trastuzumab alone or in combination with taxanes after anthracycline-containing chemotherapy [8, 23, 60–62]. Although cardiac function should be monitored during treatment [16, 23], the benefit of trastuzumab treatment outweighs the cardiac risk in most instances. Individual evaluation is nonetheless fundamental; cases that deserve special attention are those of patients at lower-risk stages of the disease (early stages) or those with increased cardiovascular risk. Cardiac monitoring is particularly relevant in these subgroups [10]. Several risk factors for the development of cardiotoxicity have been highlighted, such as anthracycline use, high body mass index, hypertension, antihypertensive therapy, coronary artery disease, congestive heart failure, left ventricular ejection fraction lower than 55 % and older age [23, 62, 63]. The interruption or discontinuation of trastuzumab treatment is generally required in cases of cardiotoxicity, severe infusion reactions and pulmonary toxicity [6, 23, 43, 58].
The safety of trastuzumab in pregnancy and lactation has not yet been established and therefore its use should be avoided unless its benefit outweighs the risk to the fetus. Women should not breastfeed during trastuzumab treatment and for 7 months after the last dose. Furthermore, trastuzumab is also contraindicated in patients with hypersensitivity to murine proteins [6, 23].
7 Resistance to Trastuzumab
Although trastuzumab markedly improved outcomes for HER2-positive BC, resistance to treatment remains a major obstacle in the management of these patients. Approximately 65 % of patients with HER2-positive BC do not respond to initial trastuzumab treatment and about 70 % of those who initially responded experience progression of the disease a year after the initial treatment. This means that trastuzumab is subject to two kinds of treatment resistance: a primary or inherent resistance and a secondary or acquired resistance [18, 64, 65].
Resistance mechanisms have been hypothesised to have three main levels: (1) steric effects; (2) alternative elevations of other tyrosine kinase receptors; and (3) intracellular alterations in the HER2 downstream signalling [18, 19].
Steric effects are normally a result of structural modifications in HER2 protein, which impede trastuzumab binding. For example, HER2 can mutate into a truncated receptor lacking the extracellular domain. This mutated isoform promotes the continuous activation of oncogenic signalling and avoids trastuzumab effects. Also, the elevated expression of mucin-4 (a cell surface protein) can yield a steric hindrance of HER2 and mask the trastuzumab binding site [18–20].
Over-expression of other tyrosine kinase receptors, such as insulin-like growth factor receptor 1 or hepatocyte growth factor receptor (encoded by c-MET and the hepatocyte growth factor receptor gene), promotes ectopic activation of the PI3K pathway. This additional activation of downstream signalling can compensate for the signalling inhibition mediated by trastuzumab, resulting in resistance. Notably, it has been reported that the over-expression of c-MET and hepatocyte growth factor receptor in human BC is correlated with poor prognosis. More importantly, trastuzumab up-regulates the c-MET expression in vitro and, therefore, this over-expression of c-MET can represent a critical mechanism involved in the acquired resistance to trastuzumab [18, 19].
Lastly, intracellular alterations in the HER2 downstream signalling, specifically the loss of phosphatase and tensin homologue (PTEN) and activation of mutations in PI3K, have been associated with a constitutive activation of the PI3K/Akt pathway. In fact, clinical studies have shown that tumours with a PTEN deficit and/or PI3K-activating mutations achieve worse therapeutic outcomes with trastuzumab, namely response rates and PFS [18, 66].
There are presently no validated, predictive biomarkers of trastuzumab efficacy in HER2-positive BC that can be used clinically. An Indian research group has developed HerceptinR, the first database with mechanisms of trastuzumab resistance at the genetic level in BC patients [64]. It is envisaged that HerceptinR will be seminal in designing biomarkers to identify patients eligible for trastuzumab treatment and in identifying effective chemotherapy for a particular patient [64]. On the research front, efforts are also focused on trastuzumab-based combinations targeting multiple checkpoints within the same oncogenic pathway or multiple sites across a signalling network, in order to enhance the anti-proliferative effect of treatment and/or to overcome the treatment resistance [65, 66]. These combinations include inhibitors of multiple growth factor receptors (e.g. pertuzumab, lapatinib, afatinib and neratinib), blockage of HER2 downstream effectors (e.g. everolimus, ridaforolimus, and current agents under investigation [dactolisib, pictrelisib and buparlisib]), histone deacetylase (e.g. panobinostat) and agents against type I insulin-like growth factor receptor and angiogenesis (e.g. bevacizumab, pazopanib and cixutumumab) [65, 66]. Multiple clinical trials have been performed and many are ongoing [65, 66]. Hence, it is expected that some of these approaches will be added to the therapeutic armamentarium for BC in the near future.
8 Trastuzumab Administration
As previously mentioned, intravenous and subcutaneous formulations of trastuzumab are now available for the treatment of BC, although not in all countries (Table 2). The two formulations have demonstrated comparable efficacy and safety profiles; a pertinent issue is therefore to establish which one is preferable.
Intravenous trastuzumab doses have been systematically adjusted to body weight, while the subcutaneous formulation consists of a fixed dose, irrespective of the patient’s body weight [23]. Several studies suggest that body size does not significantly influence the pharmacokinetics of trastuzumab [24, 25, 35]. Additionally, subcutaneous administration does not require a loading dose [24, 25, 32, 35]. Furthermore, patients prefer subcutaneous administration, mainly due to the reduced administration time and less pain and discomfort (Table 2) [10, 25, 29–31, 33, 34]. Taken together, these findings indicate that subcutaneous administration should be the preferred option for trastuzumab in the treatment of HER2-positive BC. It is expected that this administration route will become standard in the near future.
The intrathecal route has been studied recently to attain adequate concentrations of trastuzumab in the central nervous system, with the purpose of increasing the response of tumours with brain metastasis [35]. Multiple case reports have described the therapeutic success of intrathecal trastuzumab in these cases [67–76]. However, large clinical trials with an adequate design are urgently needed to unequivocally prove the value of intrathecal trastuzumab in the treatment of BC. In summary, although this route is still experimental, it appears to be promising for the management of patients with metastatic HER2-positive BC. Since brain metastasis can develop in BC patients treated with trastuzumab, we are of the opinion that intrathecal trastuzumab may also be valuable in combination with systemic trastuzumab in a non-metastatic BC setting, with the aim of preventing the development of metastasis. We believe that this unanswered issue deserves future investigation.
9 Conclusion and Future Trends
Trastuzumab is an anti-HER2 agent available in intravenous and subcutaneous formulations, which have similar efficacy and safety. Currently, trastuzumab remains the first-line option for the treatment of early and advanced HER2-positive BC, in mono- or combined therapy, due to its established safety and efficacy profile.
The major challenge of trastuzumab-based treatment is undoubtedly resistance to the drug. Further improvement of clinical and therapeutic outcomes are expected in coming years, building on the combination of drugs targeted to different biologically relevant pathways, and associated with the predictive use of therapeutic and disease biomarkers. The molecular and immunotherapeutic paradigm has changed the management of BC dramatically, and further developments are expected in this area.
References
Benson JR, Jatoi I. The global breast cancer burden. Future Oncol. 2012;8:697–702.
Coughlin S, Ekwueme D. Breast cancer as a global health concern. Cancer Epidemiol. 2009;33:315–8.
Corbex M, Bouzbid S, Boffetta P. Features of breast cancer in developing countries, examples from North-Africa. Eur J Cancer. 2014;50:1808–18.
Duffy MJ. The war on cancer: are we winning? Tumour Biol. 2013;34:1275–84.
Tinoco G, Warsch S, Glück S, Avancha K, Montero AJ. Treating breast cancer in the 21st century: emerging biological therapies. J Cancer. 2013;4:117–32.
Lambert L. Biological drugs in breast cancer: increasing understanding for the pharmacist. S Afr Pharm J. 2014;81:24–7.
Garnock-Jones KP, Keating GM, Scott LJ. Spotlight on trastuzumab as adjuvant treatment in human epidermal growth factor receptor 2 (HER2)-positive early breast cancer. BioDrugs. 2010;24:207–9.
Patani N, Mokbel K. Herceptin and breast cancer: an overview for surgeons. Surg Oncol. 2010;19:11–21.
McKeage K, Lyseng-Williamson KA. Trastuzumab: a pharmacoeconomic review of its use in early breast cancer. Pharmacoeconomics. 2008;26:699–719.
Pinto AC, Ades F, de Azambuja E, Piccart-Gebhart M. Trastuzumab for patients with HER2 positive breast cancer: delivery, duration and combination therapies. Breast. 2013;22:S152–5.
Gradishar WJ. Emerging approaches for treating HER2-positive metastatic breast cancer beyond trastuzumab. Ann Oncol. 2013;24:2492–500.
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–83.
Incorvati JA, Shah S, Mu Y, Lu J. Targeted therapy for HER2 positive breast cancer. J Hematol Oncol. 2013;6:38.
De P, Hasmann M, Leyland-Jones B. Molecular determinants of trastuzumab efficacy: what is their clinical relevance? Cancer Treat Rev. 2013;39:925–34.
Cardoso F, Costa A, Norton L, Senkus E, Aapro M, André F, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Ann Oncol. 2014;25:1–18.
Senkus E, Kyriakides S, Penault-Llorca F, Poortmans P, Thompson A, Zackrisson S, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):7–23.
Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:8–30.
Vu T, Claret FX. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol. 2012;2:62.
Emde A, Köstler WJ, Yarden Y. Therapeutic strategies and mechanisms of tumorigenesis of HER2-overexpressing breast cancer. Crit Rev Oncol Hematol. 2012;84(Suppl 1):49–57.
Bartsch R, Wenzel C, Zielinski CC, Steger GG. HER-2-positive breast cancer: hope beyond trastuzumab. BioDrugs. 2007;21:69–77.
Rexer BN, Arteaga CL. Optimal targeting of HER2-PI3K signaling in breast cancer: mechanistic insights and clinical implications. Cancer Res. 2013;73:3817–20.
Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94:259–67.
European Medicines Agency. Summary of product characteristics: Herceptin. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000278/WC500074922.pdf. Accessed 9 Feb 2016.
Azanza J-R, Sádaba B, Gómez-Guiu A. Monoclonal antibodies: pharmacokinetics as a basis for new dosage regimens? J Oncol Pharm Pract. 2015;21:370–6.
Leveque D. Subcutaneous administration of anticancer agents. Anticancer Res. 2014;34:1579–86.
Wynne C, Harvey V, Schwabe C, Waaka D, McIntyre C, Bittner B. Comparison of subcutaneous and intravenous administration of trastuzumab: a phase I/Ib trial in healthy male volunteers and patients with HER2-positive breast cancer. J Clin Pharmacol. 2013;53:192–201.
Plosker GL, Keam SJ. Spotlight on trastuzumab in the management of HER2-positive metastatic and early-stage breast cancer. BioDrugs. 2006;20:259–62.
Ismael G, Hegg R, Muehlbauer S, Heinzmann D, Lum B, Kim S-B, et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I–III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 2012;13:869–78.
Pivot X, Gligorov J, Müller V, Barrett-Lee P, Verma S, Knoop A, et al. Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study. Lancet Oncol. 2013;14:962–70.
Pivot X, Gligorov J, Muller V, Curigliano G, Knoop A, Verma S, et al. Patients’ preferences for subcutaneous trastuzumab versus conventional intravenous infusion for the adjuvant treatment of HER2-positive early breast cancer: final analysis of 488 patients in the international, randomized, two-cohort PrefHer study. Ann Oncol. 2014;25:1979–87.
Jackisch C, Müller V, Maintz C, Hell S, Ataseven B. Subcutaneous administration of monoclonal antibodies in oncology. Geburtshilfe Frauenheilkd. 2014;74:343–9.
Hourcade-Potelleret F, Lemenuel-Diot A, McIntyre C, Brewster M, Lum B, Bittner B. Use of a population pharmacokinetic approach for the clinical development of a fixed-dose subcutaneous formulation of trastuzumab. CPT Pharmacomet Syst Pharmacol. 2014;3:1–9.
Jackisch C, Müller V, Dall P, Neumeister R, Park-Simon T-W, Ruf-Dördelmann A, et al. Subcutaneous trastuzumab for HER2-positive breast cancer—evidence and practical experience in 7 German centers. Geburtshilfe Frauenheilkd. 2015;75:566–73.
Ryan S, North R, Harvey V, Cox L. Medical resource utilization for administration of trastuzumab in a New Zealand oncology outpatient setting: a time and motion study. Clin Outcomes Res. 2015;7:423–30.
Levêque D, Gigou L, Bergerat JP. Clinical pharmacology of trastuzumab. Curr Clin Pharmacol. 2008;3:51–5.
Wynne CJ, Ellis-Pegler RB, Waaka DS, Schwabe C, Lehle M, Heinzmann D, et al. Comparative pharmacokinetics of subcutaneous trastuzumab administered via handheld syringe or proprietary single-use injection device in healthy males. Cancer Chemother Pharmacol. 2013;72:1079–87.
Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012;4:CD006243.
Ismaili N, Elmajjaoui S, Tahri A, Benjaafar N, Errihani H, Belbaraka R. Trastuzumab in early breast cancer. Presse Med. 2013;42:1069–80.
Petrelli F, Barni S. Meta-analysis of concomitant compared to sequential adjuvant trastuzumab in breast cancer: the sooner the better. Med Oncol. 2012;29:503–10.
Du F, Yuan P, Zhu W, Wang J, Ma F, Fan Y, et al. Is it safe to give anthracyclines concurrently with trastuzumab in neo-adjuvant or metastatic settings for HER2-positive breast cancer? A meta-analysis of randomized controlled trials. Med Oncol. 2014;31:340.
Del Barco S, Ciruelos E, Tusquets I, Ruiz M, Barnadas A, SEOM. SEOM clinical guidelines for the systemic treatment of early breast cancer 2013. Clin Transl Oncol. 2013;15:1011–7.
Cardoso F, Costa A, Norton L, Cameron D, Cufer T, Fallowfield L, et al. 1st International consensus guidelines for advanced breast cancer (ABC 1). Breast. 2012;21:242–52.
Food and Drug Administration. Herceptin (Trastuzumab) Label. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.pdf. Accessed 9 Feb 2016.
Cortazar P, Justice R, Johnson J, Sridhara R, Keegan P, Pazdur R. US Food and Drug Administration approval overview in metastatic breast cancer. J Clin Oncol. 2012;30:1705–11.
Perez EA, Romond EH, Suman VJ, Jeong J-H, Davidson NE, Geyer CE, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–73.
Perez EA, Romond EH, Suman VJ, Jeong J-H, Sledge G, Geyer CE, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–52.
Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER. Lancet. 2010;375:377–84.
Buzdar AU, Suman VJ, Meric-Bernstam F, Leitch AM, Ellis MJ, Boughey JC, et al. Fluorouracil, epirubicin, and cyclophosphamide (FEC-75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by FEC-75 plus trastuzumab as neoadjuvant treatment for patients with HER2-positive breast cancer (Z1041): a random. Lancet Oncol. 2013;14:1317–25.
Ewer M, Suman VJ, Buzdar A, McCall LM, Meric-Bernstam F, et al. ACOSOG Z1041 (Alliance): cardiac events (CE) among those receiving neoadjuvant antracyclines (A) and taxanes with trastuzumab (T) for HER2+ breast cancer [abstract]. J Clin Oncol. 2013;31(Suppl):526.
Pivot X, Romieu G, Debled M, Pierga J-Y, Kerbrat P, Bachelot T, et al. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 2013;14:741–8.
Mavroudis D, Saloustros E, Malamos N, Kakolyris S, Boukovinas I, Papakotoulas P, et al. Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann Oncol. 2015;26:1333–40.
Albanell J, Ciruelos EM, Lluch A, Muñoz M, Rodríguez CA. Trastuzumab in small tumours and in elderly women. Cancer Treat Rev. 2014;40:41–7.
Antolín-Novoa S, Blanco-Campanario E, Antón A, Gallegos-Sancho MI, Pérez-Carrión R, Peláez I, et al. Adjuvant regimens with trastuzumab administered for small HER2-positive breast cancer in routine clinical practice. Clin Transl Oncol. 2015;17:862–9.
O’Sullivan CC, Bradbury I, Campbell C, Spielmann M, Perez EA, Joensuu H, et al. Efficacy of adjuvant trastuzumab for patients with human epidermal growth factor receptor 2-positive early breast cancer and tumors ≤2 cm: a meta-analysis of the randomized trastuzumab trials. J Clin Oncol. 2015;33:2600–8.
Lambert JM, Chari RVJ. Ado-trastuzumab Emtansine (T-DM1): an antibody-drug conjugate (ADC) for HER2-positive breast cancer. J Med Chem. 2014;57:6949–64.
Ballantyne A, Dhillon S. Trastuzumab emtansine: first global approval. Drugs. 2013;73:755–65.
Maly JJ, Macrae ER. Pertuzumab in combination with trastuzumab and chemotherapy in the treatment of HER2-positive metastatic breast cancer: safety, efficacy, and progression free survival. Breast Cancer. 2014;8:81–8.
Mehta A, Tripathy D. Co-targeting estrogen receptor and HER2 pathways in breast cancer. Breast. 2014;23:2–9.
Ishizuna K, Ninomiya J, Ogawa T, Tsuji E. Hepatotoxicity induced by trastuzumab used for breast cancer adjuvant therapy: a case report. J Med Case Rep. 2014;8:417.
Sandoo A, Kitas GD, Carmichael AR. Endothelial dysfunction as a determinant of trastuzumab-mediated cardiotoxicity in patients with breast cancer. Anticancer Res. 2014;34:1147–51.
Jitawatanarat P, Connor TLO, Kossoff EB, Levine EG, Chittawatanarat K. Breast cancer safety and tolerability of docetaxel, cyclophosphamide, and trastuzumab compared to standard trastuzumab-based chemotherapy regimens for early-stage human epidermal growth factor receptor 2-positive breast cancer. J Breast Cancer. 2014;17:356–62.
Barroso-Sousa R, Santana IA, Testa L, de Melo Gagliato D, Mano MS. Biological therapies in breast cancer: common toxicities and management strategies. Breast. 2013;22:1009–18.
Xue J, Jiang Z, Qi F, Lv S, Zhang S, Wang T, et al. Risk of trastuzumab-related cardiotoxicity in early breast cancer patients: a prospective observational study. J Breast Cancer. 2014;17:363–9.
Ahmad S, Gupta S, Kumar R, Varshney GC, Raghava GPS. Herceptin resistance database for understanding mechanism of resistance in breast cancer patients. Sci Rep. 2014;4:4483.
Vu T, Sliwkowski MX, Claret FX. Personalized drug combinations to overcome trastuzumab resistance in HER2-positive breast cancer. Biochim Biophys Acta. 2014;1846:353–65.
Singh JC, Jhaveri K, Esteva FJ. HER2-positive advanced breast cancer: optimizing patient outcomes and opportunities for drug development. Br J Cancer. 2014;111:1888–98.
Zagouri F, Sergentanis TN, Bartsch R, Berghoff AS, Chrysikos D, de Azambuja E, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res Treat. 2013;139:13–22.
Bartsch R, Berghoff AS, Preusser M. Optimal management of brain metastases from breast cancer: issues and considerations. CNS Drugs. 2013;27:121–34.
Patil A, Sherbet GV. Therapeutic approach to the management of HER2-positive breast cancer metastatic to the brain. Cancer Lett. 2015;358:93–9.
Bousquet G, Darrouzain F, de Bazelaire C, Ternant D, Barranger E, Winterman S, et al. Intrathecal trastuzumab halts progression of CNS metastases in breast cancer. J Clin Oncol (Epub 29 Dec 2014).
Mego M, Sycova-Mila Z, Obertova J, Rajec J, Liskova S, Palacka P, et al. Intrathecal administration of trastuzumab with cytarabine and methotrexate in breast cancer patients with leptomeningeal carcinomatosis. Breast. 2011;20:478–80.
Oliveira M, Braga S, Passos-Coelho JL, Fonseca R, Oliveira J. Complete response in HER2+ leptomeningeal carcinomatosis from breast cancer with intrathecal trastuzumab. Breast Cancer Res Treat. 2011;127:841–4.
Ferrario C, Davidson A, Bouganim N, Aloyz R, Panasci LC. Intrathecal trastuzumab and thiotepa for leptomeningeal spread of breast cancer. Ann Oncol. 2009;20:792–5.
Colozza M, Minenza E, Gori S, Fenocchio D, Paolucci C, Aristei C, et al. Extended survival of a HER-2-positive metastatic breast cancer patient with brain metastases also treated with intrathecal trastuzumab. Cancer Chemother Pharmacol. 2009;63:1157–9.
Platini C, Long J, Walter S. Meningeal carcinomatosis from breast cancer treated with intrathecal trastuzumab. Lancet Oncol. 2006;7:778–80.
Stemmler HJ, Schmitt M, Harbeck N, Willems A, Bernhard H, Lässig D, et al. Application of intrathecal trastuzumab (Herceptin™) for treatment of meningeal carcinomatosis in HER2-overexpressing metastatic breast cancer. Oncol Rep. 2006;15:1373–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributions
Sofia Maximiano—acquisition, analysis and interpretation of data, drafting of the manuscript and technical support; Paulo Magalhães—analysis and interpretation of data, drafting of the manuscript and critical revision of the manuscript for important intellectual content; Mara Pereira Guerreiro—analysis and interpretation of data and critical revision of the manuscript for important intellectual content; Manuel Morgado—concept and study design and critical revision of the manuscript for important intellectual content.
Funding and conflict of interest
Sofia Maximiano, Paulo Magalhães, Mara Pereira Guerreiro and Manuel Morgado state that they have no conflicts of interest regarding the publication of this article. Research funding played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Rights and permissions
About this article
Cite this article
Maximiano, S., Magalhães, P., Guerreiro, M.P. et al. Trastuzumab in the Treatment of Breast Cancer. BioDrugs 30, 75–86 (2016). https://doi.org/10.1007/s40259-016-0162-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-016-0162-9