Abstract
Background and Objective
Gastric/gastrointestinal cancers are associated with high mortality worldwide. G-protein coupled receptor (GPCR) superfamily members such as gastrin/cholecystokinin-B receptor (CCK-BR) are involved in progression of gastric tumors, thus CCK-BR is considered as a potential target for immunotherapy. However, production of functional monoclonal antibodies (mAbs) against GPCR seems to be very challenging, in part due to its integration in cell membranes and inaccessibility for selection. To tackle this problem, we implemented phage display technology and a solution-phase biopanning (SPB) scheme for production of mAbs specific to the native conformation of CCK-BR.
Methods
To perform the SPB process, we utilized a synthetic biotinylated peptide corresponding to the second extracellular loop (ECL2) of CCK-BR and a semi-synthetic phage antibody library. After enzyme-linked immunosorbent assay (ELISA) screening, the CCK-BR specificity of the selected single-chain variable fragments (scFvs) were further examined using immunoblotting, whole-cell ELISA, and flow cytometry assays.
Results
After performing four rounds of selection, we identified nine antibody clones which showed positive reactivity with the CCK-BR peptide in an ELISA assay. Of these, eight clones were unique scFv antibodies and one was a VL single domain antibody. Specificity analysis of the selected scFvs revealed that five of the selected scFvs recognized a denatured form of CCK-BR, while the majority of the selected scFvs were able to recognize the native conformation of CCK-BR on the surface of human gastric adenocarcinoma cells and cervical carcinoma HeLa cells.
Conclusion
For the first time, we report on the establishment of a diverse panel of scFv antibody fragments that are specific to the native conformation of CCK-BR. Based on these results, we suggest the selected scFv antibody fragments as potential agents for diagnosis, imaging, targeting, and/or immunotherapy of cancers that overexpress CCK-BR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Gastric cancer is considered as a high prevalence malignancy with a high rate of mortality [1, 2]. As the second most frequent cause of cancer-related deaths, its mortality rate is over a million/year [3, 4]. Many reports demonstrated that hypergastrinemia subsequent to Helicobacter pylori infection (the so-called endocrine loop) is the most frequent cause of gastric cancers (i.e., adenocarcinoma, carcinoids) [5–8]. Further, it has been shown that gastrin secreted by some already established tumors (e.g., esophageal adenocarcinoma, hepatocellular carcinoma, colorectal cancer, pancrease adenocarcinoma and ovarian stromal tumors) can impose mitogenic effect(s) on aberrant cells through an autocrine/paracrine loop [9–12]. The proliferative effect of gastrin on tumor cells was shown to be mediated through cholecystokinin-B receptor (CCK-BR), which belongs to the G-protein coupled receptor (GPCR) superfamily. Thus, enormous endeavors have been devoted to interrupting signal transduction mediated by the gastrin/CCK-BR pathway using various approaches including gastrin-specific antibodies [13], G17DT (Gastrimmune) as a cancer vaccine [14, 15], and polyclonal antibodies directed against N-terminal domain of CCK-BR, either in gastric cell lines [16] or tumor-xenograft animals [17]. However, these treatment modalities may be associated with inadvertent upregulation of ligands [18], and a non-response to active immunization in a small number of cases [19]. Although targeting of CCK-BR by monoclonal antibodies (mAbs) may provide improved clinical outcomes in immunotherapy of gastric/gastrointestinal cancers, no fully human mAb against CCK-BR has thus far been approved for clinical use.
Recombinant antibody fragments and mAbs have been shown to provide significant therapeutic effects in cancer therapy. So far, 34 therapeutic mAbs have been commercialized either in Europe or the US [20], and many others are under pre-clinical investigations or various stages of clinical trials [21]. Some of these therapeutic mAbs, which are produced based on hybridoma technology (11 % murine, 21 % chimeric and 36 % humanized), contain different amounts of mouse genetic material that may elicit immunogenic reactions such as human anti-mouse antibody response (HAMA) [22, 23]. To circumvent such immunogenic responses, mouse mAbs have been engineered as chimeric, humanized, and fully human mAbs. Of these approaches, combinatorial human antibody libraries in different display platforms (e.g., phage display, ribosome display, mRNA display, and cell displays such as Escherichia coli, yeast, and mammalian cell display) have been employed as robust high throughput tools for producing fully human therapeutic mAbs [24, 25]. Among these display platforms, phage display technology (PDT) introduced by Smith [26] appears to be the most widely used approach [27, 28]. In PDT, genes encoding antibody fragments mainly in single-chain variable fragments (scFvs) [29] or Fab [28] formats are incorporated into phage/phagemid vector [30–32] adjacent to one of the genes encoding coat proteins of the filamentous bacteriophages (e.g., M13), mostly minor coat protein 3 (g3p). Of these, the scFv antibody fragment is the most commonly used format for construction of phage antibody libraries [33]. It is a single recombinant molecule that possesses solely antigen-binding domains constructed of the variable regions of light and heavy chains [34, 35]. Because they lack the Fc functional domain, scFv-based antibody fragments (e.g., monobody, diabody, bi-specific antibody, triabody, and immunoconjugates) exert their anti-tumor effects through mechanisms independent from Fc domain-mediated effector functions. Their anti-tumor mechanisms include (a) interruption of tumorigenesis pathways such as neutralizing growth factors involved in angiogenesis or tumor cell growth, blocking or inhibiting receptors mediating proliferation signals, stimulating death receptors, and accelerating receptor internalization [36, 37], (b) modulation of immune responses such as redirecting effector immune cells towards tumor cells via bispecific scFvs or chimeric scFv-T-cell receptors, or blocking negative regulatory signals mediated by T cells [37, 38], and (c) delivery of therapeutic agents such as drugs, enzymes, toxins, radioisotopes, cytokines, gene constructs, and nanoparticles to the target site [39]. In comparison to intact canonical mAbs, scFvs have lower molecular weight (approximately 25 kDa), hence they show better tumor penetration, homogenous distribution, and less toxicity [40–42]. Taken all together, scFvs are deemed to be a suitable alternative to intact mAbs regarding immunotherapy of solid tumors.
CCK-BR belongs to class-A/I (rhodopsin-like) GPCR superfamily. It possesses an extracellular N-terminus, an intracellular C-terminus, seven membrane-spanning α-helical segments that are linked together consecutively through three extracellular segments (E1, E2, E3), and three cytoplasmic segments (C1, C2, C3) [43]. Despite the fact that the GPCR superfamily is one of the most interesting drug targets for the pharmaceutical and biotechnology industries, no FDA-approved therapeutic mAb against GPCRs is available. This is partially due to their low expression yield and difficult purification, small extracellular domain for antibody binding, receptor dimerization, conformational fluctuation, and extensive glycosylation [44].
To circumvent these downsides, a combination of PDT with an appropriate biopanning method has been exploited using different antigen formats such as purified receptor [45], synthetic peptides [46, 47], whole cell selection [48, 49], and paramagnetic proteoliposomes [50]. Thus, in the current study, we aimed to implement PDT through a solution-phase biopanning (SPB) approach for the isolation of fully human scFv antibody fragments specific to the native conformation of CCK-BR.
2 Materials and Methods
2.1 Phage Antibody Library
Semi-synthetic human single-fold scFv libraries I + J (Tomlinson I + J), KM13 helper phage, TG1 Tr E.coli for phage propagation and HB2151 E.coli for production of soluble scFvs were purchased from Source BioScience (Nottingham, UK). Diversity of the library was approximately 1.4 × 108 transformants. The phage antibody library was constructed by side chain diversification of complementarity-determining regions (CDRs) [i.e., CDR2 and CDR3 of a single framework from VH (V3-23/DP-47 and JH4b)] and VL (O12/O2/DPK9 and Jκ1) while CDR1 was kept constant. The diversifications were incorporated at 18 residues that are highly diverse in primary repertoire and function as antigen binding sites. Sequences encoding antibody fragments were cloned next to gene 3 protein in phagemid vector, pIT2, with His and myc tags.
2.2 Cell Lines, Antibodies and Reagents
Human gastric adenocarcinoma (AGS), cervical carcinoma (HeLa) and mouse embryo fibroblast (NIH 3T3) cell lines were provided by the Iranian National Cell Bank (Cell Bank Department, Pasture Institute, Teheran, Iran). All cell lines were maintained in Roswell Park Memorial Institute (RPMI) 1640 media supplemented with 10 % fetal bovine serum at 37 °C in 5 % CO2.
A peptide (Biotin-mini-PEG-PVYTVVQPVGPRVLQCVHRWPSARVRQTWS) corresponding to the ECL2 of human CCK-BR (residues 190–219) was synthesized with biotin and mini PEG as a linker at the N-terminus by Biomatik (Cambridge, Ontario, Canada). Peptide purity (96.48 %) and sequence were verified by high-performance liquid chromatography and mass spectrometry.
2.3 Solution-Phase Biopanning
Phage displaying scFvs were rescued from the phage antibody library I (1011 cfu/mL) according to the supplier’s protocol and used for affinity selection as described previously [51, 52]. Briefly, the rescued phage and Dynabeads M-280 Streptavidin (Invitrogen, Karlsruhe, Germany) were separately blocked with blocking buffer (phosphate buffered saline (PBS) containing 3 % bovine serum albumin (BSA) and 0.05 % Tween 20) at room temperature (RT) for 30 min. The blocked phages were incubated at RT for 2 h with decreasing concentrations of the biotinylated peptide (100 nM and 50 nM for selection rounds 1–2 and 3–4, respectively). The phage-peptide mixture was added to the blocked Dynabeads M-280 Streptavidin and incubated at RT for 20 min while rotated on an over-head rotator to capture peptide-bound phage-scFvs. The captured peptide-bound phage-scFvs were recovered using a magnetic particle concentrator, DynaMag-2 (Invitrogen, Karlsruhe, Germany), washed (4×) with PBST (PBS 0.1 % Tween 20) and then with PBS (2×). Washing steps were increased to 12 (for round 2) and 26 (for rounds 3 and 4) times. After 10 min incubation at RT with 0.5 mL of bovine pancreas trypsin (1 mg/mL) [Sigma-Aldrich Co., Taufkirchen, Germany], the bound phages were eluted and the supernatant containing the eluted phages was separated from the beads by DynaMag-2. To amplify the separated phages, TG1 E.coli bacteria were infected by the eluted phages at 37 °C for 30 min. The bacteria were cultured overnight (at 37 °C) and then infected with KM13 helper phage (at 37 °C for 30 min) to rescue phage-displayed scFv antibodies for the next rounds of selection.
Prior to rounds 1 and 2 of the selection process, the blocked phages were pre-absorbed at RT for 1 h with 100 μL of the previously blocked Dynabeads M-280 Streptavidin in order to exclude non-specific phage binders to streptavidin.
2.4 Polyclonal Phage Enzyme-Linked Immunosorbent Assay (ELISA)
Polystyrene 96-well plates were indirectly coated with biotinylated peptide through immobilized biotinylated BSA and streptavidin as described previously [44]. Positive and negative control plates were coated at 4 °C overnight with 100 μL/well of ImmunoPure biotinylated BSA (29130, Thermo Scientific Pierce, Rockford, IL, USA) at a final concentration of 2 μg/mL in PBS. After washing (3×) for 5 min with PBST, the plates were incubated at RT for 1 h with 100 μL/well of streptavidin (S888, Invitrogen, Karlsruhe, Germany) at a final concentration of 1 mg/mL in PBS containing 0.5 % gelatin while shaking gently. All incubations were followed by washing steps (3×) for 5 min with PBST. The positive and negative control plates were respectively incubated at RT for 1.5 h with 100 μL/well of the biotinylated peptide (100 nM) and 2 % M-PBS (PBS containing 2 % non-fat dried skimmed milk). After blocking at RT for 2 h with 200 μL/well of 2 % M-PBS, the PEG-precipitated polyclonal phages (10 μL) resulting from each round of selection were combined with 2 % M-PBS (100μL), added to both of the plates and incubated at RT for 1 h. Detection of the peptide-bound phages was performed through incubation at RT for 1 h with 100 μL/well of primary anti-M13 mAb (27-9420-01, GE Healthcare, Amersham, UK) and secondary goat anti-mouse IgG conjugated horseradish peroxidase (HRP) (M30107, Invitrogen, Karlsruhe, Germany) at dilution of 1:3000 in 2 % M-PBS. Staining was developed by tetramethylbenzidine (TMB) peroxidase substrate solution and stopped by adding 5 % H2SO4 (1 M).The optical density (OD) was read at OD450–OD650 [53].
2.5 Soluble Antibody Fragment ELISA
Soluble mAb fragments were produced in 96-well cell culture plates as described by Marks et al. [54] with some modifications. Briefly, 1 mL of the non-suppressor strain HB2151 in exponential growth phase (OD600 = 0.4) was infected with 10 μL of the phages (2.5 × 105cfu) eluted from the fourth round of selection and incubated at 37 °C for 45 min. After preparation of serial dilutions, the infected HB2151 bacteria were grown on TYE-AG at 37 °C overnight to obtain single colonies. The single colonies were cultured in the cell culture plates (as master plates) containing 100 μL/well of 2× yeast extract, tryptone containing 100 μg/ml ampicillin and 1 % glucose (2×YT-AG) with 10 % (v/v) phosphate buffer (0.17 M KH2PO4, 0.72 M K2HPO4) [55] and incubated on a shaker at 37 °C overnight. A small aliquot (5 μL) from the master plate was transferred to the second plate containing 200 μL phosphate-buffered 2×YT-A (PBS-2×YT) and shaken at 37 °C until the OD600 reached 0.9 (approximately 3 h). Expression of antibody fragments in the culture supernatant was induced by adding 25 μL/well of 2×YT-A containing 9 mM isopropyl β-D-thiogalactopyranoside (IPTG) and 0.9 M sucrose at final concentrations of 1 mM and 100 mM, respectively. After shaking at 30 °C overnight, the cultured plates were incubated at 4 °C for 30 min with 3×PE buffer (periplasmic extraction buffer: 60 % (w/v) sucrose, 150 mM Tris and 3 mM ethylene diamine tetraacetic acid (EDTA), pH 8.0 [56], in order to extract periplasmic scFvs into the culture supernatant. After centrifugation at 2000 ×g, 4 °C for 15 min, 80 μL/well of the supernatant containing antibody fragments were added to the antigen-coated plates that already contained 20 μL/well of 12 % M-PBS and incubated at RT for 1.5 h. The antigen coating and detection processes were carried out using the same method mentioned for polyclonal phage ELISA, except that HRP-Protein A (10-1023, Invitrogen, Karlsruhe, Germany) was used at a dilution of 1:6000 for detection of peptide-binding scFvs instead of anti-M13 mAb.
2.6 Sequence Analysis
Double-stranded phagemid DNA from each positive clone in the soluble antibody fragment ELISA assay was extracted and purified by QIAprep Spin Miniprep Kit (27104, Qiagen, Zist Baran, Tehran, Iran). Sequencing was performed by ABI3730XL DNA sequencer (Applied Biosystems, Darmstadt, Germany) using LMB3 primer. The sequencing data were analyzed by Chromas 2.33 sequencing software (Technelysium Pty Ltd, Queensland, Australia) and then amino acid sequences were aligned in a VBASE2 database [57] to compare CDRs and identify unique antibody fragment clones.
2.7 Expression of Periplasmic Single-Chain Variable Fragments (scFv)
Expression of periplasmic scFv antibodies was accomplished as small scale using 100 mL of phosphate-buffered 2×YT-AG [55, 58]. The individual HB2151 colonies containing scFv genes were grown in a shaker incubator (250 rpm) at 37 °C to reach an OD600 of 0.9. The bacteria were centrifuged at 2800 ×g for 10 min and then resuspended in phosphate-buffered 2×YT-A containing 0.4 M sucrose and 1 mM IPTG. After induction of scFv expression at 30 °C for 4 h, the bacteria were harvested by centrifugation at 2800 ×g, at 4 °C for 10 min. To extract the periplasmic scFvs, the bacterial pellets were incubated in 1:20 volume of ice-cold 1×PE buffer (i.e., periplasmic extraction buffer: 20 % (w/v) sucrose, 50 mM Tris and 1 mM EDTA, pH 8) on ice for 30 min. The supernatants containing periplasmic scFvs were obtained by centrifuging at 20000 ×g, at 4 °C for 15 min, followed by dialyzing at 4 °C overnight against PBS using a dialysis tubing cellulose membrane with 12400 Da cut-off (D9652, Sigma-Aldrich Co., Taufkirchen, Germany).
2.8 Affinity Chromatography
The dialyzed samples were subjected to an affinity chromatography procedure using Protein-A Sepharose 4B conjugate (10-1041, Invitrogen, Karlsruhe, Germany) according to the manufacturer’s instructions. Briefly, 1 mL of protein-A Sepharose suspension was placed into a 2-mL liquid chromatography column and then equilibrated with 10-column volumes of PBS, pH 7.2. The dialyzed samples were passed through the column (3×) and the column was washed with PBS until its absorbance at 280 nm reverted to zero. The bound scFvs were eluted with 0.2 M glycine-HCl (pH 3), collected as 0.5 mL aliquots and read at 280 nm. The eluted fractions containing scFv antibody fragments were collected for further analyses. The concentration of purified scFvs were determined by dividing absorbance at 280 (A280) to the molar extinction coefficient (ε 1mg/mL), in which ε was calculated for each purified scFvs using online ProtParam freeware (http://web.expasy.org/protparam/).
2.9 SDS-PAGE and Western Blotting
Expression and purification states were monitored by both sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. Total cell lysates and time-point periplasmic extracts of the selected scFv clones (both induced and un-induced conditions) were run on two separate 12 % SDS-polyacrylamide gels under reducing condition using a Miniprotean Tetra Cell system (Bio-Rad, Munich, Germany) to explore the location and induction of scFv expression. To this end, one of the parallel run gels was stained by Coomassie Brilliant Blue G-250 and the other one was used for immunoblotting by the semi-dry Trans-Blot system (Bio-Rad, Munich, Germany) as described previously by Schmiedl et al. [59]. All other procedures were performed according to the manufacturer’s instructions. Briefly, the membranes were quenched at RT for 2 h with 5 % M-PBS, probed at RT for 1 h with c-Myc (9E10) mAb (Sc-40, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:1000 in 2 % M-PBS, and stained at RT for 1 h with goat anti-mouse IgG conjugated HRP (1:5000). All incubations were followed by washing with PBST (3×) at RT for 10 min. Visualization was carried out by enhanced chemiluminescence Western blotting kit (2133,GE Healthcare, Amersham, UK) and X-ray film. Meanwhile, the purification states of the selected scFvs were also evaluated by SDS-PAGE and immunoblotting.
2.10 Immunoblotting with Soluble scFvs
For specificity analysis of the soluble scFvs, the recombinant CCK-BR protein (H000000887-P01, Novus Biological, Cambridge, UK) was expressed in a cell-free expression system run on (0.5 μg/lane) 10 % SDS-PAGE under reducing conditions, and immunoblotting was performed using polyvinylidene difluouride (PVDF) membrane (10 V, 45 min). After blocking with 5 % M-PBS overnight, the PVDF membranes were incubated with the purified scFvs (30 μg/mL) at 4 °C and washed (3×) for 5 min with PBST. Detection and visualization of the soluble scFvs were performed as mentioned above, except that c-Myc (9E10) mAb was used at a dilution of 1:500 instead of 1:1000.
2.11 Whole Cell ELISA
Specific binding of the purified soluble scFv antibody fragments to CCK-BR antigen expressing cells were verified by cell-based ELISA [48]. Briefly, the cell lines (i.e., AGS, HeLa, and NIH 3T3) were cultured in T-75 cm2 flasks at 90 % confluence and, after washing, dissociated in 10 mL of PBS – 2.5 mM EDTA solution at 37 °C for 10 min. The cell pellets were washed and resuspended in 2 % M-PBS, then 150 μL of the cell suspension (containing about 3.3 × 105 cells) was placed in each well and incubated under gentle shaking for a short period of time. The cell suspension was spun at 500 ×g for 4 min, and then resuspended in 100 μL/well of the purified scFvs at a final concentration of 10 μg/mL in 2 % M-PBS. After incubation under gentle shaking for 1 h, the cell suspensions were spun at 500 ×g for 4 min, washed and subjected for detection of bound scFvs by incubating with 100 μL/well of protein A-HRP (1/5000 in 2 % M-PBS). The cell suspensions were transferred to a fresh plate which had been previously pre-incubated with 200 μL/well of 2 % M-PBS and washed, then staining was developed by TMB (110 μL/well). Following incubation in the dark at RT for approximately 5 min, the cell suspensions were spun at 500 ×g for 4 min, and 100 μL of the supernatants were transferred to a fresh plate which previously contained 50 μL/well of 5 % H2SO4. The optical density was read at OD450–OD650. Washing for each step was performed twice with 180 μL/well of PBS and all incubations were performed at RT for 1 h, unless otherwise stated.
2.12 Flow Cytometry
Specific binding of the selected scFvs was further studied in AGS, HeLa and NIH 3T3 cells using fluorescent-activated cell sorting (FACS) flow cytometry. The pre-blocked cells in FACS buffer (1 % BSA and 0.1 % Na3 N in PBS) were incubated with 100 μL of the soluble scFvs for 1 h on ice. After washing with 1 mL buffer, the cells were incubated with 100 μL of 5 μg/mL mouse anti-Myc tag mAb (9E10) in the FACS buffer. Following washing as above, the cells were incubated with 100 μL of goat anti-mouse IgG fluorescein isothiocyanate (FITC)-labeled antibody (1 μg/mL) for 30 min on ice. The cells were washed twice and resuspended in 500 μL of PBS containing 1 μg/mL of propidium iodide (Sigma-Aldrich Co., Taufkirchen, Germany) to exclude dead cells. To assess the fluorescence intensity, 10,000 events were measured for each sample using FACSCalibur™ and analyzed with CellQuest™ Pro software (Becton Dickinson Biosciences, San Jose, CA, USA).
3 Results
3.1 Monitoring the Biopanning Process
Polyclonal phage ELISA was performed to evaluate successful accomplishment of the biopanning process. The amplified phages after each round of selection were incubated with the indirectly-coated biotinylated peptide through biotin-BSA and streptavidin. As shown in Fig. 1, the polyclonal phage ELISA indicated an initiation of enrichment for peptide-bound phage antibodies after three rounds of the selection without any notable background signal standing for non-specific binding. After the first and the second rounds of the selection process, no significant positive signal was observed above background. The enrichment of specific phage clones was improved up to 14.52-fold after four rounds of selection. The results of the monoclonal phage ELISA further confirmed successful accomplishment of the biopanning process, in which the percent of positive hits increased from 3.13 % in round 3 to 11.97 % in round 4.
3.2 ELISA Screening for the Cholecystokinin-B Receptor Peptide-Specific Soluble Antibody Fragments
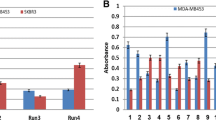
For specific binding of the soluble antibody fragments to the biotinylated peptide, ELISA was used to screen all 192 individual antibody clones obtained from selection round 4. For this purpose, the polyclonal phages eluted from selection round 4 were used to infect non-suppressor HB2151 E.coli that possess the potential to produce soluble antibody fragments without g3p. Generally, soluble antibody ELISA screening gives rise to a number of false-negative results, in part due to low yield of soluble antibody fragments in high throughput screening systems. Therefore, to increase scFv antibody production in microtiter plates, we utilized a modified media (PBS-2×YT containing sucrose) and 3×PE buffer that favored the liberation of periplasmic antibody fragments into media. The bacterial supernatants containing soluble antibody fragments were incubated with both positive- (i.e., coated peptide) and negative-background (i.e., without peptide coating) ELISA plates. Antibody clones were considered as positive hits if they showed a signal at least 3-fold greater than the negative background. As shown in Fig. 2, nine antibody clones (i.e., A6-1, F6-1, E11-1, G3-1, C1-2, C4-2, D3-2, E9-2, and E12-2) displayed a strong reaction with peptide, while a very low signal was observed for BSA-streptavidin as a negative control.
Enzyme-linked immunosorbent assay (ELISA) screening for soluble antibody clones. Individual soluble antibody clones from round 4 were screened for positive binding to the biotinylated peptide. Bacterial supernatants containing soluble single-chain variable fragment (scFv) antibody fragments were incubated with the target plates squentially coated with biotin-bovine serum albumin (BSA), streptavidin and the biotinylated peptide, and with the negative-background plates coated with biotin-BSA and streptavidin. OD optical density
3.3 Integrity and Diversity Analysis of scFv Antibody Sequences
To identify the presence of intact VH–VL genes in antibody clones and also related diversity, sequencing was performed for all the antibody clones selected from the screening step by ELISA. Sequencing results showed that eight antibody clones (i.e., A6-1, F6-1, E11-1, C1-2, C4-2, D3-2, E9-2, and E12-2) harbored the full-length size of scFv gene, and one clone (G3-1) carried only the VL gene.
Sequencing and multiple alignments of amino acid sequences indicated a diverse panel for scFv isolates. All eight full-length scFv antibodies showed a distinct sequence with most diversity occurring in the VL-CDR3 region. Based on diversity dispersion in CDR regions, we classified eight scFv antibody fragments into three groups as follows: a) scFvs C1-2 and E9-2 with diversity in VH-CDR2, VH-CDR3, VL-CDR2, and VL-CDR3 regions; b) scFvs F6-1, E12-2, and A6-1with diversity in VL-CDR2 and VL-CDR3 regions; and c) scFvs C4-2, E11-1, and D3-2 with diversity in the VL-CDR3 region.
3.4 Expression and Purification of Soluble scFv Antibodies
After identification of the individual scFv clones specific to the CCK-BR peptide, we produced sufficient quantities of scFv antibodies as reagents for downstream processes. Thus, eight scFv antibody clones, which displayed ELISA-positive reactions, were used for small scale production of antibody fragments in the periplasmic fraction. After 4 h induction, the bacterial cultures were subjected to extraction of the periplasmic scFv antibodies. SDS-PAGE and Western blotting analyses (Fig. 3a and b, respectively) showed a clear and consistent band (with molecular weight of approximately 28 kDa) in both induced cell lysates and time-point periplasmic extracted samples. Protein band width and intensity gradually increased in a time-dependent manner. Since the phage scFv library was constructed from human VH3-gene family possessing affinity for protein A, so the extracted scFvs from the periplasmic fraction were purified by protein-A Sepharose.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analyses of the selected single-chain variable fragment (scFv) antibody fragments. Expression analysis of scFvs by (a) Coomassi Brilliant Blue staining and (b) Western blotting. Purification analysis of scFvs by (c) SDS-PAGE and (d) Western blotting. Arrowheads illustrate the scFv antibody fragments. 1 h–4 h samples of periplasmic fractions after 1–4 hours of induction, E eluted fraction, F flow-through fraction, IN induced samples of total cell lysate after 4 hours culture, MM molecular mass marker (kDa), P periplasmic fraction, UN uninduced samples of total cell lysate after 4 hours culture, UNP uninduced periplasmic fraction after 4 hours culture, W washed fraction
Fig. 3c and d shows the purification process of the selected scFvs through SDS-PAGE and immunoblotting, respectively. We purified a protein band corresponding to the predicted molecular weight of the scFv, however, a small amount of the extracted scFvs was lost in flow-through and washing steps, probably due to low binding affinity of protein-A Sepharose. Based on maximum optical density at 280 nm, we categorized the purified scFvs into three groups as follows: a) scFvs with OD less than 0.05 (D3-2, A6-1); b) scFvs with OD less than 0.1 (C4-2, E9-2, F6-1); and c) scFvs with OD more than 0.1 (C1-2, E12-2, E11-1). The expression yields of the scFv clones A6-1, E11-1, F6-1, C1-2, C4-2, D3-2, E9-2, and E12-2 were 0.22, 0.63, 0.3, 0.6, 0.2, 0.16, 0.54, and 0.75 mg/L of culture, respectively.
3.5 Binding Specificity of Soluble scFv Antibody Fragments
The binding potential of the purified scFv antibody fragments to the recombinant CCK-BR antigen and native conformation of CCK-BR were respectively verified by Western blotting and whole-cell ELISA. As shown in Fig. 4, Western blot specificity analysis revealed five scFvs (E11-1, C1-2, C4-2, D3-2, and E9-2) recognized a defined protein band of 80 kDa corresponding to the expected molecular weight of recombinant CCK-BR.
Whole-cell ELISA was used to clarify whether the purified scFv antibody fragments were able to bind to the conformational structure of CCK-BR. Two CCK-BR-positive cell lines (AGS and HeLa) and one CCK-BR-negative cell line (NIH 3T3) were incubated with 10 μg/mL of the purified scFvs. Fig. 5 represents the scFv clones C1-2, D3-2, E9-2, and E12-2 that produced potent signals with both AGS and HeLa cell lines but not with NIH 3T3 cell line. On the other hand, the scFvs clones E11-1, F6-1, and C4-2 were positive with AGS without significant signal with HeLa and NIH 3T3 cell lines. ScFv clone A6-1 showed significant binding activity with both AGS and NIH 3T3 cell lines, but not with HeLa cell line. Whole-cell ELISA assays indicated that seven out of eight scFvs with positive reactivity to the biotinylated peptide were able to specifically recognize the CCK-BR antigen on the surface of the CCK-BR-positive cell lines.
Whole-cell enzyme-linked immunosorbent assay (ELISA) of the selected single-chain variable fragment (scFv) antibody fragments. Human gastric adenocarcinoma (AGS) and cervical carcinoma (HeLa) (cholecystokinin-B receptor [CCK-BR]-positive) and NIH 3T3 (CCK-BR-negative) cells were stained by the selected scFv antibody fragments (10 μg/mL), and then by protein A-horseradish peroxidase (HRP). OD optical density
3.6 Flow Cytometry Analysis
We also implemented flow cytometry analysis to revalidate the specific binding of the selected scFvs to the CCK-BR-positive cells (Fig. 6). We found significant binding activity of the selected scFvs in the CCK-BR positive cells (Fig. 6d–f), but not in the CCK-BR negative cells (Fig. 6a–c). Of the selected scFvs, D3-2, E9-2, E12-2, and A6-1 produced the highest binding potential against both AGS and HeLa cell lines, in which the responsiveness of the AGS cells to the scFvs were greater than HeLa cells (data not shown).
Fluorescent-activated cell sorting (FACS) flow cytometry analysis of the selected single-chain variable fragment (scFv) antibody fragments. Data represent the binding activity of the selected scFvs (E9-2, E12-2, and D3-2) to the cholecystokinin-B receptor (CCK-BR)-negative NIH 3T3 cells (a–c) and CC-KBR-positive human gastric adenocarcinoma (AGS) cells (d–f). The region 2 (R2) shows the scFv-bound cells. Binding of the selected scFvs was detected by 9E10 monoclonal antibody (mAb) followed by fluorescein isothiocyanate (FITC)-labeled anti mouse antibody
4 Discussion
The GPCR superfamily is a major drug target for the pharmaceutical industry. Accordingly, up until now, more than 400 small molecule drugs have been approved to target over 60 different GPCR proteins, which constitute just a small proportion of the GPCR superfamily (with over 367 members) [60]. Most of the approved small molecule drugs used for targeting GPCR proteins show poor pharmacokinetic properties, lower selectivity, and higher side effects; therefore, developing therapeutic mAbs against GPCRs seems to be a promising alternative strategy [60]. As yet, however, no GPCR-targeting immunotherapy has been approved, even though the GPCR superfamily plays pivotal roles in various diseases such as cancer, inflammation, and metabolic disorders. Selection of mAbs against GPCRs (e.g., CCK-BR) appears to be somewhat problematic. In fact, low expression and instability of purified GPCRs resulting from their structurally tight integration with plasma membrane bio-elements make them very difficult target antigens for development of biologically functional mAbs. Another dilemma related to the development of GPCR-targeting mAbs seems to be less accessibility of immunogenic epitopes for antibody binding because of the small extent of N-terminal and extracellular domains as well as masking protein core by post-translational modifications such as N-glycosylation [61].
To date, many researchers have recruited various technologies to overcome problems regarding production of mAbs against GPCRs. In this respect, some leading companies involved in the production of mAbs have capitalized on PDT along with different selection platforms. They have exploited selection processes such as whole cell panning, solution or solid-phase selection, and paramagnetic proteoliposomes for developing mAbs against a few GPCRs such as C3aR,C5aR, CCR4,CCR5, and CXCR4 (well reviewed by Hutchings et al. [60]). Although production of mAbs against some specified GPCRs appeared to be somewhat successful, a generic reproducible selection platform applicable to different GPCR members has not been achieved yet. It seems that the intrinsic properties of GPCR proteins are the major obstacle for successful selection.
In the current study, we aimed to verify the potential of PDT for production of fully human scFv antibody fragments against native conformation of CCK-BR using a semi-synthetic phage scFv antibody library and a versatile SPB approach. In this respect, ECL2 domain has been introduced as the most diverse and accessible loop in the class A GPCRs, and is deemed to be involved in activation of the class A GPCR receptors via ligand recognition, ligand binding, and signaling pathways [43]. Having considered this fact, we assumed that the ECL2 domain of CCK-BR can be an appropriate selection target to develop biologically functional mAbs. Hence, to select mAbs specific to CCK-BR, we used ‘human single-fold scFv library I’ for biopanning in a solution-phase scheme against a biotinylated synthetic peptide encompassing the ECL2 domain of CCK-BR. We then validated the specificity of the isolated clones using Western blot, whole-cell ELISA, and flow cytometry assays.
After the fourth round of the selection steps, we achieved a high enrichment of specific phage-scFv binders with a hit rate of 11.97 % (Fig. 1) under highly stringent conditions such as increasing number of washing steps (from 6 to 26 times for rounds 1–4) and decreasing the peptide concentration during successive rounds of selection. To obtain high affinity scFv antibodies, we designed a SPB scheme in which the peptide concentration was decreased in successive rounds of selection (i.e., 100 nM in rounds 1 and 2; 50 nM in rounds 3 and 4). We have also successfully carried on the selection process to the fifth round with the peptide concentration at about 10 nM (data not shown). Having used this approach, we speculate that the selected scFvs may have affinities less than 50 nM. Likewise, it has previously been shown that reducing concentration of target to a nanomolar level during subsequent rounds of selection provides selection of ligands with sub-nanomolar affinities [62].
The screening results confirmed strong binding of nine soluble antibody fragments (Fig. 2). Despite exploiting some modifications to improve production of soluble scFv antibodies on a microplate platform and thereby to increase the efficiency of the soluble antibody ELISA assay [63], the resulting positive hit rate (4.68 %) was lower than that of the monoclonal phage ELISA (11.97 %). We speculate that different reasons may attribute to such an outcome during the high-throughput screening process. First, monoclonal phage ELISA may be associated with many false-positive signals [64, 65]. Second, a soluble scFv ELISA assay is often coupled with false-negative signals due to either low yield of soluble scFvs produced on microtitre plates or lower sensitivity of soluble scFv ELISA assay [63, 66]. Further, the presence of extra amber (TAG) stop codon in CDRs of scFv antibodies isolated from semi-synthetic phage antibody libraries (e.g., Tomlinson I + J) seems to be a major obstacle for production of soluble scFv in HB2151(a non-suppressor strain), and thereby for soluble scFv ELISA screening assay [32, 67, 68].
Sequencing analyses revealed nine selected antibody fragments as eight unique scFvs and one VL single domain antibody. This diverse panel of full-length scFv antibody fragments implies that they may recognize distinct epitopes and/or bind to the same epitopes, probably with different affinities.
The selected scFv antibody fragments were produced in small scale. After purification, they were verified in terms of binding to whole CCK-BR antigen. Periplasmic expression yield of the selected scFvs using phagemid vector, pIT2, was approximately 0.16–0.75 mg/L of culture. Western blot analysis (Fig. 4) confirmed that five out of the eight ECL2-reactive scFvs (i.e., E11-1, C1-2, C4-2, D3-2, E9-2) were able to specifically recognize the recombinant CCK-BR antigen. For potential binding of the selected ECL2-reactive scFvs to intact conformation of CCK-BR antigen, we conducted the cell-based ELISA assay using live and unfixed cells according to Hoogenboom et al. [48]. ScFv clone A6-1 was considered as a cross-reactive binder as shown in both AGS and NIH 3T3 cells (Fig. 5). Four of the selected scFvs (i.e., C1-2, D3-2, E9-2, and E12-2) displayed excellent binding capability to both CCK-BR-positive cell lines (i.e., AGS and HeLa cells) without any reactivity with CCK-BR-negative cell lines (i.e., NIH 3T3 cells), while three of them (i.e., E11-1, F6-1, and C4-2) were capable of binding to AGS cells (Fig. 5). Low binding activity of the latter three scFvs toward HeLa cells could be due to: a) low binding affinity of the given scFvs; b) lower expression of CCK-BR on HeLa cells; c) inability of the given scFvs to detect specific conformational epitope of CCK-BR expressed on solely HeLa cells; or d) inaccessibility of such conformational epitope of CCK-BR to E11-1, F6-1, and C4-2 scFvs. It is also likely that the post-translational modifications (e.g., N-glycosylation) mask some epitopes of CCK-BR differently in these two cell lines. Flow cytometry analysis further indicated that the major selected scFvs can specifically detect the CCK-BR antigen in both CCK-BR-positive AGS and HeLa cells, but not in the CCK-BR-negative NIH 3T3 cells. Of the selected scFvs, E9-2 showed the highest binding affinity to AGS cells (Fig. 6). It should be highlighted that we used a low amount of scFv, therefore we expect to see much greater detection with higher concentration of scFvs.
In this study, a small number of the selected scFvs showed different binding profiles in various assays used. The scFvs E12-2 and F6-1 bound to the CCK-BR-positive AGS and HeLa cells in a cell-based assay, but not to the recombinant CCK-BR in a Western blot assay. Presumably, these scFvs can detect a native conformation of the CCK-BR receptor on the cell surface without any reaction with the denatured form of the protein in Western blot analysis – such phenomenon has previously been reported [69–71]. Lebesgue et al. [69] showed the second extracellular loop of GPCR proteins, which possesses an α-helix structure in the native form of the receptor, may be displayed as dissimilar epitopes in Western blotting (i.e., denatured form) and whole-cell ELISA (i.e., native conformation). Besides, based on the nature of the assays used, a designated antigen may differently expose a defined epitope.
In view of the cell-binding assay results, we found specific binding of seven scFv antibody fragments to the native conformation of CCK-BR on the cell surface. This clearly confirms the robustness of the SPB method for isolation of conformation-specific scFvs, in comparison with the other selection platforms. For instance, solid-phase selection with immobilized antigens or peptides on solid supports may lead to either frequent isolation of antibodies that are unable to recognize native protein in a physiological context or the enriched antibodies may be specific to the substances of the solid support [51, 72–76]. In fact, direct immobilization of a designated antigen/peptide may lead to masking/disturbing of some antigenic epitopes or displaying a new subset of epitopes. Furthermore, contrary to solid-phase methodology which often fails to enrich antibody fragments with higher biding affinity, the SPB technique permits selection of rare binders with high affinity or binders with desired binding kinetic, in part due to circumventing avidity effects and controlling antigen concentration during each round of selection [46, 52].
For the first time, we report production of fully human scFv antibodies against native conformation of CCK-BR. We envision that these mAb fragments may provide many prospective translatable clinical applications as well as research uses. It can be inferred that the produced scFv antibodies have the potential to be used in structure-function and receptor-ligand interaction studies. Further, they can be used in diagnosis, imaging, targeting, and/or immunotherapy of tumors overexpressing CCK-BR such as gastric adenocarcinoma, carcinoid cancer, colorectal cancers, pancreatic carcinoma, hepatocellular carcinoma, and stromal ovarian cancer.
5 Conclusion
Production of mAbs against plasma membrane integrated proteins such as CCK-BR is considered as a challenging issue, partly because of their inaccessibility/lower accessibility for antibody selection. To generate an appropriate mAb against CCK-BR, we have successfully used PDT through a SPB technique and isolated several scFv antibody fragments with high specificity to CCK-BR. To the best of our knowledge, no immunotherapy modality is available to target the CCK-BR receptor and this work is the first study reporting successful selection of a diverse panel of fully human scFvs with potentially specific binding toward native conformation of CCK-BR on the cell surface. While proposing the SPB strategy as a hallmark step in production of fully human scFv antibody fragments for hardly accessible antigen targets like CCK-BR, we suggest the selected scFvs could be used as targeting and/or therapeutic agents toward preclinical and clinical applications.
References
Sasako M, Inoue M, Lin JT, et al. Gastric cancer working group report. Jpn J Clin Oncol. 2010;40(Suppl. 1):i28–37.
Hartgrink HH, Jansen EP, van Grieken NC, et al. Gastric cancer. Lancet. 2009;374:477–90.
Yamashita K, Sakuramoto S, Watanabe M. Genomic and epigenetic profiles of gastric cancer: potential diagnostic and therapeutic applications. Surg Today. 2011;41:24–38.
Arkenau HT. Gastric cancer in the era of molecularly targeted agents: current drug development strategies. J Cancer Res Clin Oncol. 2009;135:855–66.
Burkitt MD, Varro A, Pritchard DM. Importance of gastrin in the pathogenesis and treatment of gastric tumors. World J Gastroenterol. 2009;15:1–16.
Polk DB, Peek RM Jr. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403–14.
McCaig C, Duval C, Hemers E, et al. The role of matrix metalloproteinase-7 in redefining the gastric microenvironment in response to Helicobacter pylori. Gastroenterology. 2006;130:1754–63.
Varro A, Noble PJ, Pritchard DM, et al. Helicobacter pylori induces plasminogen activator inhibitor 2 in gastric epithelial cells through nuclear factor-kappaB and RhoA: implications for invasion and apoptosis. Cancer Res. 2004;64:1695–702.
Smith AM, Watson SA, Caplin M, et al. Gastric carcinoid expresses the gastrin autocrine pathway. Br J Surg. 1998;85:1285–9.
Stubbs M, Khan K, Watson SA, et al. Endocytosis of anti-CCK-B/gastrin receptor antibody and effect on hepatoma cell lines. J Histochem Cytochem. 2002;50:1213–7.
Watson SA, Clarke PA, Smith AM, et al. Expression of CCKB/gastrin receptor isoforms in gastro-intestinal tumour cells. Int J Cancer. 1998;77:572–7.
Varro A, Noble PJ, Wroblewski LE, et al. Gastrin-cholecystokinin(B) receptor expression in AGS cells is associated with direct inhibition and indirect stimulation of cell proliferation via paracrine activation of the epidermal growth factor receptor. Gut. 2002;50:827–33.
Barderas R, Shochat S, Timmerman P, et al. Designing antibodies for the inhibition of gastrin activity in tumoral cell lines. Int J Cancer. 2008;122:2351–9.
Gilliam AD, Watson SA, Henwood M, et al. A phase II study of G17DT in gastric carcinoma. Eur J Surg Oncol. 2004;30:536–43.
Ajani JA, Hecht JR, Ho L, et al. An open-label, multinational, multicenter study of G17DT vaccination combined with cisplatin and 5-fluorouracil in patients with untreated, advanced gastric or gastroesophageal cancer: the GC4 study. Cancer. 2006;106(9):1908–16.
McWilliams DF, Grimes S, Watson SA. Antibodies raised against the extracellular tail of the CCKB/gastrin receptor inhibit gastrin-stimulated signalling. Regul Pept. 2001;99:157–61.
Watson SA, Clarke PA, Morris TM, et al. Antiserum raised against an epitope of the cholecystokinin B/gastrin receptor inhibits hepatic invasion of a human colon tumor. Cancer Res. 2000;60:5902–7.
Hancock WW. Chemokines and transplant immunobiology. J Am Soc Nephrol. 2002;13:821–4.
Brett BT, Smith SC, Bouvier CV, et al. Phase II study of anti-gastrin-17 antibodies, raised to G17DT, in advanced pancreatic cancer. J Clin Oncol. 2002;20:4225–31.
Reichert JM. Marketed therapeutic antibodies compendium. MAbs. 2012;4:413–5.
Stockwin LH, Holmes S. Antibodies as therapeutic agents: vive la renaissance! Expert Opin Biol Ther. 2003;3:1133–52.
Hong H, Kim S. Antibody engineering. Biotechnol Bioprocess Eng. 2002;7:150–4.
Kim SJ, Park Y, Hong HJ. Antibody engineering for the development of therapeutic antibodies. Mol Cells. 2005;20:17–29.
Mondon P, Dubreuil O, Bouayadi K, et al. Human antibody libraries: a race to engineer and explore a larger diversity. Front Biosci. 2008;13:1117–29.
Sergeeva A, Kolonin MG, Molldrem JJ, et al. Display technologies: application for the discovery of drug and gene delivery agents. Adv Drug Deliv Rev. 2006;58:1622–54.
Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–7.
Bradbury AR, Marks JD. Antibodies from phage antibody libraries. J Immunol Methods. 2004;290:29–49.
Hoet RM, Cohen EH, Kent RB, et al. Generation of high-affinity human antibodies by combining donor-derived and synthetic complementarity-determining-region diversity. Nat Biotechnol. 2005;23:344–8.
Soderlind E, Strandberg L, Jirholt P, et al. Recombining germline-derived CDR sequences for creating diverse single-framework antibody libraries. Nat Biotechnol. 2000;18:852–6.
O’Connell D, Becerril B, Roy-Burman A, et al. Phage versus phagemid libraries for generation of human monoclonal antibodies. J Mol Biol. 2002;321:49–56.
Knappik A, Ge L, Honegger A, et al. Fully synthetic human combinatorial antibody libraries (HuCAL) based on modular consensus frameworks and CDRs randomized with trinucleotides. J Mol Biol. 2000;296:57–86.
Tohidkia MR, Barar J, Asadi F, et al. Molecular considerations for development of phage antibody libraries. J Drug Target. 2012;20:195–208.
Benhar I. Design of synthetic antibody libraries. Expert Opin Biol Ther. 2007;7:763–79.
Nelson AL. Antibody fragments: hope and hype. MAbs. 2010;2:77–83.
Majidi J, Barar J, Baradaran B, et al. Target therapy of cancer: implementation of monoclonal antibodies and nanobodies. Hum Antibodies. 2009;18:81–100.
Ben-Kasus T, Schechter B, Sela M, et al. Cancer therapeutic antibodies come of age: targeting minimal residual disease. Mol Oncol. 2007;1:42–54.
Sanz L, Blanco B, Alvarez-Vallina L. Antibodies and gene therapy: teaching old ‘magic bullets’ new tricks. Trends Immunol. 2004;25:85–91.
Weiner LM, Murray JC, Shuptrine CW. Antibody-based immunotherapy of cancer. Cell. 2012;148:1081–4.
Binyamin L, Borghaei H, Weiner LM. Cancer therapy with engineered monoclonal antibodies. Update Cancer Ther. 2006;1:147–57.
Beckman RA, Weiner LM, Davis HM. Antibody constructs in cancer therapy: protein engineering strategies to improve exposure in solid tumors. Cancer. 2007;109:170–9.
Adams GP, Schier R. Generating improved single-chain Fv molecules for tumor targeting. J Immunol Methods. 1999;231:249–60.
Smith J, Kontermann RE, Embleton J, et al. Antibody phage display technologies with special reference to angiogenesis. FASEB J. 2005;19:331–41.
Peeters MC, van Westen GJ, Li Q, et al. Importance of the extracellular loops in G protein-coupled receptors for ligand recognition and receptor activation. Trends Pharmacol Sci. 2011;32:35–42.
de Haard HJ, van Neer N, Reurs A, et al. A large non-immunized human Fab fragment phage library that permits rapid isolation and kinetic analysis of high affinity antibodies. J Biol Chem. 1999;274:18218–30.
Freson K, Van Geet C, Hoylaerts M, et al. Anti-VPAC1 antibodies and theire uses. In: WO Patent WO/2009/000,894; 2008.
Huang L, Sato AK, Sachdeva M, et al. Discovery of human antibodies against the C5aR target using phage display technology. J Mol Recognit. 2005;18:327–33.
Hawlisch H, Frank R, Hennecke M, et al. Site-directed C3a receptor antibodies from phage display libraries. J Immunol. 1998;160:2947–58.
Hoogenboom HR, Lutgerink JT, Pelsers MM, et al. Selection-dominant and nonaccessible epitopes on cell-surface receptors revealed by cell-panning with a large phage antibody library. Eur J Biochem. 1999;260:774–84.
Sui J, Bai J, St Clair Tallarico A, et al. Identification of CD4 and transferrin receptor antibodies by CXCR4 antibody-guided Pathfinder selection. Eur J Biochem. 2003;270:4497–506.
Mirzabekov T, Kontos H, Farzan M, et al. Paramagnetic proteoliposomes containing a pure, native, and oriented seven-transmembrane segment protein, CCR5. Nat Biotechnol. 2000;18:649–54.
Henderikx P, Kandilogiannaki M, Petrarca C, et al. Human single-chain Fv antibodies to MUC1 core peptide selected from phage display libraries recognize unique epitopes and predominantly bind adenocarcinoma. Cancer Res. 1998;58:4324–32.
Hawkins RE, Russell SJ, Winter G. Selection of phage antibodies by binding affinity: mimicking affinity maturation. J Mol Biol. 1992;226:889–96.
Emadi S, Barkhordarian H, Wang MS, et al. Isolation of a human single chain antibody fragment against oligomeric alpha-synuclein that inhibits aggregation and prevents alpha-synuclein-induced toxicity. J Mol Biol. 2007;368:1132–44.
Marks JD, Hoogenboom HR, Bonnert TP, et al. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J Mol Biol. 1991;222:581–97.
Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 3rd ed. Cold Spring Harbor (NY): CSHL press; 2001.
Konthur Z, Wilde J. Evaluation of recombinant antibodies on protein microarrays applying the multiple spotting technique. Antibody Eng 2010; 447–60.
Retter I, Althaus HH, Münch R, et al. VBASE2, an integrative V gene database. Nucleic Acids Res. 2005;33:D671–4.
Kipriyanov SM, Moldenhauer G, Little M. High level production of soluble single chain antibodies in small-scale Escherichia coli cultures. J Immunol Methods. 1997;200:69–77.
Schmiedl A, Breitling F, Winter CH, et al. Effects of unpaired cysteines on yield, solubility and activity of different recombinant antibody constructs expressed in E. coli. J Immunol Methods. 2000;242:101–14.
Hutchings CJ, Koglin M, Marshall FH. Therapeutic antibodies directed at G protein-coupled receptors. MAbs. 2010;2:594–606.
Gupta A, Decaillot FM, Gomes I, et al. Conformation state-sensitive antibodies to G-protein-coupled receptors. J Biol Chem. 2007;282:5116–24.
Sidhu SS, editor. Phage display in biotechnology and drug discovery. Boca Raton (FL): CRC Press; 2005.
Hust M, Steinwand M, Al-Halabi L, et al. Improved microtitre plate production of single chain Fv fragments in Escherichia coli. N Biotechnol. 2009;25:424–8.
Goffinet M, Chinestra P, Lajoie-Mazenc I, et al. Identification of a GTP-bound Rho specific scFv molecular sensor by phage display selection. BMC Biotechnol. 2008;8:34.
Jensen KB, Larsen M, Pedersen JS, et al. Functional improvement of antibody fragments using a novel phage coat protein III fusion system. Biochem Biophys Res Commun. 2002;298:566–73.
Wang X, Campoli M, Ko E, et al. Enhancement of scFv fragment reactivity with target antigens in binding assays following mixing with anti-tag monoclonal antibodies. J Immunol Methods. 2004;294:23–35.
Wu S, Ke A, Doudna JA. A fast and efficient procedure to produce scFvs specific for large macromolecular complexes. J Immunol Methods. 2007;318:95–101.
Barderas R, Shochat S, Martinez-Torrecuadrada J, et al. A fast mutagenesis procedure to recover soluble and functional scFvs containing amber stop codons from synthetic and semisynthetic antibody libraries. J Immunol Methods. 2006;312:182–9.
Lebesgue D, Wallukat G, Mijares A, et al. An agonist-like monoclonal antibody against the human beta2-adrenoceptor. Eur J Pharmacol. 1998;348:123–33.
Teufel M, Pompejus M, Humbel B, et al. Properties of bacteriorhodopsin derivatives constructed by insertion of an exogenous epitope into extra-membrane loops. EMBO J. 1993;12:3399–408.
Verdot L, Bertin B, Guilloteau D, et al. Characterization of pharmacologically active anti-peptide antibodies directed against the first and second extracellular loops of the serotonin 5-HT1A receptor. J Neurochem. 1995;65:319–28.
Zhang Y, Pool C, Sadler K, et al. Selection of active scFv to G-protein-coupled receptor CCR5 using surface antigen-mimicking peptides. Biochemistry. 2004;43:12575–84.
Hall BL, Boroughs J, Kobrin BJ. A novel tumor-specific human single-chain Fv selected from an active specific immunotherapy phage display library. Immunotechnology. 1998;4:127–40.
Schwab C, Bosshard HR. Caveats for the use of surface-adsorbed protein antigen to test the specificity of antibodies. J Immunol Methods. 1992;147:125–34.
Adey NB, Mataragnon AH, Rider JE, et al. Characterization of phage that bind plastic from phage-displayed random peptide libraries. Gene. 1995;156:27–31.
Butler JE, Ni L, Nessler R, et al. The physical and functional behavior of capture antibodies adsorbed on polystyrene. J Immunol Methods. 1992;150:77–90.
Acknowledgments
This work was supported by the Digestive Disease Research Center (DDRC) at Tehran University of Medical Sciences and the Research Center for Pharmaceutical Nanotechnology (RCPN) at Tabriz University of Medical Sciences. The authors are grateful to Mr. Abolfazl Barzegari, Dr. Hossein Zareh and Dr. Safar Farajnia (Tabriz University of Medical Sciences) and Dr. Masoumeh Rajabi Bazli (Shaid Behesti University of Medical Sciences) for their useful comments.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tohidkia, M.R., Asadi, F., Barar, J. et al. Selection of Potential Therapeutic Human Single-Chain Fv Antibodies against Cholecystokinin-B/Gastrin Receptor by Phage Display Technology. BioDrugs 27, 55–67 (2013). https://doi.org/10.1007/s40259-012-0007-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-012-0007-0