Abstract
Objective
Previous studies showed that offering BRCA mutation testing to population subgroups at high risk of harbouring the mutation may be cost effective, yet no evidence is available for low- or middle-income countries (LMIC) and in Asia. We estimated the cost effectiveness of BRCA mutation testing in early-stage breast cancer patients with high pre-test probability of harbouring the mutation in Malaysia, an LMIC in Asia.
Methods
We developed a decision analytic model to estimate the lifetime costs and quality-adjusted life-years (QALYs) accrued through BRCA mutation testing or routine clinical surveillance (RCS) for a hypothetical cohort of 1000 early-stage breast cancer patients aged 40 years. In the model, patients would decide whether to accept testing and to undertake risk-reducing mastectomy, oophorectomy, tamoxifen, combinations or neither. We calculated the incremental cost-effectiveness ratio (ICER) from the health system perspective. A series of sensitivity analyses were performed.
Results
In the base case, testing generated 11.2 QALYs over the lifetime and cost US$4815 per patient whereas RCS generated 11.1 QALYs and cost US$4574 per patient. The ICER of US$2725/QALY was below the cost-effective thresholds. The ICER was sensitive to the discounting of cost, cost of BRCA mutation testing and utility of being risk-free, but the ICERs remained below the thresholds. Probabilistic sensitivity analysis showed that at a threshold of US$9500/QALY, 99.9% of simulations favoured BRCA mutation testing over RCS.
Conclusions
Offering BRCA mutation testing to early-stage breast cancer patients identified using a locally-validated risk-assessment tool may be cost effective compared to RCS in Malaysia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
As the cost of BRCA mutation testing becomes more affordable, evidence on its cost effectiveness in low- or middle-income countries (LMIC) is necessary. |
Using local data on BRCA mutation testing uptake, choices of interventions among patients tested positive and cancer survival, we demonstrated that BRCA mutation testing reduces the cases of ipsilateral/contralateral breast cancer and may be cost effective compared to the status quo of routine clinical surveillance in Malaysia. |
1 Background
BRCA mutation carriers have higher risk of breast cancer and ovarian cancer than non-carriers [1,2,3]. Nevertheless, individuals found to be carriers could reduce their risks by opting for risk-reducing interventions [1] such as mastectomy, oophorectomy, tamoxifen chemoprevention or their combinations. While BRCA mutation testing has seen a rapid increase in utilization in high income countries such as the USA [4], it has not been routinely available in the low- or middle-income countries (LMIC) due to its high cost. The lack of access to BRCA mutation testing in LMIC results in missed opportunities for cancer prevention, particularly among those at high risk of harbouring the mutation such as those with early onset cancers [5].
As low-cost testing is becoming available, policy makers in the LMIC now have the opportunity to consider adopting the test. However, to the best of our knowledge, there has been no evidence on the cost effectiveness of a BRCA mutation testing strategy in an LMIC [6, 7]. Evidence from high-income countries suggests that population-based testing (prevalence of BRCA mutation 0.02–0.06% [8]) is likely too expensive to be justified by the health gains achieved. This will likely apply to the LMIC that may not even have sufficient resources to establish and sustain the necessary lab facilities to cater for the whole population. Indeed, testing a population subgroup with a high pre-test probability of harbouring BRCA mutation such as the Ashkenazi Jewish population [9] (prevalence of BRCA mutation 2.5%) and those identified using risk prediction tools [10] (≥ 10% pre-test probability based on family history and age of cancer onset [5, 11]) has been shown to be high value for money. This evidence suggests that a BRCA mutation testing strategy in an LMIC should also focus on high-risk population subgroups.
Offering testing to patients with existing access to the healthcare system minimizes the logistic costs and challenges by reaching out to those who could benefit, and hence is more feasible than population-based testing in an LMIC. In Malaysia, BRCA mutation testing is recommended for breast cancer patients in the local clinical guidelines [12]. A risk-prediction tool to identify patients with a high pre-test probability of BRCA mutation is also validated locally [13]. However, testing is only offered in one local hospital [14, 15]. This presents an opportunity to assess the cost effectiveness of the BRCA mutation testing program. Using data from the testing program as well as those in the literature, our study examined the cost effectiveness of BRCA mutation testing in breast cancer patients with a high pre-test probability of BRCA mutation compared to routine clinical surveillance (RCS) in Malaysia, an LMIC. Our findings address the paucity of evidence in an LMIC and may advise strategies for the adoption of BRCA mutation testing in similar settings.
2 Methods
2.1 Model Overview
We prepared this article according to the CHEERS checklist [16].
We developed a decision analytic model to estimate the lifetime costs and quality-adjusted life-years (QALYs) accrued through BRCA mutation testing or RCS from the health system’s perspective. The model was adapted from published studies [10, 17] and in consultation with a genetic counsellor, a breast surgeon and a gynaecologist. In the base-case scenario, a hypothetical cohort of 1000 newly diagnosed and treated early-stage (Stages 1 or 2) unilateral breast cancer patients aged 40 years entered the model. These patients must not have previously undergone oophorectomy and must be at high risk of having BRCA mutation as identified based on locally-validated criteria [13, 18]: (a) early-onset breast cancer (≤ 40 years old) and ≥ 1 additional case(s) of breast cancer in first- or second-degree relatives; (b) breast cancer (≥ 40 years old) and ≥ 2 additional cases of breast cancer in first- or second-degree relatives; (c) bilateral breast cancer (< 50 years old) or (d) a personal or family history of breast and ovarian cancer.
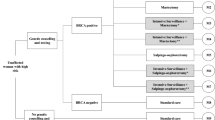
The two-part model began with a decision tree (Fig. 1a), where breast cancer patients received either BRCA mutation testing or routine clinical surveillance (RCS, usual care in institutions without BRCA mutation testing). Two types of breast cancer patients entered the model—those with only one remaining breast, because mastectomy of the affected breast was part of the breast cancer treatment before they underwent testing) and those with two remaining breasts (because they had unilateral or bilateral breast conservation surgery). Under the BRCA mutation testing arm, patients may accept or decline testing. For those who accepted testing, their tests may return positive (true or false-positive) or negative (true or false-negative) results. Those with positive test results would then undertake risk-reducing mastectomy (RRM), risk-reducing bilateral salpingo-oophorectomy (RRBSO), tamoxifen chemoprevention, combinations of these or neither (Fig. 1b). We assumed that patients who opted for RRM removed all remaining breasts, i.e. the contralateral breast for those who already had mastectomy prior to entering the model or both breasts for those who had breast-conserving surgery prior to entering the model.
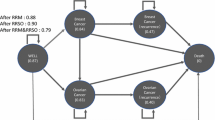
Subsequently, all patients entered the Markov model with six health states: ipsilateral breast cancer (IBC, recurrence or new primary breast cancer in the same breast), contralateral breast cancer (CBC, new breast cancer in the opposite breast), ovarian cancer (OC), at risk of IBC, CBC and/or OC (depending on the choice of risk-reducing interventions), risk-free (from IBC, CBC and OC) and death (Fig. 2). We assumed that patients would be risk-free from future breast and ovarian cancer if they underwent RRM and RRBSO, respectively. Patients moved through these health states in an annual cycle. We chose a 1-year cycle length as it reflected the nature of transition of both cancers that did not progress fast. On the other hand, patients who declined testing and those with negative test results underwent RCS, similar to patients under the RCS arm. If a patient developed a cancer, the model assumed that she either survived or died within the stage but did not recover from it (Fig. 2). Similar to all previous studies [6], our model did not differentiate testing of BRCA1 and BRCA2 mutations as there was no difference in the recommended treatment under current clinical guidelines.
Markov model. Patients who undertook both RRM and RRBSO would enter the Markov model in State B (risk-free from IBC, CBC and OC), whereas those who undertook either procedure or did nothing would enter State A (at risk) of the decision tree. Depending on the combination of interventions, patients at risk then transitioned to one of the cancer states (IBC, CBC or OC) or death in an annual cycle. The model assumed that patients in State B remain cancer free and die from other causes. IBC ipsilateral breast cancer, CBC contralateral breast cancer, OC ovarian cancer, RRM risk-reducing mastectomy, RRBSO risk-reducing salpingo-oophorectomy
We populated the model with data compiled from multiple data sources and literature searches (Supplementary File 1), prioritizing local data and data from an Asian population. Briefly, BRCA mutation testing uptake, probabilities of test results and choice of risk-reducing interventions were based on a local cohort of BRCA patients [15], cancer survival data were based on local registries [19] and cost data based on a local hospital. Utility data were derived from a mixture of Asian [20] and Caucasian populations [21,22,23,24]. Meanwhile, transition probabilities and relative risks were based on published meta-analyses [1] or large cohort studies [3, 25,26,27] from other population due to a paucity of data on Asian populations. Supplementary File 2 presents all parameters used in the model.
2.2 Likelihood of Events
To reflect the local population, we derived the BRCA mutation testing uptake rate, probabilities of test results and choice of risk-reducing interventions from the Malaysian Breast Cancer Genetic Study (MyBrCa) [15], an ongoing study recruiting breast cancer patients for genetic counselling and testing based on locally validated criteria [13, 18]. While the BRCA mutation testing uptake rate and probabilities of test results were the same regardless of the breast status (one or two remaining breasts), patients with only a contralateral breast (those who had removed the affected breast prior to BRCA mutation testing) were more willing to undergo risk-reducing interventions than those with two remaining breasts. For instance, 21.1% of patients with two remaining breasts had RRM, among whom 71.4% also opted for RRBSO [15]. Meanwhile, all patients with only a contralateral breast who had RRM (22.2%) also opted for RRBSO [15]. Similarly, no patients with two remaining breasts who had RRBSO (13.3%) opted for tamoxifen, whereas 8.3% of those with only a contralateral breast who had RRBSO (47.6%) opted for tamoxifen [15] (Supplementary File 2).
We adopted the true-positive rate of 99% and true-negative rate of 99% from a previous study [17], as the sensitivity and specificity of commercial genetic tests have been reported to be high [28].
There were two groups of untested patients—those who declined testing as well as those under the RCS arm. These patients fulfilled the BRCA mutation testing criteria [13, 18] and may have different cancer risks from the general population. We assumed the proportion of BRCA + patients in both groups was the same as those who were tested.
To date, there has been no study on the transition probabilities and relative risks of IBC, CBC and OC for BRCA + breast cancer patients in Asia. Therefore we adapted these estimates from published studies of other populations [3, 25,26,27] based on our literature search (Supplementary File 1). For consistency, we used data from meta-analyses [1] and standardized the population from which the estimates were derived as much as possible.
Within the Markov model, there were two risk groups—BRCA mutation carriers (BRCA test true positive and false negative, hereafter ‘BRCA+’) and non-carriers (true negative and false positive, hereafter ‘BRCA−‘). We obtained data on transition probabilities of IBC [27], CBC [26] and OC [25] for BRCA + from North America. The corresponding probabilities for BRCA- were estimated using the inverse of relative risks of BRCA + versus BRCA- obtained from a recent meta-analysis [1] and a large cohort study [3]: contracting IBC [relative risk (RR) 1.32, 95% confidence interval (CI) 0.70–2.46], CBC (RR 2.90; 95% CI 1.85–4.53), and OC (BRCA1: RR 44.83, 95% CI 23.87–76.66; BRCA2: RR 15.15, 95% CI 4.92–35.36). We used RRs pooled from cohort studies from the meta-analysis [1].
We assumed similar effects of risk-reducing interventions for BRCA + and BRCA- patients—undertaking RRBSO would reduce the risk of IBC by 58% (hazard ratio (HR) 0.42; 95% CI 0.22–0.81) and CBC by 48% (HR 0.52; 95% CI 0.37–0.74) [1]. Tamoxifen alone reduces the risk of CBC (HR 0.42; 95% CI 0.27–0.63) but has no significant effect on IBC (HR 0.39; 95% CI 0.09–1.69) [1]. Meanwhile, tamoxifen after RRBSO has no significant effect on CBC (HR 0.83; 95% CI 0.24–2.89) [1].
Annual survival probabilities of breast cancer and OC were estimated from 5-year survival data in a local cancer registry [19].
For two variables—the transition probability of OC [25] and relative risk of BRCA + vs BRCA- for OC [3]—we used the proportion of BRCA subtypes data in MyBrCa to combine the individual RR for BRCA1 and BRCA2 to obtain an overall RR as an overall relative risk was not reported.
2.3 Costs
For this study, we adopted the health system’s perspective with a boundary at the health system’s cost only. All costs included costs of the services as well as the workforce required to deliver the service. The costs were first inflated to Malaysian ringgit (MYR) 2016 values using the healthcare component of local consumer price index [29] where necessary. Subsequently, they were converted to 2016 US$ using the historical Central Bank of Malaysia exchange rate on 1 July 2016 (US$1 = MYR3.9913) [30].
We used direct medical care costs obtained from University Malaya Medical Centre (UMMC) database as well as the Ministry of Health Medical Cost of Services Order 2014 [31]. Public hospitals in Malaysia are tax-funded and government-run; they are the major providers of acute curative care in the country [32]. UMMC is one of the three public tertiary breast centres [32] where patients receive government subsidy similar to those at other public hospitals.
Meanwhile, the cost of BRCA mutation testing (inclusive of full BRCA sequencing, multiple ligand-dependent probe amplification, MLPA and genetic counselling) was estimated from MyBrCa [15]. The genetic counselling session would involve a genetic counsellor and a clinical geneticist who would share information on familial cancer, the test itself, and explanations of implications of a positive result, including inheritance and risk to family members.
Patients in both arms incurred the cost of clinic visits for surveillance—four times in the first year after diagnosis, twice a year from the second to fifth year, and once a year from the sixth year onwards, regardless of risk-reducing interventions. Patients who accepted BRCA mutation testing would incur a one-off cost of testing. In addition, patients in the ‘at-risk’ stage would also utilize annual mammogram and MRI screening if they did not undergo RRM; annual transvaginal ultrasound and CA125 test for OC if they did not undergo RRBSO. A diagnosis of breast cancer would require ultrasound, CT scan, biopsy and histopathology, whereas a diagnosis of OC would involve la aparoscopic day-care procedure as well as biopsy and histopathology. We assumed patients entering a cancer state to undergo surgical removal of tumour and radiotherapy. Cost of chemotherapy was the average cost for patients using various chemotherapy regimes in UMMC. We did not consider the cost of targeted therapy for breast cancer as access to targeted therapy is limited in public hospitals in Malaysia due to budget constraints [33].
2.4 Utility
We performed a literature search for the utility of each health state for the QALY estimation. As we could not locate any literature on utility values for local patients, we adopted utility values from other countries, prioritizing literature from Asian countries as much as possible.
The health-state utility values for ‘IBC’ and ‘CBC’ states were obtained from the quality of life in breast cancer patients in Singapore [20], which share a similar population composition and culture to Malaysia, whereas the utility values for ‘OC’ state [21] was adopted from North America due to the paucity of local and regional data.
Based on a systematic review [22], there have only been two studies [23, 24] eliciting utility values of breast care patients on risk-reducing interventions for BRCA mutations. We based the utility values of different ‘at-risk’ states on these two studies where patients who were ‘risk free’ had the lowest utility values due to the removal of both breasts and ovaries. As these studies did not directly elicit utility values for those who had RRBSO only, we used the difference in utility values between RRM only and RRBSO only in a previous study [17] to estimate the utility values for those who performed RRBSO. Likewise, we used the difference in utility values between ‘at risk (do nothing/monitor only)’ and ‘at risk (tamoxifen only)’ in a previous study [24] to estimate the utility values for those who had RRBSO and tamoxifen.
2.5 Base-Case Analysis
In accordance with the Malaysian Pharmacoeconomic Guideline 2012 [34], we adopted the health system’s perspective and discounted all costs and health benefits at a rate of 3% per year. All analyses were performed using Microsoft Excel 2010® (Microsoft Corp., Redmond, WA, USA). The outcomes of interest were incremental cost effectiveness ratio (ICER), measured in terms of quality-adjusted life-years (QALYs) and life-years saved (LYS).
There was no official cost-effectiveness threshold for reimbursement in Malaysia. We used one-time gross domestic product (GDP) [35], i.e. US$9500 as the threshold, which was also recommended by Malaysian Health Technology Assessment Section (MaHTAS) [36]. We also examined the ICER using a lower threshold of US$5000 as local studies [37, 38] revealed lower willingness to pay per QALY (US$4993 to US$7286) in the community.
2.6 Sensitivity Analyses
To determine the robustness of estimates from the base case, we performed one-way sensitivity analyses on all variables listed in Supplementary File 2. We altered the proportions, probabilities and utilities within their 95% CIs and cost by ± 30% of the point estimate. Discounts on cost and outcome of 0–5% were also included.
In addition, we examined the outcomes if we exclusively tested only patients with one or two remaining breasts. While we assumed that any subsequent cancer would be detected early in our base case, some patients diagnosed with advanced cancer may receive chemotherapy; some may also opt for no active treatment and receive end-of-life care in outpatient and inpatient settings. We therefore assessed the effect of adding these costs to the base-case analysis.
We performed a probabilistic sensitivity analysis (PSA) to address the uncertainty in the assumptions underlying the model by allowing all input parameter values to vary simultaneously over their respective standard errors within the model. This analysis required 1000 iterations. For cost parameters, we calculated the standard errors when we varied the base case estimate by ± 30%. All input parameters were assigned a probability distribution to reflect the feasible range of values each parameter could attain. We used a beta distribution for probability and utility variables, log normal distribution for relative risks and gamma distribution for costs [39]. To illustrate the relationship between the values of threshold and the probability of favouring each strategy, we presented a cost-effectiveness acceptability curve.
3 Results
3.1 Base-Case Analysis
In the base case, offering BRCA mutation testing for breast cancer patients fulfilling testing criteria would cost US$4815 per patient over a lifetime, higher than US$4574 for RCS. However, the testing arm also accrued higher QALYs (11.2 vs. 11.1 QALYs per patient) than the RCS arm. This resulted in an ICER of US$2275/QALY gained for testing compared to RCS, well below the threshold of US$9500/QALY gained. Total life-years per patient in the testing and RCS arms were 18.5 and 18.2, respectively, with a corresponding ICER of US$814/life-year saved (Table 1). Offering BRCA mutation testing reduced the cases of breast (two IBC and 18 CBC less in the testing arm than in the RCS arm), but increased the cases of ovarian cancer (18 more OC cases in the testing arm) (Table 2).
3.2 Sensitivity Analyses
The results of sensitivity analyses (Fig. 3) indicate that variations in proportions, transition probabilities, costs and utility values have little influence on the overall results.
One-way sensitivity analyses for ten variables which produced the ten highest variations in the incremental cost effectiveness ratio (ICER). This analysis suggests that variation in proportions, probabilities, costs and utility values only had modest effects on the model outcomes. RRM risk-reducing mastectomy, RRBSO risk-reducing bilateral salpingo-oophorectomy, RR relative risk, CBC contralateral breast cancer, IBC ipsilateral breast cancer, OC ovarian cancer, 2RB patients with two remaining breasts before being offered BRCA mutation testing, 1RB patients with one remaining breast before being offered BRCA mutation testing
While the ICERs were positive (i.e. higher cost and higher QALYs gained) at both upper and lower limits of these variables, they remained below both thresholds of US$5000 and US$9500/QALY gained. Similarly, ICERs remained below both thresholds when we exclusively tested patients with two remaining breasts (US$2871/QALY gained) or patients who had removed the affected breast (US$1841/QALY gained) as well as when the model accounted for the cost of chemotherapy (US$2113/QALY gained), terminal care (US$1856/QALY gained) or both (US$1694/QALY gained) (Tables 1 and 3).
The model was sensitive to the discounting of cost, cost of BRCA mutation testing, utility of being risk-free (undergone both RRM and RRBSO), proportion of patients tested BRCA mutation positive and CBC relative risk reduction for those who had RRBSO. Figure 3 depicts ten model inputs with the highest ICER variations. The ICERs were highest when outcome was discounted at 5% (US$3231/QALY gained) and at the higher limit of cost of BRCA mutation testing (US$3014/QALY gained), but still remained below US$5000.
The PSA (Supplementary File 3) shows that at a threshold of US$9500/QALY gained, BRCA mutation testing was cost effective compared to RCS in 99.9% of the simulations. The corresponding percentage at the threshold of US$5000 was 96.5%.
4 Discussion
Our study found that offering BRCA mutation testing to breast cancer patients at high risk of having BRCA mutation incurred higher costs over a lifetime horizon, but saved more life-years and accrued higher QALYs than RCS, with ICERs of US$814/life-year saved and US$2275/QALY gained. The ICERs remained similar even if patients incurred higher costs of treatment from chemotherapy and end-of-life treatment, indicating our findings were robust.
Our analyses contribute to the literature by examining the cost effectiveness of testing in early-stage breast cancer patients with a high pre-test probability of having BRCA mutation identified using a locally-validated tool. This mode of testing is practical in low-resource settings as patients with breast cancer are likely to be in contact with a health facility, where expertise to administer the tool and to perform the test are more readily available, thus easier to implement than offering the test to healthy individuals, as examined previously [8, 10]. To the best of our knowledge, this is also the first study to examine the cost effectiveness of a BRCA program in an LMIC as well as the first to demonstrate the effect of BRCA mutation testing on the number of IBC, CBC and OC. Other strengths of our study include the use of local data on BRCA mutation testing uptake, choices of interventions among patients tested positive, and cancer survival. Some previous studies assumed 100% uptake of BRCA mutation testing and/or risk-reducing interventions [17, 40, 41], which may not be realistic.
Our ICER was lower than those reported in the USA and Europe, likely attributable to the higher proportion of BRCA + in our eligible population (13.7%) and lower costs of risk-reducing interventions and cancer treatment. Offering the test to the general population (0.02–0.06% BRCA +) would cost up to US$2 million/QALY gained compared to no testing [8], likely beyond the willingness to pay (WTP) and affordability of most health systems. In contrast, offering the test to healthy female or ovarian cancer patients with pre-test probability > 10% would yield lower ICERs (US$3500–33,000/QALY) [42]. The only other study assessing cost effectiveness of BRCA mutation testing for breast cancer patients [17] has ICERs between US$8085 and US$112,908/QALY gained compared to no testing. In this study [17], breast cancer surgery alone cost US$17,000 (US$876 in our study). Indeed, lowering the probability of BRCA + to 3% in our model (beyond the 95% CI used in the sensitivity analyses) would increase the ICER to US$11,100/QALY gained; a probability of 0.02% would yield an ICER of US$ 1.7 million/QALY gained. This highlights the importance of identifying patients with a high pre-test probability before offering the BRCA mutation testing where shared decision making between clinicians and patients play a pivotal role. This may also represent a risk of the actual ICER being above the WTP threshold if the selection of patients for testing was not done properly, for example due to a lack of trained personnel in a low resource setting.
Considering the age-standardized annual incidence of 38.7 breast cancer per 100,000 female population and 5410 new cases in 2012 [43], the overall effect of BRCA mutation testing on reducing breast cancer cases is small (20 cases in 1000 eligible patients, or 0.02%; Table 2). Nevertheless, the impact was much larger among those who accepted the test and tested positive [25.0% = 20/80 i.e. (1000 eligible × 0.578 uptake × 0.137 tested positive)]. One unexpected finding was the higher number of ovarian cancer cases in BRCA mutation testing than the RCS arm (31 vs. 23 cases), which may be due to high proportions of BRCA + patients opting for RRM or tamoxifen but not RRBSO (45% and 75% among those with one and two remaining breasts, respectively), hence still remained susceptible to ovarian cancer.
4.1 Limitations
As is the case with all modelling studies, our results depend on the data and assumptions used. Our model assumed that patients would have either an additional breast cancer (ipsilateral or contralateral) or an ovarian cancer, but not both, due to the lack of evidence on transition probabilities for these patients to acquire further cancers, similar to a previous study which offered BRCA mutation testing for breast cancer patients [17]. Accounting for these transitions may increase the overall cost, particularly in the RCS arm as no BRCA carriers in the RCS arm would have the opportunity to undergo any risk-reducing interventions, leading to more cancer cases and higher treatment costs. While this may reduce the ICER as observed in our sensitivity analyses when we added cost of end-of-life care and chemotherapy into the model (Table 3), we expect the magnitude of reduction to be small due to the low cancer survival rate, especially that of ovarian cancers. Due to the lack of local data, we used transition probabilities, relative risks and utility values derived from Caucasian populations. However, varying these estimates within their 95% CI showed that our base-case ICER was robust.
Although the risk reduction with tamoxifen among those without RRBSO did not have high evidence studies to date [1], we incorporated it in the model as some patients in our setting opted for tamoxifen alone without RRBSO. Future studies may be necessary to ascertain the effect size of such an intervention. Local data in BRCA mutation testing uptake, choices of risk-reducing interventions and costs were based on a single institution (the only institution in the country offering BRCA mutation testing at the point of writing), which may not be generalizable to all health facilities if the BRCA mutation testing strategy is rolled out nationally. We did not explicitly factor in an increase or decrease of utility as a result of positive or negative test results as there has been no empirical evidence on the impact of knowing BRCA status and health-related quality of life. In addition, the test result is genetic information that represents a risk or association, not a medical diagnosis. Thus, any impact on patients’ utility would likely be small and transient.
Our model also assumed patients made decisions for testing and risk-reducing interventions within a year after being tested positive and would be compliant to routine surveillance. We also only considered risks and benefits for breast cancer patients without cascade testing among relatives. The model did not account for costs of establishing laboratories and training to perform the test; neither did it account for possible injuries and non-fatal complications due to risk-reducing interventions, e.g. higher cardiovascular risks with tamoxifen. Similarly, our model assumed patients would become disease free after RRM/RRBSO, although patients may still be susceptible to local or systemic recurrences from the primary cancer.
We adapted our model from previous studies in consultation with local clinicians to attain face validity. However, due to the potential implication on BRCA mutation testing programs in LMICs, future studies should consider calibrating the model when more data are available from longitudinal cohorts such as MyBrCa. We were unable to do this for the current study due to limited outcome data at the time of writing.
4.2 Policy Implications
Our findings would be applicable for patients recently diagnosed with breast cancer at early stages (Stages 1 or 2), for whom removing either or both breasts is not part of their routine treatment. With the modest cost per life-year saved and cost per QALY gained, it may be worthwhile for policy makers to reimburse BRCA mutation testing for high-risk patients alongside efforts to increase uptake of population surveillance and to improve access to better breast cancer treatment. Our small incremental cost (US$241, Table 1) implies that providing BRCA mutation testing to our target patients may not incur a substantially higher budget impact than RCS (current usual care). Nevertheless, there may be significant costs involved in establishing and maintaining the laboratory facilities with trained personnel, which our model did not account for.
5 Conclusion
In conclusion, our study provides the first evidence on cost effectiveness of BRCA mutation testing among female breast cancer patients with a high pre-test probability of harbouring a BRCA mutation in an LMIC, using local data on test uptake, choices of risk-reducing interventions and cancer survivals. The BRCA mutation testing program had a modest incremental cost of US$814/life-year saved and US$2275/QALY gained over a lifetime horizon. For the 1000 eligible patients tested, it prevented two IBC and 18 CBC, but resulted in an increase of eight ovarian cancers as patients who undertook RRM were still susceptible to ovarian cancer. While the reduction in breast cancer cases is small relative to its annual incidence, it represents up to 26.3% reduction among patients who accepted the test and tested positive.
References
Valachis A, Nearchou AD, Lind P. Surgical management of breast cancer in BRCA-mutation carriers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;144(3):443–55.
Mavaddat N, et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst. 2013;105(11):812–22.
Ingham SL, et al. Ovarian cancer among 8,005 women from a breast cancer family history clinic: no increased risk of invasive ovarian cancer in families testing negative for BRCA1 and BRCA2. J Med Genet. 2013;50(6):368–72.
Guo F, et al. Use of BRCA Mutation Test in the US, 2004–2014. Am J Prev Med. 2017;52(6):702–9.
Evans DGR, et al. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. J Med Genet. 2004;41(6):474.
D’Andrea E, et al. Which BRCA genetic testing programs are ready for implementation in health care? A systematic review of economic evaluations. Genet Med. 2016;18(12):1171–80.
Oosterhoff M, van der Maas ME, Steuten LMG. A systematic review of health economic evaluations of diagnostic biomarkers. Appl Health Econ Health Policy. 2016;14(1):51–65.
Tengs TO, Berry DA. The cost effectiveness of testing for the BRCA1 and BRCA2 breast-ovarian cancer susceptibility genes. Dis Manag Clin Outcomes. 2000;1:15–24.
Manchanda R, et al. Cost-effectiveness of population screening for BRCA mutations in Ashkenazi jewish women compared with family history-based testing. J Natl Cancer Inst. 2015;107(1):380.
Holland ML, Huston A, Noyes K. Cost-effectiveness of testing for breast cancer susceptibility genes. Value Health. 2009;12(2):207–16.
Antoniou AC, et al. Predicting the likelihood of carrying a BRCA1 or BRCA2 mutation: validation of BOADICEA, BRCAPRO, IBIS, Myriad and the Manchester scoring system using data from UK genetics clinics. J Med Genet. 2008;45(7):425–31.
Ministry of Health Malaysia and Academy of Medicines Malaysia. Management of Breast Cancer, 2nd Ed. Ministry of Health Malaysia: Putrajaya; 2010. http://www.moh.gov.my/penerbitan/CPG2017/6915.pdf.
Thirthagiri E, et al. Evaluation of BRCA1 and BRCA2 mutations and risk-prediction models in a typical Asian country (Malaysia) with a relatively low incidence of breast cancer. Breast Cancer Res. 2008;10(4):R59.
Yoon SY, et al. Genetic counseling for patients and families with hereditary breast and ovarian cancer in a developing Asian country: an observational descriptive study. Fam Cancer. 2011;10(2):199–205.
Cancer Research Malaysia. Breast Cancer Current Research and Programme: MyBrCa and MyMammo. 2016 05/06/2016]. http://www.cancerresearch.my/our-research/breast-cancer-research/more-than-a-mammo-programme/. Accessed 20 June 2017.
Husereau D et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ Br Med J 2013;346:f1049.
Kwon JS, et al. Expanding the criteria for BRCA mutation testing in breast cancer survivors. J Clin Oncol. 2010;28(27):4214–20.
Toh GT, et al. BRCA1 and BRCA2 germline mutations in Malaysian women with early-onset breast cancer without a family history. PLoS One. 2008;3(4):e2024.
Allemani C, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2). The Lancet. 2015;385(9972):977–1010.
Tan X-Y, et al. Assessment of preference for hormonal treatment-related health states among patients with breast cancer. Value Health Reg Issues. 2014;3:27–32.
Havrilesky LJ, et al. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol. 2009;113(2):216–20.
Peasgood T, Ward SE, Brazier J. Health-state utility values in breast cancer. Expert Rev Pharmacoecon Outcomes Res. 2010;10(5):553–66.
Grann VR, et al. The quality of life associated with prophylactic treatments for women with BRCA1/2 mutations. Cancer J Sci Am. 1999;5(5):283–92.
Cappelli M, et al. Measuring women’s preferences for breast cancer treatments and BRCA1/BRCA2 testing. Qual Life Res. 2001;10(7):595–607.
Metcalfe KA, et al. The risk of ovarian cancer after breast cancer in BRCA1 and BRCA2 carriers. Gynecol Oncol. 2005;96(1):222–6.
Metcalfe K, et al. Predictors of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2011;104(9):1384–92.
Metcalfe K, et al. Risk of ipsilateral breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat. 2011;127(1):287–96.
Nelson HD, et al. Genetic risk assessment and brca mutation testing for breast and ovarian cancer susceptibility: systematic evidence review for the US preventive services task force. Ann Intern Med. 2005;143(5):362–79.
Department of Statistics Malaysia. Malaysia economics statistics—time series 2015. 2015 30/12/2015 10/05/2016]. https://www.statistics.gov.my/index.php?r=column/ctimeseries&menu_id=NHJlaGc2Rlg4ZXlGTjh1SU1kaWY5UT09. Accessed 20 June 2017.
Central Bank of Malaysia. Kuala Lumpur US$/MYR Reference Rate. 2016 20/06/2017]. http://www.bnm.gov.my/index.php?ch=statistic&pg=kualalumpurusdmyrreferencerate. Accessed 20 June 2017.
Attorney General’s Chambers of Malaysia. Fees (Medical Cost of Services) Order 2014. Putrajaya: Attorney General’s Chambers of Malaysia; 2014.
Clinical Research Centre (CRC). National Healthcare Establishment and Workforce Statistics (Hospital) 2011. Kuala Lumpur: Clinical Research Centre, Ministry of Health Malaysia; 2013.
Lim GCC, et al. Closing the global cancer divide—performance of breast cancer care services in a middle income developing country. BMC Cancer. 2014;14:212.
Ministry of Health Malaysia. Pharmacoeconomic guideline for Malaysia. Kuala Lumpur: Pharmaceutical Services Division; 2012.
Hutubessy R, et al. Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Effect Resour Alloc C/E. 2003;1:8.
Malaysian Health Technology Assessment Section. Establishing a cost-effectiveness threshold value for health technologies Newsletter, Vol 17. Putrajaya: Malaysian Health Technology Assessment Section (MaHTAS) Medical Development Division Ministry of Health Malaysia; 2015.
Shafie AA, et al. Exploring the willingness to pay for a quality-adjusted life-year in the state of Penang, Malaysia. ClinicoEcono Outcomes Res CEOR. 2014;6:473–81.
Lim YW, et al. PHP201—Determination of cost-effectiveness threshold for Malaysia. Value Health. 2014;17(7):A438.
Briggs A, Claxton K, Sculpher M. Chapter 4: making decision model probabilistic. In: Grey A, Briggs A, editors. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2011.
Heimdal K, Maehle L, Moller P. Costs and benefits of diagnosing familial breast cancer. Dis Mark. 1999;15(1–3):167–73.
Balmana J, et al. Genetic counseling program in familial breast cancer: analysis of its effectiveness, cost and cost-effectiveness ratio. Int J Cancer. 2004;112(4):647–52.
Kwon JS, et al. Preventing future cancers by testing women with ovarian cancer for BRCA mutations. J Clin Oncol. 2010;28(4):675–82.
International Agency for Research on Cancer (IARC). GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012: Malaysia. 2012 20/12/2016]. http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. Accessed 20 June 2017.
Data Availability Statement
All data used in the analyses are referenced and described in the text and listed in Supplementary File 2. All other information is available from the corresponding authors on reasonable request.
Author information
Authors and Affiliations
Contributions
SYY, SHT and NC conceptualized the research idea; KKL and NC formulated the research questions, and designed and performed the analysis; NAMT, YLW and MKT assisted in model building by providing their clinical inputs, FHS and MD provided secondary data on resource consumption, and assisted in data analysis and interpretation of findings. KKL prepared the first draft of the manuscript. All authors were responsible for critically revising the manuscript and agreed on the final content before submission.
Corresponding author
Ethics declarations
Funding
No funding was involved for this study.
Human or animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
All authors (KKL, SYY, NAMT, FHS, MD, YLW, MKT, SHT, NC) declare no conflicts of interest related to BRCA mutation testing.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lim, K.K., Yoon, S.Y., Mohd Taib, N.A. et al. Is BRCA Mutation Testing Cost Effective for Early Stage Breast Cancer Patients Compared to Routine Clinical Surveillance? The Case of an Upper Middle-Income Country in Asia. Appl Health Econ Health Policy 16, 395–406 (2018). https://doi.org/10.1007/s40258-018-0384-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-018-0384-8